To evaluate the use and clinical effect of intermittently scanned continuous glucose monitoring (isCGM) in adults with type 1 diabetes (T1D) in a public health service scenario.
MethodCross-sectional retrospective analysis of all patients with T1D and medical indication for isCGM use from a cohort followed since 2010 at Castilla-La Mancha Public Health Service (Spain). Primary outcome was HbA1c change during the first year of follow-up after isCGM initiation. Secondary outcomes included evaluation of self-monitoring of blood glucose (SMBG), isCGM and insulin use, along with glycometric indexes.
ResultsA total of 945 T1D patients were analyzed. Median age was 49.5 years (IQ range 19.0 years) and T1D duration of 28.9 years (IQ range 14.0 years). The most frequent insulin therapy alternatives were multiple daily injections (85%) followed by insulin pump (11%). Eighty percent of the patients were active isCGM users with a 90% of adherence to the device. Patients showed a mean daily scan frequency of 10.1±6.4scans/day. Daily SMBG reduced by −3.5 test/day [95% CI −3.7, −3.2; P<0.001]. We detected an HbA1c reduction of −0.3% (−4mmol/mol) [95% CI −0.2, −0.4 (−3, −5); P<0.001] at the end of the follow-up. An inverse correlation between HbA1c levels at the end of the follow-up and daily frequency of isCGM scanning (R=−0.34, P<0.001) was observed. Dropout rate was 4%, and 4% of patients were not willing to use isCGM.
ConclusionsAdult patients with T1D improved glycaemic control after isCGM initiation in a public health service scenario. Despite described clinical benefits, a higher than expected percentage of patients were not using isCGM technology. NCT05095610.
Evaluar el uso y efecto clínico de la monitorización continua de glucosa intermitente (MCGi) en pacientes adultos con diabetes mellitus tipo 1 (DM1) en un sistema público de salud.
MétodoEstudio transversal retrospectivo para analizar a todos los pacientes adultos con DM1 e indicación médica MCGi de la cohorte DIACAM, seguida desde 2010 en el Servicio Público de Salud de Castilla-La Mancha (España). El objetivo principal fue el cambio en los valores de hemoglobina glicosilada (HbA1c) al año del inicio de la MCGi. Los objetivos secundarios analizados incluyeron: autodeterminación de la glucemia capilar (AGC), uso de la insulina y MCGi, y variables glicométricas.
ResultadosSe analizaron 945 pacientes con DM1. La edad mediana fue 49,5 años (IQR 19,0 años), con una mediana de duración de DM1 de 28,9 años (IQR 14,0 años). Las opciones de tratamiento insulínico más frecuentes fueron: múltiples dosis diarias de insulina (85%) seguido de bomba de insulina (11%). El 80% de los pacientes eran usuarios activos de la MCGi con un 90% de adherencia al dispositivo. Los pacientes mostraron una frecuencia de 10,1±6,4 escaneos de glucosa diarios. Observamos una reducción de la frecuencia de AGC de -3,5 test/día [95% IC -3,7, -3,2; p<0,001]. Detectamos una reducción de los valores de HbA1c de -0,3% (-4 mmol/mol) [95% IC -0,2, -0,4 (-3, -5); p<0,001] al final del seguimiento. Se encontró una relación inversa entre las cifras de HbA1c al final del seguimiento y la frecuencia diaria de escaneos de glucosa (R=-0,34, p<0,001). La tasa de abandonos de la MCGi fue del 4%, un 4% adicional de pacientes no deseaba usar la MCGi.
ConclusionesDetectamos una mejora en el control glucémico en pacientes adultos con DM1 tras un año de seguimiento en un sistema público de salud. Pese a los beneficios clínicos descritos, un porcentaje mayor a lo esperado de pacientes no estaba utilizando la MCGi. NCT05095610.
Tight control of blood glucose in people living with type 1 diabetes (T1D) delays onset of macrovascular and microvascular chronic complications, although glucose levels need to be closely monitored to prevent hypoglycaemia.1,2 Self-monitoring of blood glucose (SMBG) was critical for safe and effective glycemic therapy adjustments for T1D patients.3–5 However, repeated daily glucose checks are painful, inconvenient, and can be challenging to maintain in the long term. Therefore, factory-calibrated continuous glucose monitoring (CGM) systems were developed to avoid frequent SMBG. Most frequent extensive used CGM is intermittently scanned continuous glucose monitoring (isCGM), also called Flash glucose monitoring. This device allows scanning as often as needed for knowing interstitial glucose concentration during a 14-day period.6 From 2014 onwards, isCGM through FreeStyle Libre (Abbott Diabetes Care, Witney, UK) was introduced onto the market. Flash technology improves quality of life, satisfaction and glycemic control in people with T1D, especially reduces the time adults with well controlled T1D spent in hypoglycaemia.7–10 Therefore, isCGM has been subsequently reimbursed by different health public systems.
Real-world clinical benefits of Flash glucose monitoring have previously been published.11–13 The widest view came from the Swedish National Diabetes Registry where isCGM was associated with a small and sustained improvement in glycated haemoglobin A1c (HbA1c) and lower rates of severe hypoglycaemic events among 14.372 adult T1D patients.12 Other public registries had also confirmed similar HbA1c reduction.14,15 In Spain, higher scan rates with flash glucose monitoring were associated with greater time in range (TIR), lower time above range (TAR), and time bellow range (TBR) of interstitial glucose.16 However, lack of information about the possible benefits of isCGM beyond glycemic indexes from greater number of patients seems evident in our country. Only the DIACAM1 2020 study, based on a well-known public health system cohort, showed that the use of technology (such as isCGM) was a determining factor in achieving a sustained diabetes control improvement.17 In our region, Flash monitoring started being public reimbursed for specific groups of T1D patients in October 2017. Finally, it was public reimbursed for all T1D patients older than 4 year-old from April 2019.18
The aim of the present study was to evaluate the use and effect of Flash glucose monitoring over glycemic control in adult with T1D from the DIACAM1 cohort.
MethodsStudy design and ethicsA cross-sectional multicenter observational study was designed to assess isCGM use and its clinical effects among adult T1D patients from a complete Spanish public health service. The protocol was approved by the Castilla-La Mancha Public Health System (Servicio de Salud de Castilla-La Mancha, SESCAM) Ethic Committee and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The protocol was publicly registered at ClinicalTrials.gov (NCT05095610). All participants provided written informed consent.
As previously reported, DIACAM1 cohort gathered information from the regional public health system in Castilla-La Mancha. Castilla-La Mancha Region (79,463km2) is the South-central Spanish region regarding a population size of 2,043,063 inhabitants (last formal national census held in 2021), with 14 hospitals treating adult T1D patients distributed through eight health care areas.17 Here, we present data from five of these areas (Albacete, Ciudad Real, Cuenca, Puertollano and Toledo) representing the 68.3% (1,394,722 inhabitants) of the whole population.19
ParticipantsAll adult (≥16yrs) T1D patients with active follow-up from the aforementioned public health areas belonging to DIACAM1 cohort were analyzed. All studied patients followed the possible indication for isCGM use at the beginning of the follow-up to preserve the intention-to-treat analysis.
Variables and outcomesThe primary end-point was mean change in HbA1c from the last visit before to isCGM initiation to one year after the beginning. The secondary outcomes included changes during the one-year follow-up in1: insulin use2; isCGM use: adherence, scan frequency, reasons for lack of use3; glycometric indexes, including: TAR>250mg/dL (>13.9mmol/L), TAR 180–250mg/dL (10.0–13.9mmol/L), TIR 70–180mg/dL (3.9–10.0mmol/L), TBR 54–70mg/dL (3.0–3.9mmol/L), and TBR<54mg/dL (<3.0mmol/L) of interstitial glucose; mean interstitial glucose (MIG); glucose management index (GMI), and variation coefficient (VC)2; body mass index (BMI). The data from patients which did not began isCGM were collected from last visit until the end of the data collection period. The data were gathered from September 2021 to April 2022. Variables were collected through electronic medical records and the specialized online webpage Libreview (https://www2.libreview.com). The final CGM data were obtained from the last two-weeks period before the one-year follow-up visit.
Statistical analysisCross-sectional and longitudinal/retrospective analysis for patients meeting inclusion criteria (adult T1D patients being subsidiary of isCGM treatment) were performed. Quantitative variables are expressed as means and standard deviation (SD) or median and range; qualitative variables are presented as total number of events and percentage. Kolmogorov–Smirnov test was used to evaluate the normality of quantitative variables. A paired Student's t-test or a Wilcoxon signed-rank test were used for the analysis of differences. Comparisons between proportions were analyzed using a Chi-squared test. Mann–Whitney U and Wilcoxon signed-rank nonparametric tests were used to analyze statistical differences between groups and differences between baseline and study end, respectively. A bivariate analysis was performed to determine which variables could be candidates in a linear regression model with the variable difference in HbA1c during the follow-up as the dependent variable. Those that obtained the lowest P-values in this analysis and those that were considered to have the highest biological plausibility were evaluated in a multivariable linear regression model for the HbA1c difference. Significance was taken at P<0.05. Analyses were performed with IBM SPSS software version 28.0 for Windows (SPSS Inc., Chicago, IL) and graphics with R 4.1.2 (R Statistics, Vienna, Austria).
ResultsA total of 945 T1D patients meeting inclusion criteria were analyzed. The patients showed a median age of 49.5 years (IQ range 19.0 years) and T1D duration of 28.9 years (IQ range 14.0 years). The most frequent insulin therapy alternatives were multiple daily injections (85%), followed by insulin pump (11%), and premixed insulin therapy (4%). Rest of baseline characteristics can be observed in Table 1.
Demographics and baseline characteristics.
| Total (n=945) | |
|---|---|
| Sex (female/male) (%) | 49/51 |
| Age (years) (median, IQ, range) | 49.5 (19) |
| Diabetes duration (years) (median, IQ range) | 28.9 (14) |
| Diabetes chronic complications (%) | |
| Microvascular | 66 |
| Macrovascular | 7.3 |
| Total | 67 |
| Educational level (%) | |
| None | 7 |
| Primary education | 40 |
| Secondary education | 32 |
| University | 21 |
Eighty percent of the patients were active isCGM users with an 90% of adherence to the device at the end of the follow-up. Patients showed a mean daily scan frequency of 10.1±6.4 scans/day (median 10, IQ range 8). Daily SBGM reduced by −3.5 test/day [95% CI −3.7, −3.2; P<0.001] during the study among Flash users whereas glucose testing (scans plus SBGM) increased by 8.1test/day [95% CI 6.6, 9.6; P<0.001].
Sixteen percent of the patients did not begin isCGM owing to the use of real-time CGM (rtCGM) (50%), lack of will to use isCGM information (29%), and not willing to wear a viewable Flash device (21%). Hence, 8% of all T1D adult patients from the cohort did not commence using isCGM because of the lack of will to use or wear the device. The observed dropout Flash rate was 4% mainly due to the beginning of rtCGM (49%), local adverse reactions (24%), not willing to continue wearing the Flash device (23%) and glue-adhesion problems (3%).
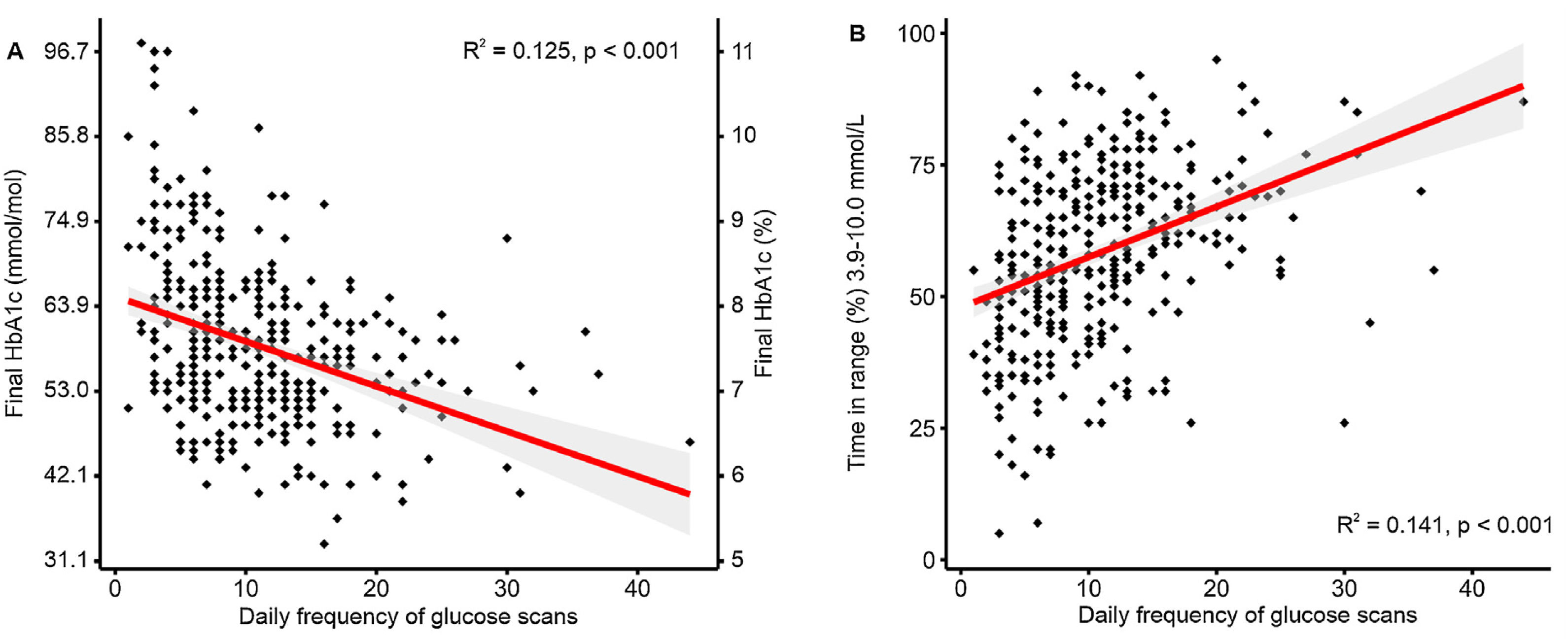
Diabetes controlHbA1c levels decreased along the study by −0.3% (−4mmol/mol) [95% CI −0.2, −0.4 (−3, −5); P<0.001]. An HbA1c reduction greater than 0.5% (6mmol/mol) was detected in 36.6% of the patients. HbA1c lower than 7% (53mmol/mol) was observed in 22% of the patients at the end of the follow-up. We observed an inverse correlation between HbA1c levels at the end of the follow-up and daily frequency of isCGM scanning (R=−0.34, P<0.001) (Fig. 1A). We also detected a similar −0.3% (−4mmol/mol, [95% CI −0.2, −0.4 (−3, −5); P<0.001]) after excluding those non-Flash-user patients during the follow-up.
The bivariate analysis determined current age, BMI, SBGM daily frequency, and basal insulin daily dose before isCGM initiation as candidates (lowest P-values) in a linear regression model with the variable change in HbA1c as the dependent variable. These variables and those that were considered to have the highest biological plausibility (educational level, age at diabetes diagnosis, type on intensive insulin treatment, presence of chronic diabetes complications) were evaluated in the simple linear regression models for the HbA1c decrease. However, the multivariate analysis did not show an association between change in HbA1c with the described variables (P=0.532).
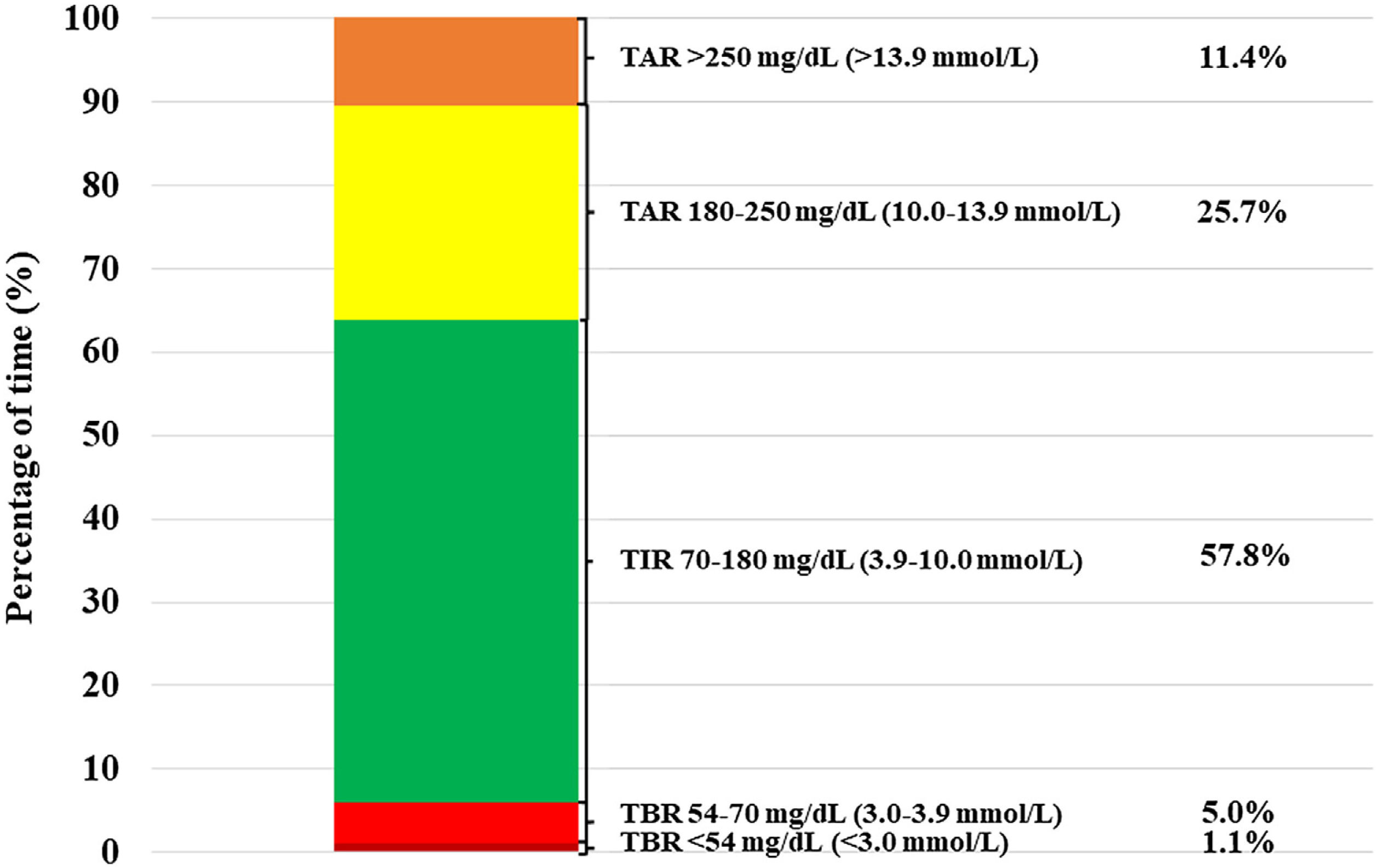
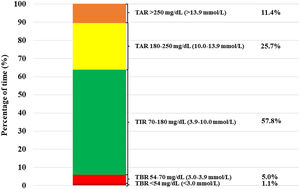
Patients treated with isCGM achieved a TIR of 57.8% at the end of the follow-up. Furthermore, we detected a direct correlation between daily frequency of isCGM scanning and final TIR 70–180mg/dL (3.9–10.0mmol/L) levels among isCGM users (R=−0.38, P=0.001) (Fig. 1B). On the other hand, observed TBR<70mg/dL (3.9mmol/L) was 6.1%. Daily hypoglycemic event frequency was 10.0±8.2events/day (median 8, IQ range 10). Frequent hypoglycemia, defined as TBR<70mg/dL (3.9mmol/L)≥10%, was observed in 13.8% of the patients. The rest of glycometric indexes are shown in Fig. 2.
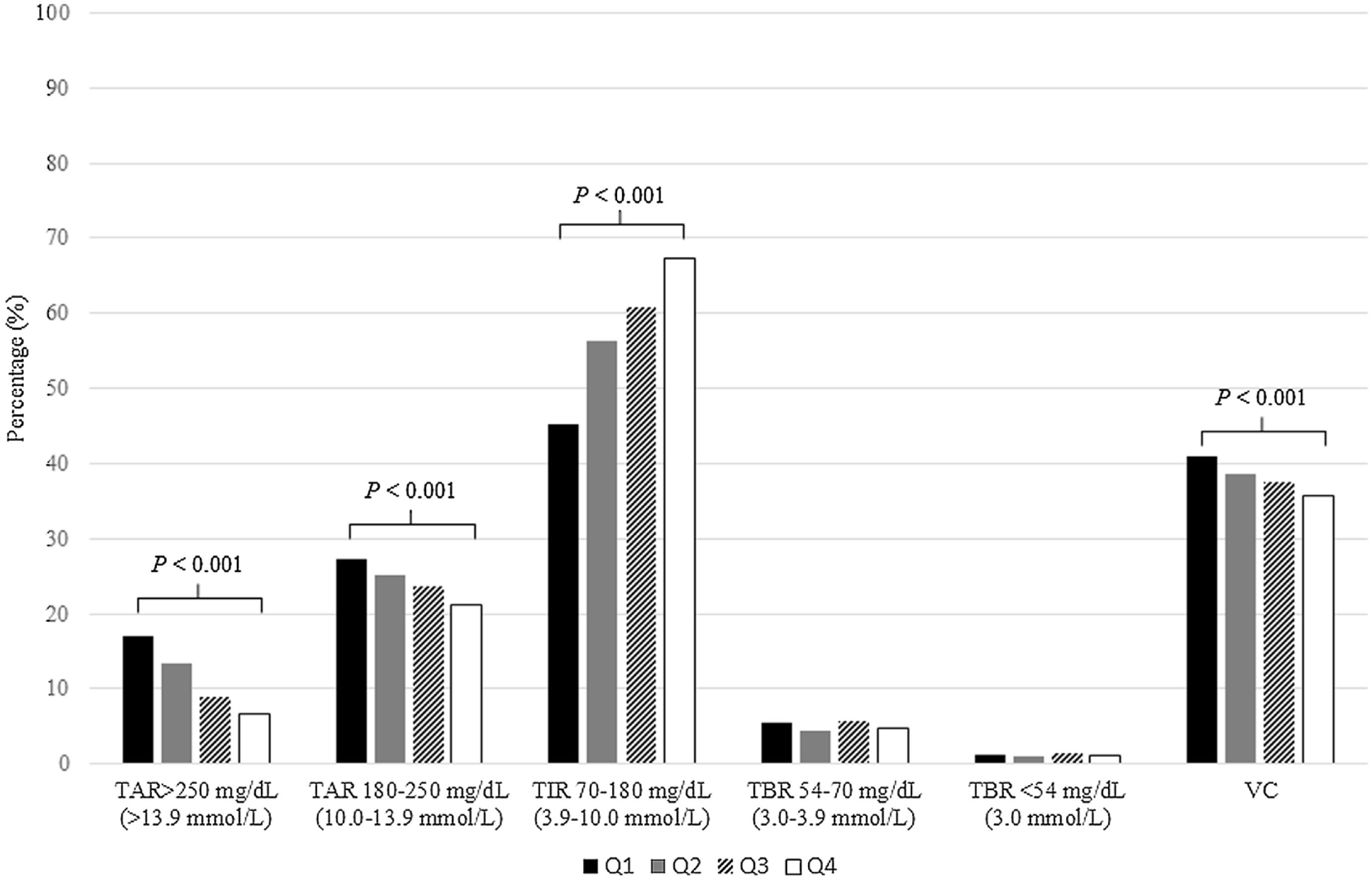
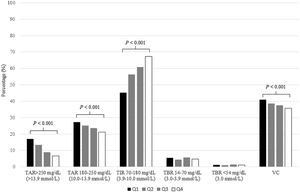
After distributing studied patients according to quartile scan frequency groups (Q1<6scans/day, Q2 6–10 scans/day, Q3 10–14scans/day and Q4>14scans/day), we detected than those with higher quartiles attained lower TAR>250mg/dL (13.9mmol/L) (P<0.001), TAR 180–250mg/dL (10.0–13.9mmol/L) (P<0.001) and VC (P<0.001). Furthermore, same group of patients achieved higher TIR 70–180mg/dL (3.9–10.0mmol/L) (P<0.001). Conversely, TBR was independently related with daily scan frequency (P>0.05). Glycometric results according to scan frequency quartile can be observed in Fig. 3.
Patients showed a MIG and GMI of 164±32 (9.1±1.8mmol/L) and 7.3±0.8% (56.3±9mmol/mol), respectively. Finally, glycemic variability expressed as VC of interstitial glucose was 37.8%.
Insulin use and weightTotal daily insulin dose was scarcely reduced from the beginning to the study end (−0.01UI/kg/day [95% CI −0.02, 0.00, P=0.068]). We did not detect differences throughout the study in the amount of basal o bolus insulin. However, a small BMI increase was observed at the end of the follow-up (0.2kg/m2 [95% CI: 0.1, 0.3; P<0.001]). Rest of the insulin use and BMI change along the study can be observed in Table 2.
Evolution of insulin doses and anthropometric results.
| Baseline | 1-year | MDC (95% CI) | P | |
|---|---|---|---|---|
| Basal insulin (%) | 54.7 | 54.3 | −0.4 (−6.3, −0.3) | 0.13 |
| Bolus insulin (%) | 45.3 | 45.7 | 0.4 (6.3, 0.3) | 0.13 |
| TDD (UI/kg/day) | 0.69±0.25 | 0.68±0.25 | −0.01 (−0.02, 0.00) | 0.068 |
| BMI, kg/m2 | 27.6±4.6 | 27.8±4.7 | 0.2 (0.1, 0.3) | <0.001 |
Data are expressed as percentages or mean±standard deviation. BMI, body mass index; MDC, mean difference in change; CI, confidence; TDD, total daily dose.
The novelty of our present results resides in the longitudinal description of the situation and clinical effect of isCGM among adult T1D patients from a cohort based on a public health system. Up to date, this is the widest view of isCGM situation in this group of patients in a Mediterranean scenario. The main findings were a significant −0.3% (−4mmol/mol) HbA1c reduction along the one-year follow-up despite one in six patients were not using the reimbursed isCGM technology. Remarkably, even patients with longstanding diabetes (30 yrs. after T1D diagnosis) could benefit from isCGM use.
Bolinder et al. described an unprompted SMBG frequency reduction after isCGM initiation together with a maintained daily frequency scanning of 15.1scans/day.7 Studied patient glucose testing increased threefold.7 Subsequent real-world data confirmed observed high daily scan frequency (16.3scans/day), although it was slightly lower among English or Spanish users (12.9scans/day and 13scans/day, respectively).14,16,20 Here, we observed the expected SMBG reduction with even a lower daily scan frequency (10.1scans/day) which could be explained by our longest follow-up and resulting lower adherence to the device. In fact, similar scan frequencies have been described in previous reports with same follow-up such as the FUTURE Study (9.7scans/day).13
Although initial IMPACT study could not demonstrate an HbA1c reduction through isCGM (mainly due to baseline low HbA1c), succeeding observational studies showed lowest HbA1c levels after isCGM initiation.14,21 Most recent meta-analysis of clinical trials and real-world observational studies described a −0.56% HbA1c reduction across a short 2–4 months period in adult T1D patients.22 Remarkably, HbA1c levels was maintained during a one-year period after isCGM initiation in the FUTURE study.13 Our patients benefited from isCGM use with a −0.3% (−4mmol/mol) HbA1c reduction along the study. Even longer 2-year follow-up from the Swedish National Diabetes Registry showed a slight −0.11% (−1.2mmol/mol) difference between Flash users and patients only using conventional SMBG.12 Therefore, it seems possible that isCGM effect over HbA1c may be affected by Flash treatment duration. Anyway, baseline higher HbA1c values and achieved scan frequency persist as key factors over better HbA1c results.
A real-world European Flash analysis showed that patients approximately achieved 60% of TIR coupled with 30% of TAR >180mg/dL (10.0mmol/L) being patients with highest scan frequency those attaining better glycometric data.20 Moreover, similar results were corroborated in an Spain-focus subanalysis.16 Our present results confirmed these findings from a public health system point of view with congruous glycometric results and greater clinical benefits in highest scan frequency group of patients. The IMPACT study demonstrated that Flash glucose testing reduced the time adults with well controlled T1D spent in hypoglycemia.7 Aforementioned European and Spanish subanalysis evidenced that Flash users nearly achieved 6% of TBR <70mg/dL (3.9mmol/L) and 2% of TBR <54mg/dL (3.9mmol/L). These results are in line with data presented in our research (5% and 1.1%, respectively).
Noticeably, one in six patients were not using isCGM at the end of the follow-up. In the FUTURE study, 8% of the participants stopped using Flash technology mainly due to skin reactions, low confidence in sensor values and frequent sensor loss.13 Eleven percent of the patients in the ABCD study reported problems with Libre device related with technical problems or local reactions.14 Unfortunately, other published real-word Flash-use studies did not report or consider dropout or sensor-related problem evaluation.11,12,20,21 We novelty described lack of will to use or wear Flash devices as patient's main reason not to begin or stop using isCGM. Furthermore, the previous use or the swapping to rtCGM seems another critical aspect in Flash avoidance in T1D patients.
Strengths of the study include its one-year follow-up duration and well-known based cohort with clinical and biological data (DIACAM1). This is also one of the few multicentre studies gathering direct information about isCGM situation and use from patients with T1D. Lastly, to our knowledge, this is the first study that shows a view of Flash use and benefits base on a Mediterranean public health service. Nevertheless, there are some limitations inherent to this study. Firstly, the fact that rtCGM results were not included in our analysis did not allow us to compare its possible clinical benefit with isCGM users. The German/Austrian Prospective Diabetes Follow-up Registry demonstrated that rtCGM versus isCGM was associated with a higher percentage of TIR and improved metabolic stability.23 Secondly, we did not gather acute diabetes complications (severe hypoglycemia or diabetes ketoacidosis) data, and neither we registered hospitalization or work leaves, so we could not evaluate Flash impact over these outcomes. The RELIEF study showed a significant lower incidence of admissions for acute diabetes complications associated with use of Flash glucose monitoring.11 Finally, diabetes burden and well-being were not measured during the study, so we could not evaluate patient reported outcomes. The FLARE-NL4 study detected an improved in well-being and a decreased disease burden among isCGM-treated patients.24
In conclusion, we observed Flash clinical benefit over glycemic control in adult T1D patients despite a more than expected percentage of patients was not using isCGM technology. We suggest that diabetes educational programmes should consider strengthening potential clinical benefits of isCGM use and answer patient's doubts about the system to reduce lack of use of this technology.
Authors’ contributionJS and JMF designed the study. JMF wrote the study protocol and published it in a public registry. PP, DCV, JMF, JS and JG gather the data. JRMR contributed to the interpretation of the results. JMF took the lead in writing the manuscript. JRMR performed statistical regression analysis and reviewed statistical analysis. All authors provided critical feedback and helped shape the final manuscript.
FundingNone.
Conflicts of interestThe authors declare that they have no conflicts of interest concerning this article.
The authors are also very grateful to Castilla-La Mancha Endocrine, Diabetes and Nutrition Society (SCAMEND) for their support in promoting this study among the society members.