Verifying the clinical effectiveness and the impact on quality-of-life parameters, fear of hypoglycaemia and satisfaction with the treatment obtained with a flash glucose monitoring (MFG) devices implantation program that includes a telematic and group educational intervention in adults with type 1 diabetes.
Patients and methodsProspective quasi-experimental study, carried out during the COVID-19 pandemic period with a 9-month follow-up at the Virgen Macarena University Hospital, Sevilla.
ResultsEighty-eight participants were included (men: 46.6%; mean age (years) 38.08, SD: 9.38); years of DM1 evolution: 18.4 (SD: 10.49); treatment with multiple doses insulin (MDI) 70.5% vs 29.5% subcutaneous insulin infusion therapy (CSII)). Baseline HbA1c was 7.74% (1.08). After the intervention, the global decrease in HbA1c was −0.45% (95% CI [−0.6, −0.25], P < 0.01), increasing to −1.08% in the group that started with HbA1c ≥ 8% (P < 0.01). A mean decrease in the Fear of Hypoglycemia 15 (FH15) test score of −6.5 points was observed (P < 0.01). In the global score of the Spanish version of Diabetes Quality Of Life (DQOL-s) test, the decrease was −8.44 points (P < 0.01). In Diabetes Treatment Satisfaction Questionnaire test (DTQ-s), global score increased in + 4 points (P < 0.01).
ConclusionsThe incorporation of an educational program in group and telematic format within the development of MFG devices implantation strategies is an effective option, with associated benefits in quality of life and fear of hypoglycemia in adult patients with DM1. This option can be implemented in usual clinical practice.
Comprobar la efectividad clínica y la repercusión sobre los parámetros de calidad de vida, miedo a hipoglucemias y satisfacción con el tratamiento obtenidas con un programa de implantación de sistemas de monitorización intermitente o tipo flash de glucosa (MFG) que incluye una intervención educativa telemática y grupal en adultos con diabetes mellitus tipo 1 (DM1).
Material y métodosEstudio cuasiexperimental prospectivo, realizado durante el período de pandemia COVID-19 con un seguimiento de 9 meses en el Hospital Universitario Virgen Macarena, Sevilla.
ResultadosSe analizaron 88 participantes (varones: 46,6%; edad media: 38,08 años (desviación estándar [DE]: 9,38); tiempo de evolución DM1: 18,4 años (DE: 10,49); tratamiento con múltiples dosis de insulina (MDI) 70,5 vs. 29,5% bombas de infusión subcutánea continua de insulina (ISCI)). HbA1c basal del 7,74% (DE: 1,08). Tras la intervención el descenso global de HbA1c fue del −0,45% (p < 0,01), aumentando a −1,08% en el grupo que partía con HbA1c ≥ 8% (p < 0,01). El descenso medio en la puntuación del test Fear of Hypoglycaemia (FH15) fue de −6,5 puntos (p < 0,01), en el test Diabetes Quality of Life en español (EsDQOL): −8,44 puntos (p < 0,01), y en el test Diabetes Treatment Satisfaction Questionnaire (DTQ-s): +4 puntos (p < 0,01). No se registraron eventos adversos locales ni complicaciones agudas o crónicas de la diabetes durante el seguimiento.
ConclusiónLa incorporación de un programa educativo en formato grupal y telemático sobre el uso de dispositivos de MFG dentro del desarrollo de estrategias de implantación de estos sistemas es una opción efectiva y con beneficios asociados en calidad de vida y miedo a hipoglucemias, implementable en la práctica clínica habitual en pacientes adultos con DM1.
Intensive monitoring of blood glucose in patients with type 1 diabetes mellitus (DM1) is one of the fundamental therapeutic aspects for achieving an optimal degree of metabolic control and reducing chronic complications associated with this disease.1,2 In recent times, the application of advanced technologies to the field of health in general, and diabetes in particular, has allowed the development of new tools for self-monitoring of blood glucose, such as intermittent or flash glucose monitoring (FGM) systems.3
Numerous investigations have shown how the use of this technological resource in patients with DM1 is related to a significant improvement in metabolic control parameters. In this sense, in the IMPACT study (main clinical trial carried out on FGM systems), it was observed how the use of these devices was related to a decrease in time in hypoglycaemia, compared to capillary blood glucose self-monitoring.4 Meanwhile, recent meta-analyses have reported data suggesting that using these systems could contribute to achieving a reduction in HbA1c.5,6
The good results associated with their use, cost-effectiveness and increasing accessibility have initiated a paradigm shift in the clinical care of people with diabetes. They are often publicly financed for patients with DM.7,8
In this sense, structured diabetes education plays a fundamental role in initiating the use of advanced technologies applied to this field. In the case of FGM, a structured educational program could provide patients with greater training in the management of these systems, as well as an improvement in the management of the volume of blood glucose information, thus optimising the results obtained.8
Medical societies and leading health organisations have developed specific guidelines and recommendations for implementing FGM systems, which indicate the need and convenience of programming specific educational interventions for training in these devices.9
In addition, a telematic group format could be especially useful, simplifying logistical and material aspects and solving problems in face-to-face physical attendance at health centres.10 The epidemiological situation experienced due to the COVID-19 pandemic during 2020 and 2021 highlighted the need to have non-face-to-face healthcare strategies, especially at times when it is necessary to limit citizen mobility.11
To date, our group has not found publications assessing the clinical effectiveness of a therapeutic education programme in diabetes implemented in a telematic and group format for this technology.
For this reason, we propose this study to verify the effectiveness of glycaemic control parameters⠀ and quality of life variables obtained with a programme for implementing and quality of life variables obtained with a programme for the implementation of FGM systems that includes a telematic educational group intervention in adults with DM1, carried out during the COVID-19 pandemic in Spain.
Material and methodsStudy designA quasi-experimental design was followed, with a single prospective follow-up cohort at nine months. The study was carried out in the facilities of the Department of Endocrinology and Nutrition of the Hospital Universitario Virgen Macarena de Sevilla (HUVM) [Virgen Macarena University Hospital] in Seville between March 2020 and April 2021, in the context of the increased free distribution of FGM devices to all patients with DM1 by the Andalusian Public Health System (Spain)12 and the epidemiological situation of the COVID-19 pandemic in Spain.
PatientsThe participants were systematically selected from the service's internal patient registry applying the following inclusion criteria: older than 18 years, definitive diagnosis of DM1 (ADA 202113 criteria), treatment with multiple doses of insulin (MDI) or continuous subcutaneous insulin infusion (CSII) with self-monitoring through capillary glucose measurements, no previous experience with FGM systems, and possession of basic skills for managing a real-time videoconference system and email communication. Patients with functional physical or cognitive impairments that prevented participation in the educational intervention or previous experience using FGM systems and those who were pregnant or planning to become so were excluded.
InterventionA specific therapeutic education programme was designed in a group format (between 10–15 participants per group) to train patients in the use of FreeStyle® Libre 2 FGM devices (Abbott Laboratories). Some graphic supporting material in the form of a slide presentation covering all the device's fundamental aspects was also prepared. This was subsequently used during the sessions in all intervention groups equally. The coordination and delivery of the programme were carried out by the same healthcare professional with a diabetes educator profile.14
The educational intervention consisted of two group sessions of approximately 2 h each, with a break of 45–60 days between them, and held in virtual format via videoconference in real time. This was done using the Zoom® videoconferencing application (Zoom Video Communications, San Jose, California, USA), with the patients' prior express consent. To encourage interaction on the part of those attending each session, fixed questions were asked about the level of previous knowledge throughout the intervention. A forum space was scheduled in the final section for any open questions.
Finally, three months after the first session, the patients were reassessed in consultation with their treating physician for consolidation and adherence supervision in a third session.
Table 1 summarises the education programme’s content, the specific objectives and the work plan.
Structural diagram of the therapeutic education programme on FGM management used in the intervention.
| Scheduled date | Duration | Type of intervention | Objectives | |
|---|---|---|---|---|
| Session 1 | Start of use of FGM devices | 2 h | Basic training session.Group | 1. Differentiate between capillary and interstitial blood glucose.2. Identify situations that require measurement of capillary blood glucose.3. Proceed to the first insertion of the device. Explanation of the parts of the system and method of application.4. Interpret the data on the screen, glucose readings and trend arrows.5. Calculate of insulin dose from interstitial glucose figure and trend.6. Review concepts of basic diabetes education (actions against hypo-/hyperglycaemia).7. Inform about support web applications and contact telephone number if incidents appear. |
| Session 2 | 30−45 days after session 1 | 2 h | Advanced training session.Group | 1. Interpret data from the reports and main variables (time in range, average glucose, estimated HbA1c, hypoglycaemic episodes, sensor download, data on insulin and carbohydrates).2. Managing trend arrows.3. Make therapeutic adjustments based on the data provided by the device.4. Programming of sound alarms in the event of hyper/hypoglycaemia. |
| Session 3 | Three months after session 1 | 2 h | Review of adherence and results. Individual | 1. Solve doubts and individual therapeutic reinforcement.2. Review adherence and results.3. Analyse retrospective blood glucose data with each patient. |
FGM: intermittent or flash-type glucose monitoring.
The process of incorporating participants into the programme was carried out by telephone contact to plan the dates of the sessions, guaranteeing that at least three attempts were made before proceeding to their non-inclusion due to loss of contact.
The consumable material (FGM sensor and applicator) was sent to each participant's home by ordinary courier to be available at least 24 h before the start of the intervention. Before the end of the first session, the participants started the FGM systems for the first time. They applied the sensor following the instructions and supervision of the educator in real-time.
The date and content of each session, and individual incidents, if any, were registered in the electronic medical record of each patient, for subsequent follow-up.
VariablesThe main outcome variable was the difference in HbA1c measured before and nine months after the start of the intervention (laboratory technique: HPLC, normal range of our laboratory: 4.0–5.5%). The rest of the variables are listed below:
Baseline sociodemographic and clinical variablesBaseline or pre-intervention variables included age, sex, years with diabetes and age at onset, presence of micro- and chronic macrovascular complications, insulin therapy method, total insulin dose in 24 h (IU) and a number of severe hypoglycaemia episodes that occurred in the last year (defined as those that require help from third parties to resolve). The baseline values of mean blood glucose, coefficient of variation (CV) and the number of daily capillary blood glucose controls were extracted from the downloads from the personal glucose meters of each participant.
Blood glucose variables provided by intermittent or flash glucose monitoring systemsFrom the ambulatory glucose reports (AGP) provided by the configured FGM devices for the previous 14 days, the following variables were explored: percentage (%) of time of sensor use, number of daily scans, number of patients with the use of sensor greater than 80%, percentage of time in target range (70−180 mg/dl), percentage of time in hypoglycaemia <70 and <54 mg/dl, percentage of time in hyperglycaemia >180 and >250 mg/dl, mean blood glucose, CV, glucose management indicator (GMI), number of hypoglycaemic events, average duration (min) and presence of nocturnal hypoglycaemia.
Psychosocial variables and questionnairesVariables related to quality of life and psychosocial aspects were analysed through the following questionnaires:
The Fear of Hypoglycaemia Scale (FH-15): consists of 15 items measured using a 5-point Likert scale15 (1–5), which assesses three behavioural factors (fear, avoidance and interference). The cut-off point is 28 points (a higher score indicates fear of hypoglycaemia).16
Diabetes Quality of Life questionnaire, Spanish version (EsDQoL): a questionnaire designed by the DCCT Research Group. It contains 43 items scored using a 5-point Likert scale. It is divided into four subscales: impact of diabetes on daily life, concerns related to diabetes, satisfaction with the disease, and social concerns. The lower the score, the better the perceived quality of life.17
Diabetes Treatment Satisfaction Questionnaire (DTSQ): used to numerically quantify the degree of satisfaction with diabetes treatment at a given time. Two of its items also assess the patient's self-perception of hyper- and hypoglycaemia. It consists of eight items, scored on a Likert scale between 0 and 6 points each (the higher the score, the greater the satisfaction with the treatment).18
Schedule and analysis of resultsThe baseline variables were collected during the three months prior to the start of the intervention. After completing nine months of follow-up (taking the first educational session as a time reference), a post-intervention face-to-face visit was carried out in which the HbA1c level was measured and the psychosocial questionnaires were administered again. The before/after differences were subsequently calculated. For the analysis of the changes in mean blood glucose and CV, the mean blood glucose and CV values were extracted from the AGP reports for the previous 14 days, which were taken as post-intervention values.
In a complementary way, during months 1, 3 and 6 of the follow-up period, intermediate measurements of the variables provided by the AGP reports were made; with the aim of obtaining more information on the change observed in the metabolic control of the participants.
Once the study was completed, univariate sub-analyses were performed for the description of possible interactions, studying the influence of the following variables on the results: age groups (≤25 years, 26−45 years; 46–59 years; ≥60 years), sex, method of insulin therapy and degree of previous metabolic control.
Subsequently, a multiple linear regression model was performed in which "change in HbA1c" was included as the dependent variable and the baseline HbA1c level, sex, age, years with diabetes and insulin therapy method were included as independent variables (MDI/CSII).
Statistical analysisThe estimated sample size was 87 participants to detect differences of 0.4% of HbA1c with a confidence level of 95% (95% CI) and an estimate of 15% losses/dropouts, based on the results of the study by Gordon et al.6
For the test of normality of the sample distributions, the Shapiro-Wilk test was used. The differences between paired dichotomous variables were analysed using the McNemar-Bowker test. The continuous quantitative variables were analysed using the Student's t-test for paired variables and its non-parametric alternative (Wilcoxon test). The one-way ANOVA test and its non-parametric alternative (Kruskal-Wallis test) were used for qualitative variables of more than two categories. An analysis of variance model was used to study the variables related to hypoglycaemia (with intermediate measurements during follow-up) with repeated measures for quantitative variables. We used the Cochrane test to study the interaction of third variables on the results in the FH15 test. The multivariate linear regression model performed to evaluate predictive variables of improvement in metabolic control (HbA1c) was developed using the stepwise method. A p value <0.05 was considered statistically significant. Data analysis was performed using the software IBM SPSS® Statistics version 26.0.
Safety of the interventionBefore the intervention, the participants were given a specific telephone number to call in case of incidents regarding the operation or implementation of the FGM systems and a contact e-mail address. During the follow-up period of the study, the incidence of cutaneous adverse reactions related to the insertion of the sensor, the rate of acute complications related to diabetes decompensation (ketoacidosis, hyperglycaemia or hypoglycaemia) and the rate of chronic complications (coronary heart disease, stroke or peripheral artery disease) that would have required hospital admission and/or intra- or out-of-hospital emergency consultations.
Ethical aspectsAll participants signed an informed consent form before being recruited. A study protocol was prepared and approved by the HUVM internal biomedical research ethics committee.
ResultsPatients and baseline characteristicsInitially, 98 participants were identified as candidates to participate in the study, of which 93 were finally included based on the inclusion/exclusion criteria. The reasons for exclusion were: previous experience with the use of FGM systems (n = 4; 80%) and functional impairments that prevented participation in the telematic intervention (severe visual impairment) (n = 1; 20%).
The percentage of losses during follow-up was 5.37% (n = 5), the reasons being: non-attendance to the second educational session (n = 3; 3.23%) and active sensor time <80% (n = 2, 2.15%). The basic sociodemographic and clinical characteristics of the lost patients were similar to those of the patients who completed the study.
The baseline characteristics of the participants are shown in Table 2.
Descriptive baseline variables of the sample.
| Socio-demographic and clinical variables | Dyslipidaemia, n (%) | 15 (17) | |
| No. of patients: n (%) | 88 (100) | Arterial hypertension, n (%) | 9 (10.2) |
| Gender | Active smoker, n (%) | 14 (15.9) | |
| Females, n (%) | 47 (53.4) | Total insulin units/24 ha | 44.21 (20.86) |
| Males, n (%) | 41 (46.6) | Blood glucose control variablesa | |
| Age (years)a | 38.08 (9.38) | HbA1c (%) | 7.74 (1.08) |
| Age at start (years)a | 19.53 (12.12) | Mean blood glucose (mg/dl) | 155.79 (30.38) |
| Years with DMa | 18.4 (10.49) | Coefficient of variation (%) | 46 (13) |
| Microvascular complications, n (%) | Mean number of daily capillary blood glucose controls | 4.56 (2.99) | |
| Retinopathy | 14 (15.9) | Baseline scores of psychosocial questionnairesa | |
| Nephropathy | 2 (2.3) | FH15 score | 38.89 (13.28) |
| Neuropathy | 5 (5.7) | FH15 (≥28 points), n (%) | 41 (74.5) |
| Macrovascular complications, n (%) | EsDQoL - total | 101.43 (25.5) | |
| Ischaemic heart disease | 2 (2.3) | EsDQoL - satisfaction | 38.07 (10.19) |
| Stroke | 1 (1.1) | EsDQoL - impact | 38.26 (11.56) |
| Peripheral arterial disease | 1 (1.1) | EsDQoL - social/vocation | 14.52 (5.95) |
| Severe hypoglycaemia (last year), n (%) | 6 (6.82) | EsDQoL - diabetes concern | 10.57 (3.2) |
| Insulin therapy method | DTSQ - treatment satisfaction | 26.02 (5.77) | |
| MDI, n (%) | 62 (70.5) | DTSQ - perception of hyperglycaemia | 3.22 (1.49) |
| CSII, n (%) | 26 (29.5) | DTSQ - perception of hypoglycaemia | 2.76 (1.61) |
| BMI (kg/m2)a | 25.87 (4.53) | ||
| Obesity, n (%) | 15 (17) | ||
DM: diabetes mellitus; BMI: body mass index; CSII: continuous subcutaneous insulin infusion; MDI: multiple doses of insulin.
The number of patients with a sensor active time greater than 80% at the end of follow-up was 95.6% (n = 88), with a mean number of daily scans of 12.4 (SD: 10.5).
Metabolic control variablesAfter nine months of follow-up, a significant mean decrease in the HbA1c level (main variable) of 0.45% (95% CI: −0.6; −0.25; p < 0.01) was observed.
Table 3 summarises the main results of the study.
Results (before/after differences).
| Baseline visit | Visit at 9 months | Difference | 95% CI | p-value | |
|---|---|---|---|---|---|
| Blood glucose control variables | |||||
| HbA1c %, mean (SD) | 7.69 (1.2) | 7.17 (1) | −0.45 | (−0,6; −0,25) | <0.01 |
| Mean blood glucose (mg/dl) mean (SD)a | 155.7 (30.38) | 146.8 (24.5) | −8.9 | (−12.47−1.37) | 0.11 |
| Coefficient of variation (%), mean (SD)a | 46.2 (13) | 36.7 (7.2) | −9.5 | (−10.76; −4.24) | <0.01 |
| Distribution of patients according to HbA1c | |||||
| Optimal (≤7%), n (%) | 23 (26.13) | 38 (44.19) | +16 (18.61) | (10.2−26.9) | |
| Suboptimal (7−8%), n (%) | 34 (39.53) | 31 (35.04) | −3 (4.49) | (0.1−8.9) | <0.01 |
| Deficient (≥8%), n (%) | 31 (35.23) | 19 (17.77) | −11 (17.11) | (9.2−25.1) | |
| Psychosocial and quality of life tests | |||||
| FH15 - total score, mean (SD) | 38.9 (13.3) | 32.6 (12.7) | −6.5 | (−9.5; −4) | <0.01 |
| FH15 (≥28 points), n (%) | 41 (74.5) | 33 (60) | −14.4 | a | 0.09 |
| EsDQoL - total, mean (SD) | 101.4 (25.5) | 92.9 (22.7) | −8.4 | (−15.6; −1.29) | 0.02 |
| EsDQoL - satisfaction, mean (SD) | 38.1 (10.2) | 32.3 (7.8) | −5.77 | (−8.48; −3.07) | <0.01 |
| EsDQoL - impact, mean (SD) | 38.3 (11.6) | 34 (13) | −2.5 | (−5−0.5) | 0.09 |
| EsDQoL - diabetes concern, mean (SD) | 10.6 (3.2) | 9.9 (2.8) | +0.65 | (−0.19−1.48) | 0.13 |
| EsDQoL - social/vocation, mean (SD) | 14.52 (5.9) | 14.4 (5.3) | 0 | (−1.5−1) | 0.86 |
| DTSQ, mean (SD) | 26 (5.8) | 29.7 (4.8) | +4 | (2.5−5.5) | <0.01 |
| DTSQ - hyper, mean (SD) | 3.2 (1.5) | 2.9 (1.5) | –0.5 | (−1; 0) | 0.23 |
| DTSQ - hypo, mean (SD) | 2.8 (1.6) | 2.4 (1.3) | –0.5 | (−1;0) | 0.11 |
CV: coefficient of variation; SD: standard deviation; DTSQ; Diabetes Treatment Satisfaction Questionnaire;EsDQoL: Diabetes Quality of Life in Spanish; FH: fear of hypoglycaemia; 95% CI: 95% confidence interval.
The number of participants with optimal metabolic control increased by 18.1% at the end of the intervention, and that of patients with poor control decreased by 15.4% (p < 0.001) (Table 3).
On the other hand, in the group that started with poor control, the mean decrease in HbA1c was 1.079%, while in the group that started with suboptimal baseline control, it was 0.48%. No differences were found in the group that started with an optimal level. No significant differences were observed in the reduction of HbA1c when sub-analysed by sex, age or method of insulin therapy (Table 4).
Univariate analysis of the results adjusting for potentially interacting third variables.
| Diff not adjusted | Degree of prior control | Age groups (years)b | Sex | Insulin therapy method | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Optimal (≤7%) | Suboptimal (7−8%) | Deficient (≥8%) | p-value | ≤25 | 26−45 | 46−60 | ≥60 | p-value | Male | Female | Diff. | p-value | MDI | CSII | Diff. | p-value | ||
| n = 23 | n = 34 | n = 31 | n = 9 | n = 59 | n = 13 | n = 4 | n = 41 | n = 47 | n = 62 | n = 26 | ||||||||
| HbA1c % | −0.45 | 0.01 | −0.41 | −1.08 | <0.001a | −0.63 | −0.54 | −0.41 | −0.53 | 0.843 | −0.40 | −0.63 | 0.2 | 0.187 | −0.57 | −0.44 | 0.13 | 0.973 |
| FH15 - total score | −6.5 | −4.23 | −7.05 | −7.08 | 0.753 | −0.83 | −6.68 | −8.28 | −7.14 | 0.595 | −6.25 | −6.51 | 1 | 0.683 | −5.46 | −8.05 | 2.59 | 0.923 |
| FH15: % with >28 points | −14.4 | −15.38 | −10.52 | −17.31 | 0.033c | −33.3 | −9.75 | −23.2 | −16.4 | 0.037c | −25 | −5.45 | 24.55 | 0.038c | −8.57 | −25 | 16.43 | 0.029c |
| EsDQoL - total | −8.44 | −9 | −5.5 | −11.47 | 0.499 | −10.71 | −5.89 | −17 | −16.7 | 0.425 | −7.07 | −9.71 | 2.54 | 0.697 | −9.2 | −7.05 | 2.15 | 0.786 |
| EsDQoL - satisfaction | −5.77 | −4.15 | −5.14 | −7.63 | 0.514 | −5.43 | −4.97 | −10.14 | −9.28 | 0.755 | −5.08 | −6.43 | 1.35 | 0.621 | −4.83 | −7.53 | 2.7 | 0.514 |
| EsDQoL - impact | −2.05 | −3.85 | 0.41 | −2.31 | 0.887 | −3 | −0.87 | −4.29 | −5.56 | 0.553 | −1.88 | −1.93 | 0.5 | 0.815 | −3.09 | 0.26 | 2.83 | 0.612 |
| EsDQoL - diabetes concern | 0.65 | 0 | −0.727 | –1 | 0.664 | –1 | –0.44 | –1.71 | –1.45 | 0.790 | –0.73 | –0.57 | 0.16 | 0.857 | –0.8 | −0.364 | 0.43 | 0.626 |
| EsDQoL - social/vocation | 0 | –1 | –0.77 | –0.42 | 0.906 | –1.14 | –0.46 | –0.86 | –1.3 | 0.823 | 0.62 | –0.71 | –1.33 | 0.355 | –0.43 | 0.58 | 0.15 | 0.906 |
| DTSQ | 4 | 2.14 | 4.85 | 3.62 | 0.432 | 5.14 | 3.32 | 4 | 4.31 | 0.832 | 3.87 | 3.56 | –1 | 0.732 | 3.8 | 3.5 | 1 | 0.367 |
| DTSQ - hyper | –0.5 | 0.43 | –0.55 | –0.57 | 0.191 | 0.57 | –0.32 | –1.5 | –1.73 | 0.164 | –0.48 | –0.19 | 0 | 0.951 | –0.2 | –0.5 | –0.3 | 0.547 |
| DTSQ - hypo | –0.5 | –0.5 | −0.15 | −0.48 | 0.812 | 0 | −0.37 | −0.17 | −0.09 | 0.234 | −0.44 | −0.31 | −0.12 | 0.809 | −0.34 | −0.4 | 0 | 0.887 |
DTSQ: Diabetes Treatment Satisfaction Questionnaire; EsDQoL: Diabetes Quality of Life in Spanish; FH: fear of hypoglycaemia; CSII: continuous subcutaneous insulin infusion; MDI: multiple insulin doses.
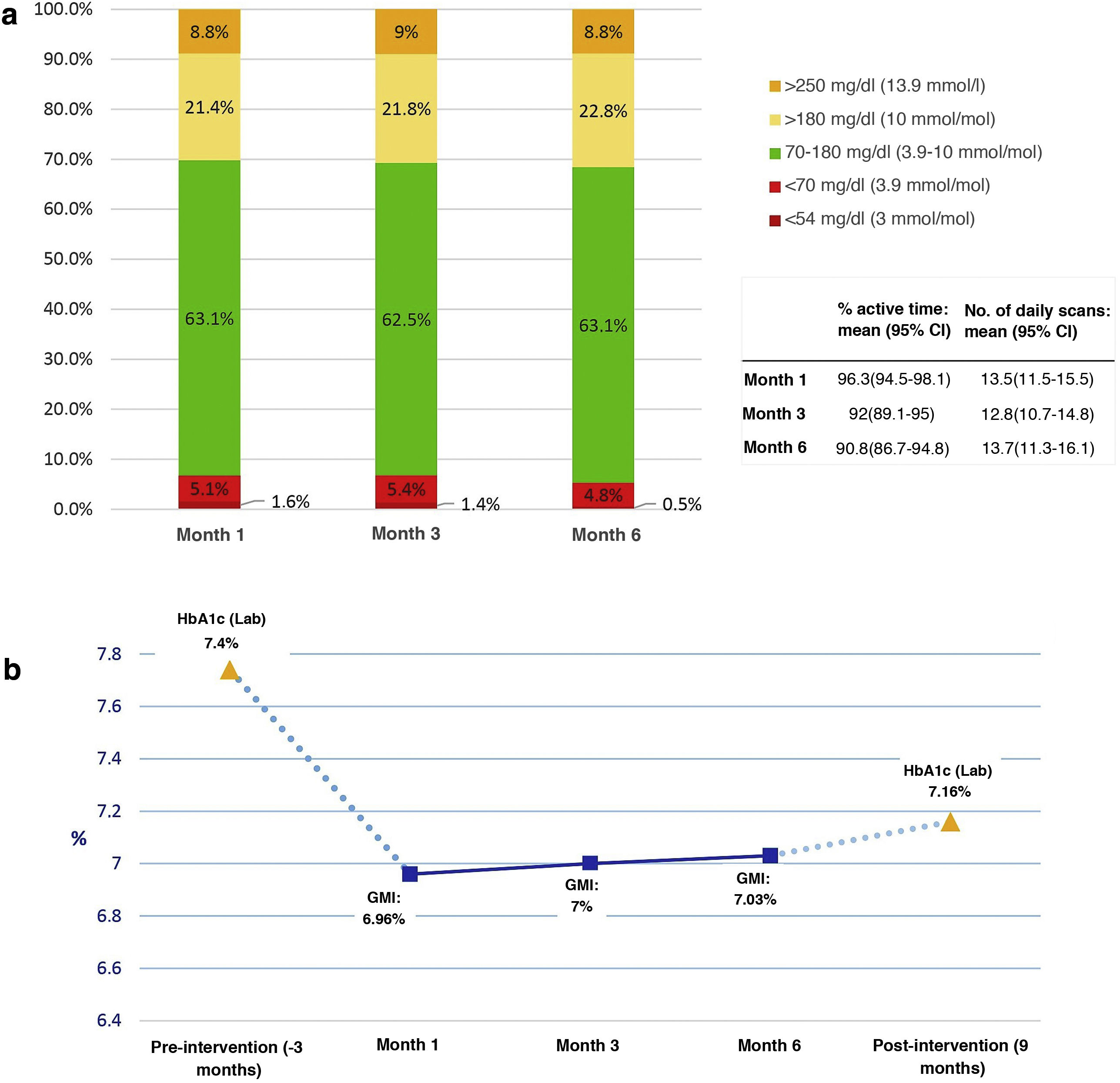
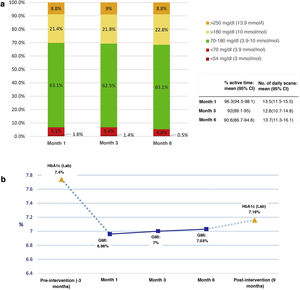
Fig. 1 shows the results of the analysis of the blood glucose variables provided by the AGP reports of the FGM systems. The percentage of active time of the sensor was greater than 90% in the readings of months 1, 3 and 6 of the follow-up, with an average greater than 12.5 readings/day in all of them.
Blood glucose data obtained from the AGP reports (previous 14-day settings) during follow-up at months 1, 3, and 6 from the first educational session. a) Percentage of mean times in ranges, variables of use of the systems; b) Evolution of IMT and comparison with baseline and post-intervention HbA1c.
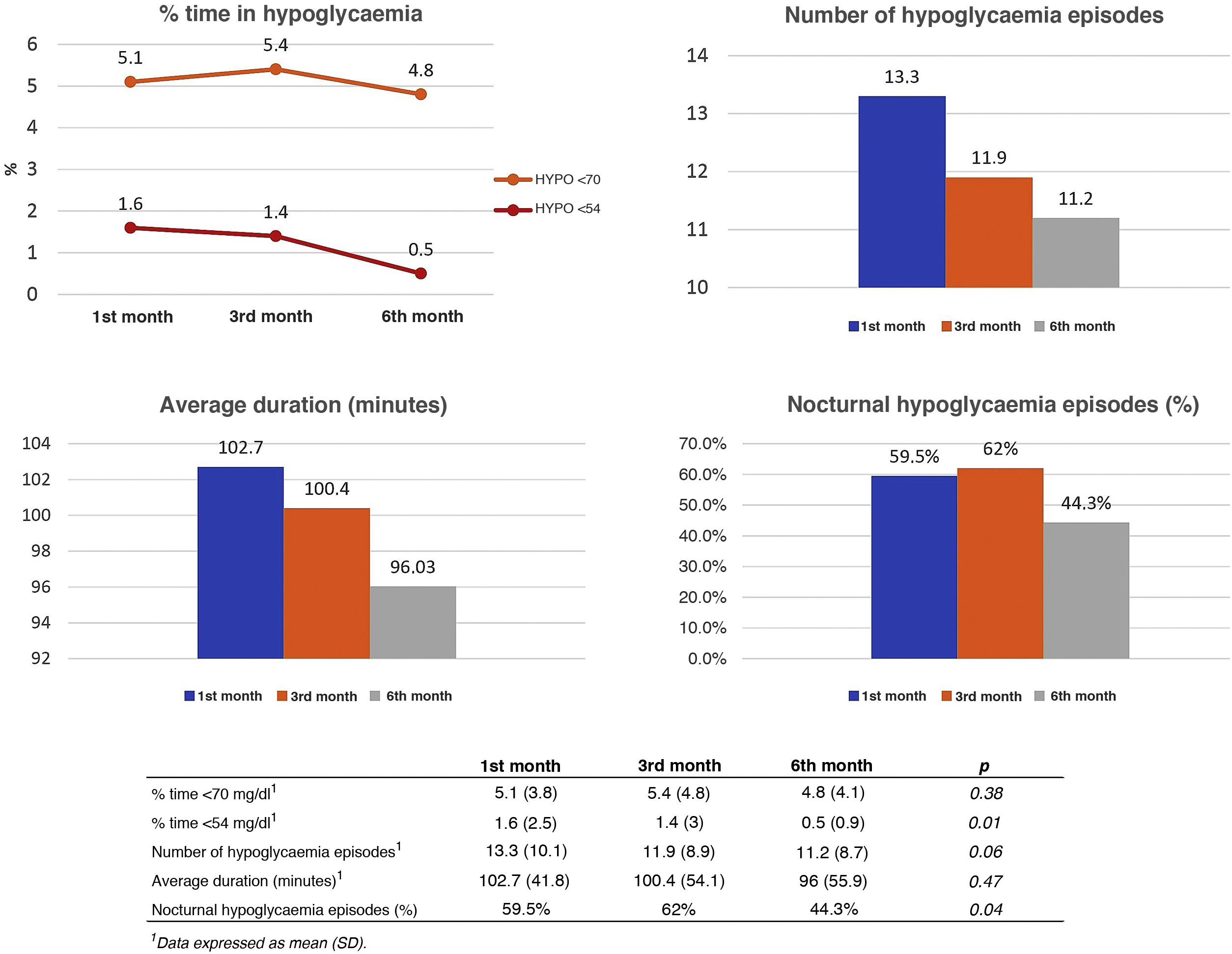
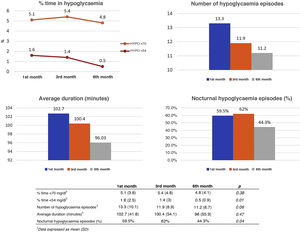
To study the impact of the intervention on the incidence of hypoglycaemia, the number of events, their mean duration, the percentage of time in hypoglycaemia, and the presence or absence of nocturnal hypoglycaemia were recorded during follow-up. A progressive and statistically significant reduction was observed in the percentage of time <54 mg/dl and in the percentage of patients with nocturnal hypoglycaemia. The results are shown in Fig. 2.
Analysis of hypoglycaemia events during follow-up at months 1, 3 and 6 from the first educational session. Data obtained from AGP reports configured for the previous 14 days. Data shown: mean percentage of time in hypoglycaemia <70 mg/dl and <54 mg/dl (upper left image), mean number of daily hypoglycaemic events (upper right image), mean duration of each event (lower left image), percentage of patients with nocturnal hypoglycaemia (lower right image).
A multiple linear regression model was performed, in which the baseline HbA1c level showed a high predictive capacity for the change in HbA1c before/after the intervention (β coefficient: −0.605; SD: 0.091; p < 0.001; VIF: 1,034). The remaining variables did not show a significant influence: age (β: 0.007; SD: 0.01; p: 0.488), sex (β: −0.187; SD: 0.191; p: 0.331), years with DM (β: 0.011, SD: 0.01, p: 0.263), method of insulin therapy (β: −0.176, SD: 0.21, p: 0.404). The coefficient of determination (R2) of the model was 0.375 (p < 0.001).
Results in psychological variables and quality of lifeAfter nine months of follow-up, a decrease was observed in the scores of the FH15 test, EsDQoL (overall and in its sub-sections), and an increase in the DTSQ questionnaire on satisfaction with treatment (Table 3).
The results were adjusted according to potentially interacting variables using univariate analysis techniques without finding significant differences (Table 4).
SafetyNo local cutaneous adverse event was recorded during the development of this study. No episodes recorded of acute decompensation or chronic complications of diabetes that required hospital admission and/or intra/out-of-hospital emergency visits during the study follow-up.
DiscussionThis study demonstrates the effectiveness of an educational intervention in a telematic and group format within a programme to implement flash glucose monitoring systems in adults with type 1 diabetes.
The benefits associated with the delivery of intervention of these characteristics in a face-to-face format have already been reported by Hermanns et al., observing better clinical and psychological results as well as a high degree of satisfaction compared to the use of these devices without therapeutic education.19
Currently, the scientific literature available on adapting to a telematic format of structured face-to-face care strategies in type 1 diabetes during the COVID-19 pandemic is scarce. Case series resulting from the experience of different authors have been reported, highlighting positive aspects such as the possibility of not giving up medical care despite mobility restrictions or the reduction of risk exposure to SARS-CoV-2.20,21 Vigerski et al. published a study that showed how a telematic programme via videoconference on training in the use of insulin pumps during the COVID-19 pandemic demonstrated similar clinical results as its face-to-face equivalent, with a high level of participant satisfaction.22
In this sense, the use of videoconferencing as a tool for medical care in general, and diabetes in particular, had already been studied previously, with associated benefits such as improved glycaemic control, safety, cost reduction and increased perception of quality of life.23,24
Favourable prior evidence and the need to ensure access to a structured diabetes education programme to initiate and optimise FGM use during the COVID-19 pandemic while ensuring lockdown and social distancing measures during this period justify our initiative, in line with the recommendations of the Sociedad Española de Endocrinología y Nutrición (SEEN) [Spanish Society of Endocrinology and Nutrition].11
The clinical results of our study in terms of improvement in the degree of metabolic control are comparable to those reported in the literature for analogous face-to-face strategies.6,25,26 We observed an overall decrease in HbA1c of 0.45% after nine months of follow-up, which increased to 1.08% in patients who started with a baseline HbA1c greater than 8%.
A recent analysis of the impact of FGM systems by the Swedish National Diabetes Registry reported a 0.11% improvement in HbA1c after two years of follow-up (0.23% in subgroups starting with ≥8.5%).25 This same trend has also been observed in a study by the Association of British Clinical Diabetologists (ABCD), in which a cohort of more than 14,000 patients with diabetes (97% DM1), who were users of FGM within the National Health System of the United Kingdom (NHS, UK), was analysed, with a decrease of 0.5% after eight months of follow-up (1.2% in participants with baseline HbA1c ≥ 8.5%).26 The global improvement quantified in a recent meta-analysis regarding HbA1c associated with using these systems was 0.4%6 for DM1.
When sub-analysing the influence of the degree of basal metabolic control on the results, it was observed how this behaved as an independent predictor of the final decrease in HbA1c, as has been reported in other studies.6,25,26 Based on these data, we conclude that patients who start with poor basal metabolic control could significantly benefit from educational interventions such as the one we present, emphasising the support that FGM technology provides in this process of intensification.
On the other hand, when analysing the variation of the IMT in the first month concerning the value of the baseline HbA1c level, we observed a decrease of 0.4% (Fig. 1b) that remained stable during the subsequent follow-up, as well as the values of the different percentages of times in control ranges. This fact could indicate that the clinical benefit of the intervention would, for the most part, occur early during the first month of follow-up, coinciding with the beginning of the use of FGM devices and after the first education session, with a maintained effect given the low level of subsequent variation.
We also observed a high impact of the intervention on psychosocial aspects such as satisfaction with treatment, quality of life or fear of hypoglycaemia.
Fear of hypoglycemia is a common psychopathological phenomenon in people with diabetes, resulting from negative subjective experiences about the symptoms derived from episodes of hypoglycaemia.27
In our study, a discrete, although statistically significant, the reduction was observed in the percentage of time <54 mg/dl throughout the follow-up and in the percentage of patients with nocturnal hypoglycaemia, in addition to a reduction in the number of hypoglycaemic events close to statistical significance. However, the fear of hypoglycaemia decreased more noticeably, with an average reduction of 6.5 points in the FH15 test and 14.4% fewer participants with scores indicative of pathological fear of hypoglycaemia (28 points). This discrepancy could be justified by the fact that patients with a pathological fear of hypoglycaemia (which in our study constituted 74.5%) paradoxically tend to suffer a small number of these events due to the adoption of hyperglycaemia avoidance behaviours that generally make it difficult for them to achieve optimal goals of metabolic control.27
Diabetes education and the use of continuous real-time and intermittent or flash-type glucose monitoring technology have been proposed as useful strategies for managing the pathological fear of hypoglycaemia.28 In our research, the results support this hypothesis and the validity of a telematic format. In our opinion, the decrease in the FH15 test scores registered in the participants of this study could be related to factors such as the acquisition of a greater degree of knowledge about the prevention and management of hypoglycaemia, the more information available about glucometers, the reduction in the number of nocturnal hypoglycaemia episodes or training in the configuration of personalised alerts in real-time during the development of the training programme, together with other specific aspects of FGM technology.
The use of FGM systems has been associated with improving the quality of life of people with diabetes, 29 although the scientific evidence in this regard is scarce.30 Hermanns et al. described how a specific group of educational interventions on the management of FGM devices was associated with better scores on quality of life questionnaires compared to the use of these systems without any educational intervention.19
In our study, the benefits obtained in this sense have been especially relevant, with improvement in the scores of the EsDQoL quality of life test, in overall score and all its sub-sections. Satisfaction with treatment also improved in a clinically significant fashion, as measured by the DTSQ questionnaire. These data reinforce the conclusions formulated by other authors, in which diabetes education provides superior results in the quality of life and satisfaction with treatment compared to the use of FGM devices without specific technical training given by a professional team.19,30
On the other hand, a group and telematic format for educational interventions concomitant to the implementation of FGM systems could have additional advantages, such as the reduction of delay times without detriment to clinical results compared to its face-to-face alternative, the simplification of logistical aspects and the need for physical facilities, in addition to facilitating greater accessibility to patients with difficulties in travelling to health centres.
However, it must be taken into account that non-face-to-face care methods, such as the one we propose in this study, constitute a valid alternative for patients able to manage real-time videoconferencing and without functional and comprehension impairments, with the programming of face-to-face strategies being required for patients who do not meet these characteristics.11
Study limitationsOur study has limitations derived from not having a control group to compare the results of the telematic intervention versus an analogous face-to-face group. The reason for this was the start of the COVID-19 pandemic and the limitation of citizen mobility imposed due to the epidemiological situation and social distancing measures, making it impossible to hold face-to-face group sessions in health centres.
Second, the lack of continuous glucose monitoring systems before implementing the FGM systems prevented us from analysing the intervention's impact on specific blood glucose variables, such as the percentage of time in the different ranges of glycaemic control. In this sense, the validity of the results referring to the pre-and post-intervention differences in mean glucose and CV could have been limited, as the data came from different sources of information (personal glucometers of each participant for the baseline data and the AGP reports of FGM devices for later ones).
On the other hand, in line with the SEEN recommendations on telemedicine in times of COVID-19, telematic interventions via videoconference similar to the one presented in our study should be carried out through web applications that guarantee the cybersecurity of the attendees, with measures such as end-to-end encryption.11
ConclusionsThe incorporation of a specific group and telematic educational programme in the development of strategies for the implementation of FGM systems represents an effective and safe alternative for patients able to manage videoconferencing in real time, with benefits both clinical and in quality of life parameters, positioning itself as a format that can be implemented as routine clinical practice in adult patients with type 1 DM.
FundingNo external funding has been received to carry out this study.
AuthorshipAll the authors declare that they have contributed substantially to the preparation of this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.