The Flash Guide (FG) for insulin dosing (A. Chico, C. González) was the first document intended for FreeStyle Libre® (FSL) user patients to help with decision-making depending on glucose level and trend. The objective of the study was to evaluate the usefulness of and the level of satisfaction with the recommendations given by the FG in a group of patients with type 1 diabetes (DM1) who were FSL users. It included 31 subjects (54% women; age 41 ± 15 years; DM duration 21 ± 14 years; 22 with FSL > 12 months) who were provided with the FG. They completed a questionnaire on decision-making depending on glucose trend in different situations (before and three months after using the FG), and a satisfaction questionnaire (ad hoc). Demographic, clinical and glycaemic control data were collected. The percentage of subjects who used glucose trend in decision-making after receiving the FG increased: for adjusting insulin (51 vs. 83; p = 0.016), action without insulin (51 vs. 90%; p = 0.001), and in special circumstances. The FG was evaluated as very useful (4.19/5). There were no significant changes in glycaemic control, although the percentage of data gathered increased significantly (89.07 vs. 94.46%; p = 0.042). In conclusion, the FG was evaluated well for managing glucose trends with FSL by the patients with DM1 analysed, increasing their use of trend in decision-making, with no changes in glycaemic control, but with more data gathered.
La Guía Flash (GF) de dosificación de insulina (A. Chico y C. González) fue el primer documento dirigido a pacientes usuarios de FreeStyle Libre® (FSL) para facilitar la toma de decisiones según glucosa y tendencia. El objetivo del estudio fue evaluar la utilidad y el grado de satisfacción del uso de las recomendaciones proporcionadas por la GF en un grupo de pacientes con diabetes tipo 1 (DM1) usuarios de FSL. Se incluyeron 31 sujetos (54% mujeres; edad: 41 ± 15 años; duración DM: 21 ± 14 años; 22 con FSL > 12 meses) a los que se les proporcionó la GF. Cumplimentaron un cuestionario sobre toma de decisiones según tendencia de glucosa en diferentes situaciones, antes y 3 meses después de usar la GF, y un cuestionario de satisfacción (ad hoc). Se recogieron datos demográficos, clínicos y de control glucémico. El porcentaje de sujetos que usaba la tendencia en la toma de decisiones después de recibir la GF aumentó: tanto para ajuste de insulina (51 vs. 83%; p = 0,016), actuación sin insulina (51 vs. 90%; p = 0,001) y en situaciones especiales. La GF fue valorada como muy útil (4,19/5). No hubo cambios significativos en el control glucémico, aunque sí aumentó significativamente el porcentaje de datos captados (89,07 vs. 94,46%; p = 0,042). Como conclusión, la GF fue bien valorada para el manejo de tendencias con el FSL por los pacientes con DM1 analizados, incrementando el uso de la tendencia en la toma de decisiones, sin cambios en control glucémico, pero con más datos captados.
The FreeStyle Libre® (FSL) flash glucose monitoring system is a device that measures interstitial glucose in a simple way. It shows a good correlation with plasma glucose and is suitable for making treatment decisions in stable situations.1–4 In situations where blood glucose changes rapidly, interstitial glucose values may be different from those of plasma glucose, with a delay of approximately 5 min in detecting the changes.5 These systems provide more information on the glucose profile than monitoring with capillary blood glucose, and benefits have been shown in metabolic control and quality of life in different populations with diabetes mellitus (DM), especially in terms of reducing hypoglycaemic episodes, particularly at night, in patients with type 1 (DM1) and type 2 diabetes mellitus (DM2).4,6 Because of the demonstrated benefits, its convenience and financing more or less across the board by the public health services, its use has become widespread among people with DM. With the new version of the FSL (FSL2), patients also have configurable hypoglycaemia and hyperglycaemia alarms to offer greater safety.
The FSL system provides retrospective information for the last 8 h, as well as trend information. The retrospective information includes the Glucose Management Indicator (GMI), which can be considered equivalent to the estimated glycosylated haemoglobin (HbA1c) value in the analysed period, mean glucose, percentage of time above, below and in range (in general: 70–180 mg/dl), number and duration of hypoglycaemic events, daily profiles, number of daily readings and proportion of data captured. Glucose trend information is provided in the form of trend arrows as follows: horizontal arrow (→) if the variation is <1 mg/dl/min, diagonal (↗ or ↘) if the variation is 1–2 mg/dl/min and vertical (↑ or ↓) if the variation is ≥2 mg/dl/min.
Proper interpretation of the glucose trend information allows the user to make more appropriate treatment decisions for blood glucose control, with the aim of preventing hyper- and hypoglycaemia, anticipating these events by calculating prandial and correction insulin doses and doses in special situations (exercise, driving, stress or illness, etc.). The literature provides various proposals about how to make treatment adjustments on the basis of the glucose value and the trend arrow, both for flash and for continuous glucose monitoring (CGM) systems.7–13 However, these recommendations are aimed primarily at healthcare professionals and not at patients, and there is also no evidence on the impact that following these recommendations has on blood glucose control and patient satisfaction. Before the recommendations for healthcare professionals regarding the management of trends with the FSL system were published, Chico et al. (material edited, unpublished, written in Spanish and distributed in Spain) had put together the only document aimed at people with DM using FSL, to facilitate decision-making based on glucose and trend in various situations (Flash Guide [FG]14). The utility and degree of acceptance by patients of that document are the reasons behind this article.
ObjectivesTo evaluate the clinical utility and the degree of satisfaction with the FG perceived by a group of patients with DM1 who use FSL. The utility was evaluated through blood glucose control parameters before and at three months: HbA1c and data obtained from the FSL (time in range, time in hypoglycaemia, time in hyperglycaemia, number of readings per day and percentage of data captured); satisfaction was assessed through ad hoc questionnaires.
Patients and methodsA prospective descriptive study was carried out that included patients with DM1 – users of FSL who were provided with the FG – from two centres (Hospital de la Santa Creu i Sant Pau and Hospital Universitario de Ciudad Real) from November 2019 to February 2020.
The subjects completed a questionnaire (Annex 1) aimed at finding out what decisions they had made in various day-to-day situations based on the glucose value and the trend, before using the FG and three months after using it, and a questionnaire (Likert scale) (Annex 2) on satisfaction with the use of this guide (ad hoc), shown in the annex. When the patients were given the FG, they received brief information about its features to facilitate understanding of the document.
The inclusion criteria were as follows: subjects with DM1; over 18 years of age; FSL users; and capable of signing the informed consent form.
Patients who had received prior specific training on the interpretation of and action based on the glucose trend or who had completed a specific educational programme aimed at using the system were excluded. The patients signed the informed consent form for data collection and the study was approved by the Independent Ethics Committee of the Hospital de la Santa Creu i Sant Pau Research Institute.
Demographic and clinical data were collected through the electronic medical records and blood glucose control data were obtained from the specific FSL online platform, LibreView™.
The statistical analysis was carried out with STATA® software v.14. A descriptive analysis was performed. Variables were compared with the Student’s t test for paired data and the Wilcoxon signed rank test.
ResultsPatientsA total of 31 patients with DM1 from two centres (21 from Hospital de la Santa Creu i Sant Pau and 10 from Hospital Universitario de Ciudad Real) participated in the study. Their demographic and clinical characteristics are shown in Table 1.
Demographic and clinical characteristics of the subjects included in the study.
| Age (years) | 41 ± 15 (18–76) |
| Gender (male/female) | 14/17 (46%/54%) |
| Years since onset of DM | 21 ± 14 |
| Baseline HbA1c | 7.17 ± 0.91% (5.6%–9.5%) |
| Cardiovascular risk factors | |
| Hypertensiona | 8 (25%) |
| Hyperlipidaemiab | 15 (48%) |
| Chronic complications | |
| Diabetic retinopathy | 10 (32%) |
| Diabetic nephropathy | 3 (9%) |
| Diabetic polyneuropathy | 0 (0%) |
| Coronary heart disease | 1 (3%) |
| Cerebrovascular disease | 0 (0%) |
| Distal arteriosclerosis obliterans | 0 (0%) |
| Treatment | |
| Basal-bolus | 19 (61%) |
| CSII | 12 (39%) |
| Use of bolus calculator | 26 (84%) |
| Use of FSL | |
| <3 months | 1 (3%) |
| 3−6 months | 2 (6%) |
| 6−12 months | 5 (16%) |
| >12 months | 22 (75%) |
| General educational course on use of FSL | 20 (64%) |
DM: diabetes mellitus; CSII: continuous subcutaneous insulin infusion; FSL: FreeStyle Libre®.
There was no statistically significant change in the degree of blood glucose control when comparing the three months prior to receiving the FG with the three months after. HbA1c went from 7.17 ± 0.91% to 7.37 ± 0.82%, with no statistically significant changes. With regard to hypoglycaemia, there was a decrease in time in hypoglycaemia (TBR), a slight increase in time in target range (TIR), and a decrease in the number of hypoglycaemia episodes and mean time in hypoglycaemia, although none of these changes was statistically significant. There was a significant increase in the percentage of data captured and a trend towards a higher number of daily readings. A summary of these results is shown in Table 2.
Blood glucose control data 3 months before and 3 months after receiving the FG.
| Before | After | Difference | |
|---|---|---|---|
| Mean interstitial glucose (mg/dl) | 159 ± 34 | 160 ± 29 | p = 0.81 |
| GMI (%) | 7.04 ± 0.96 | 7.21 ± 0.95 | p = 0.84 |
| TIR (70–180 mg/dl) (%) | 63.32 ± 17.17 | 65.60 ± 14.95 | p = 0.23 |
| TAR (>180 mg/dl) (%) | 26.25 ± 15.49 | 24.96 ± 13.09 | p = 0.54 |
| TBR (<70 mg/dl) (%) | 10.42 ± 7.81 | 9.42 ± 8.47 | p = 0.46 |
| No. of hypoglycaemia episodes | 13 ± 10.72 | 11.82 ± 8.28 | p = 0.97 |
| Time in hypoglycaemia | 96.42 ± 49.44 | 28.46 ± 37 | p = 0.13 |
| No. of readings/day | 11.41 ± 7.07 | 14.28 ± 16.43 | p = 0.3 |
| Data capture (%) | 89.07 ± 11.71 | 94.46 ± 6.7 | p = 0.015 |
FG: Flash Guide; GMI: glucose management indicator; TAR: time above range; TBR: time below range; TIR: time in range.
The FG usage survey (Annex 1) showed that, before receiving the guide, approximately half of the users used the trend arrows to adjust insulin doses, although the majority did not apply any specific criteria. Moreover, half of the patients made decisions based on the trend arrows at times when they were not taking insulin. Action taken based on trend in special situations (exercise, driving, and stress or illness) varied. At three months after receiving the FG, the number of patients who were using the trend arrows to adjust their insulin dose increased significantly, as did the number who did so with a specific criterion. There was also a significant increase in the number of patients using trend information in special situations (exercise, driving, and stress or illness). The data on the results of the usage survey are shown in Table 3.
Use of the trend arrows for decision making in the 3 months before and in the 3 months after receiving the FG.
| Before | After | p-Value | |
|---|---|---|---|
| Patients who adjust insulin dose according to trend (%) | 51 | 83 | p = 0.016 |
| Patients who use specific criteria for dose adjustment (%) | 25 | 76 | p = 0.001 |
| Patients who make decisions according to trend not related to insulin injection (%) | 51 | 90 | p = 0.001 |
| Patients who use the trend in special situations (%) | |||
| Exercise | 64 | 90 | p = 0.015 |
| Driving | 38 | 77 | p = 0.002 |
| Stress or illness | 54 | 80 | p = 0.03 |
FG: Flash Guide.
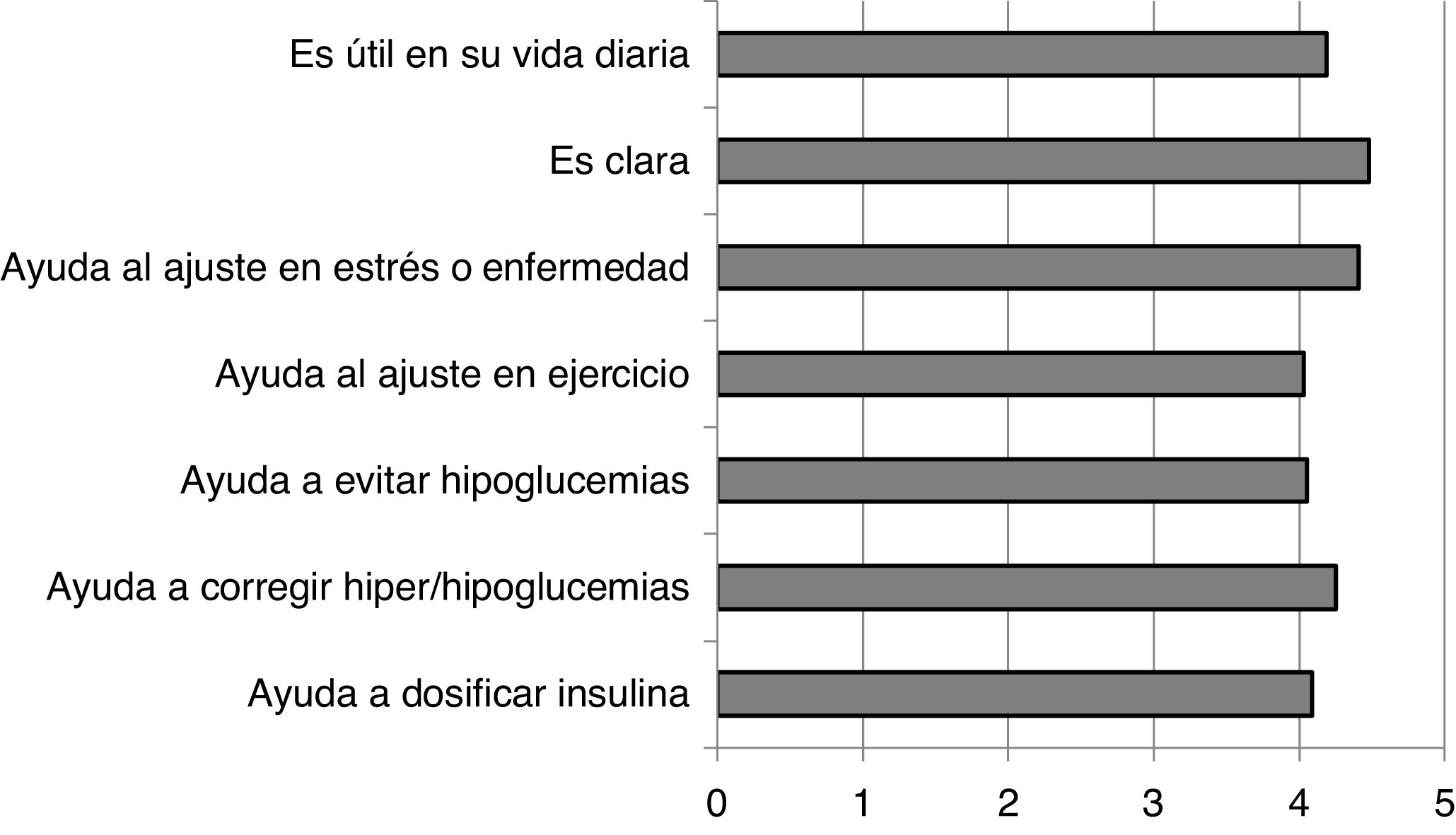
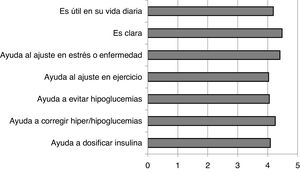
The degree of satisfaction perceived by the patients with the recommendations in the FG was very high, showing scores above 4 out of 5 in all the questions asked (Annex 2). These results are shown in Fig. 1.
DiscussionInterstitial glucose monitoring devices provide the ability to obtain immediate information on current glucose levels, as well as the direction and rate of change. This information allows people with diabetes to make therapeutic decisions related to the dosage of prandial insulin and to correct high glucose values, as well as to react more quickly and appropriately to prevent acute glycaemic episodes.12,13
Of all the proposed insulin adjustment systems based on glucose trends presented in the literature, most were developed for CGM systems and aimed at healthcare professionals and not at patients. Of these, one has been evaluated in the paediatric population.15 However, there are no specific recommendations for patients with the FSL system. This study is the first to evaluate, in this case in the adult population, specific recommendations designed for diabetes patients in terms of taking action according to glucose trends.
In the population of people with DM1 who participated in our study, after using the FG the use of trend arrows increased significantly. There was also an increase in the number of subjects who used specific criteria for therapy-related actions. In their subjective evaluation of the guide, the subjects considered the guide very useful in their day-to-day lives (4.19/5) and clear in the way it presents the information (4.48/5).
Blood glucose control did not change significantly in the before/after comparison, but there was an increase in the data captured by the FSL, a factor that has been associated with better blood glucose control in other studies with larger samples.2
Education is the basic pillar for self-management and self-efficacy in diabetes, and even more so when combined with technology. It has been shown that the benefits of using flash glucose monitoring are greater if it is accompanied by a specific educational programme.16 The aim is to achieve empowerment or self-management, enabling patients to use the new technology effectively in their day-to-day lives.17
The FG can act as a facilitator of diabetes self-management and can be used as an advanced educational strategy to simplify the information to be transmitted to patients. This will make it easier for patients to understand and therefore make it safer for them to manage their own diabetes.
Ethical considerationsThe study was approved by the Independent Ethics Committee of the Hospital de la Santa Creu i Sant Pau Research Institute. Informed consent was obtained from all the participants.
FundingThe authors declare that they received no funding to carry out this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Mateu-Salat M, Moreno-Fernández J, Mangas N, Genua I, Martínez MJ, López A, et al. Evaluación de la utilidad y satisfacción con la guía de uso del sistema flash de monitorización de glucosa (FreeStyle Libre®) en pacientes con diabetes tipo 1. Endocrinol Diabetes Nutr. 2022;69:316–321.