This study aimed to analyze the relationship of neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and monocyte-lymphocyte ratio (MLR) with clinicopathological characteristics of patients with differentiated thyroid cancer (DTC).
METHODS:This retrospective study included 390 patients with DTC who had complete blood cell counts available at the time of surgery. NLR, PLR, and MLR were calculated, and the risk of cancer-related death, structural recurrence, and response to therapy were assessed using the eighth edition of the tumor-node-metastasis classification, American Thyroid Association (ATA) Risk Stratification System, and ATA Response to Therapy Reclassification, respectively.
RESULTS:PLR was higher in patients with distant metastasis than in those without (133.15±43.95 versus 119.24±45.69, p=0.0345) and lower in patients with disease-free status (117.72±44.70 versus 131.07±47.85, p=0.0089) than in those who experienced persistent disease or death. Patients aged ≥55 years had a higher MLR than those aged <55 years (0.26±0.10 versus 0.24±0.12, p=0.0379). Higher MLR (odds ratio [OR]: 8.775, 95% confidence interval [CI]: 1.532-50.273, p=0.0147), intermediate ATA risk (OR: 4.892, 95% CI: 2.492-9.605, p≤0.0001), and high ATA risk (OR: 5.998, 95% CI: 3.126-11.505, p≤0.0001) were risk factors associated with active disease. NLR was not significantly different among the studied variables. Receiver operating characteristic curve cut-off values for NLR, PLR, and MLR were able to differentiate distant metastasis from lymph node metastasis (NLR>1.93: 73.3% sensitivity and 58.7% specificity, PLR>124.34: 86.7% sensitivity and 69.2% specificity, MLR>0.21: 80% sensitivity and 45.2% specificity).
CONCLUSION:Cut-off values of NLR, PLR, and MLR differentiated distant metastasis from lymph node metastasis with good sensitivity and accuracy. PLR was associated with disease-free status and it was higher in DTC patients with distant metastasis, persistent disease, and disease-related death. MLR was a risk factor for active disease.
Cancer-associated inflammation plays an essential role in the clinical evolution of most of the cancers, mainly by promoting tumor cell proliferation, angiogenesis, invasion, and metastasis (1-3). Increasing evidence has shown that several biomarkers related to inflammation such as C-reactive protein levels, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and monocyte-lymphocyte ratio (MLR) or lymphocyte-monocyte ratio (LMR) are indicators of poor prognosis in a variety of cancers (4) and chronic diseases (5-7). However, the importance of such an inflammatory response is not entirely clear in thyroid cancer. NLR is the most widely investigated inflammatory marker among patients with thyroid cancer, and many authors have demonstrated that a high NLR is closely related to aggressive tumors, lymph node metastasis, and poor prognosis (8-13). Similarly, a high PLR is also linked to poor prognosis, especially among patients with papillary and medullary thyroid cancer (14-16). Recently, some authors (17-20) have demonstrated that a low LMR could predict recurrence and overall survival in patients with papillary thyroid cancer (PTC) and poor survival in patients with anaplastic thyroid carcinoma. To the best of our knowledge, no study has evaluated NLR, PLR, and MLR obtained from patients with differentiated thyroid carcinoma (DTC) in addition to their relationship with different histological types and long-term clinical and laboratory outcomes.
The present study aimed to analyze the potential relationship of NLR, PLR, and MLR with clinicopathological characteristics and outcomes in patients with DTC.
MATERIALS AND METHODSSubjectsThis retrospective study involved 1,143 patients diagnosed with DTC who were followed-up at the Thyroid Disease Unit at the Clinics Hospital of the University of Campinas between January 2000 and January 2019. Ethical committee approval for the study was obtained according to the Declaration of Helsinki from the Human Research Ethics Committee in Lausanne (No. 204/14. CAAE: 58234916.3.0000.5404).
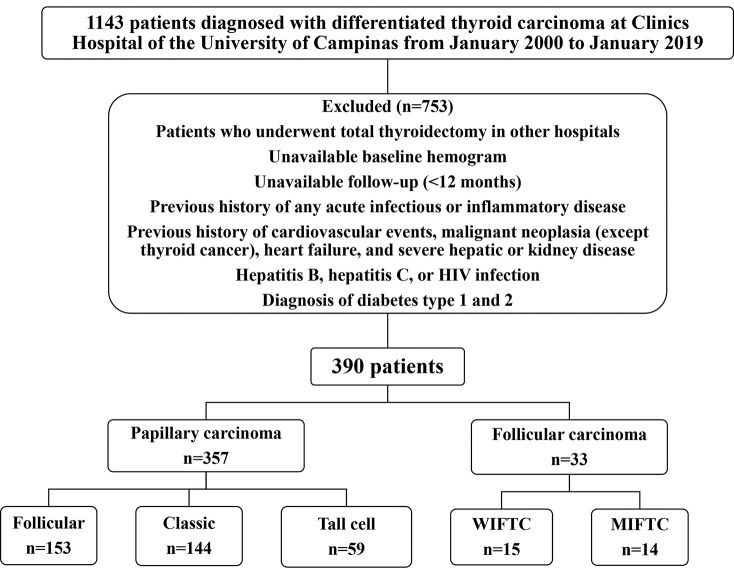
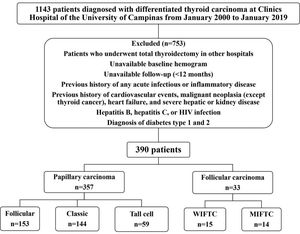
Among the 1,143 initially selected patients, 390 had adequate clinicopathological information and were followed-up. Seven hundred and fifty-three patients were excluded from the study for the following reasons: underwent total thyroidectomy in other hospitals, unavailability of baseline hemogram before the surgery, and inadequate follow-up (a minimum of 12-month follow-up was required). Patients with a previous history of any acute infectious or inflammatory diseases; history of cardiovascular events (myocardial ischemia, unstable angina, or stroke); malignant neoplasia (except thyroid cancer); heart failure (New York Heart Association class III or IV); severe hepatic or kidney disease (chronic kidney disease stages 4 or 5 or hemodialysis); hepatitis B, hepatitis C, and human immunodeficiency virus infection; and diabetes (type 1 and 2) were also excluded. Figure 1 shows the flowchart of the study.
Clinical assessmentClinical characteristics and laboratory data were collected through chart review. The collected clinical data included sex, age, age at diagnosis, ethnicity, smoking habit, duration of follow-up, use of ablative and/or adjuvant radioactive iodine therapy, findings from radioactive iodine whole-body scanning, cervical ultrasound, and computed tomography scan when available, and response to cancer treatment.
Tumor characteristics analyzed in the study included histology (papillary or follicular), PTC variants (classic, follicular, or tall cell), tumor size (largest dimension of the lesion), multifocality, invasion (extrathyroidal extension or capsular or vascular invasion), metastatic nodes, and distant metastasis.
Laboratory data included serum levels of thyroid-stimulating hormone (reference value 0.41-4.5 mUI/L), free thyroxine (reference value 0.9-1.8 m/dL), thyroglobulin antibodies (reference value <115 mUI/L), and thyroglobulin (reference value 0.2-70 ng/mL) measured using electrochemiluminescence immunoassay. Complete blood counts and automated differential counts were collected 3 days before the surgery. NLR, PLR, and MLR were calculated from the complete blood. NLR was calculated by dividing the number of neutrophils by the number of lymphocytes. Similarly, PLR and MLR were calculated by dividing the number of platelets and monocytes by the lymphocyte count, respectively.
Risk stratificationThe eighth edition of the tumor-node-metastasis (TNM) classification developed by the American Joint Committee on Cancer was used to classify thyroid tumors and to predict the risk of cancer-related death. The risk of structural recurrence was assessed using the American Thyroid Association (ATA) risk stratification system, based on which the risk can be classified as low, intermediate, or high. Response to treatment was assessed using the ATA Response to Therapy Reclassification, and the response was classified as excellent, biochemical incomplete, structural incomplete, or indeterminate. Disease outcomes were classified as disease-free status, persistent disease, or disease-related death.
Statistical analysisStatistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute Inc., Cary, NC, USA). Exploratory data analysis was performed using summary measures (mean, standard deviation, minimum, median, maximum, frequency, and percentage). The relationship of NLR, PLR, and MLR with the variables of interest was assessed using Mann-Whitney or Kruskal-Wallis tests. Univariate and multiple Cox regression analyses were used to identify the factors associated with disease-free status. Stepwise variable selection process was employed. The receiver operating characteristic (ROC) curve was used to assess the predictive accuracy of the ratios. Larger area under the ROC curve (AUC) indicated greater discriminatory power of the model. Generally, an AUC≥0.7 indicates acceptable discriminatory power of the model. The significance level was set at p<0.05.
RESULTSBaseline characteristicsTable 1 shows the detailed characteristics of 390 patients with DTC who were included in this study. Most of the included patients were female (83.08%), Caucasians (73.08%), and non-smokers (70.51%). The mean age of the patients was 46.65±15.41 years. The majority of the patients were diagnosed with PTC (91.54%) and were classified as either classic (40.45%), follicular (42.98%), or tall cell (16.57%) variants. Based on the ATA risk stratification system, 50% of the patients were classified as low-risk, 25.90% as intermediate-risk, and 24.10% as high-risk patients. Despite the high proportion of patients with intermediate and high risk, most of them (78.72%) presented an excellent response to treatment. The rates of structural incomplete response, indeterminate response, and biochemical incomplete response were 8.21%, 7.95%, and 5.13%, respectively. Our classification of disease outcomes into disease-free status (78.72%), persistent disease (19.49%), and disease-related death (1.79%) was similar to the ATA Response to Therapy Reclassification. The mean NLR, PLR, and MLR were 2.05±1.12, 120.56±45.65, and 0.25±0.11, respectively.
Baseline and histopathological characteristics and outcomes of patients with differentiated thyroid carcinoma.
| Patients' characteristics | n=390 | |
|---|---|---|
| Gender | Female | 324 (83.08%) |
| Male | 66 (16.92%) | |
| Age (years) | 46.65±15.41 | |
| Age | <55 years | 262 (67.18%) |
| ≥55 years | 128 (32.82%) | |
| Follow-up (months) | 82.83±54.17 | |
| Platelets (×103/μL) | 250±63 | |
| White blood cells (×103/μL) | 7.20±1.91 | |
| Neutrophils (×103/μL) | 4.16±1.48 | |
| Lymphocytes (×103/μL) | 2.26±0.76 | |
| Monocytes (×103/μL) | 0.52±0.20 | |
| Neutrophil-lymphocyte ratio | 2.05±1.12 | |
| Platelet-lymphocyte ratio | 120.56±45.65 | |
| Monocyte-lymphocyte ratio | 0.25±0.11 | |
| DTC characteristics | n=390 | |
| Thyroid cancer | Papillary | 357 (91.54%) |
| Follicular | 33 (8.46%) | |
| Papillary thyroid cancer variants | Classic | 144 (40.45%) |
| Follicular | 153 (42.98%) | |
| Tall cell | 59 (16.57%) | |
| Diameter of the largest tumor focus (cm) | 2.31±1.96 | |
| Microcarcinoma | 101 (25.96%) | |
| Multifocality | 202 (52.06%) | |
| Invasion | Extrathyroidal extension | 92 (23.71%) |
| Capsular | 87 (22.42%) | |
| Vascular | 83 (21.39%) | |
| No invasion | 126 (32.48%) | |
| Metastatic lymph nodes | 126 (32.31%) | |
| Distant metastasis | Lung | 29 (7.44%) |
| Bone | 4 (1.03%) | |
| Lung and bone | 4 (1.03%) | |
| T1 | 181 (46.53%) | |
| T2 | 69 (17.74%) | |
| T3 | 86 (22.11%) | |
| T4 | 53 (13.62%) | |
| N0 | 263 (67.61%) | |
| N1 | 116 (32.39%) | |
| M0 | 351 (90.23%) | |
| M1 | 38 (9.77%) | |
| TNM staging (eighth edition) | I | 296 (76.09%) |
| II | 58 (14.91%) | |
| III | 16 (4.11%) | |
| IV | 19 (4.88%) | |
| ATA risk | Low | 195 (50%) |
| Intermediate | 101 (25.90%) | |
| High | 94 (24.10%) | |
| Radioiodine therapy | 366 (94.33%) | |
| Radioiodine activities | 195.16±198.34 | |
| Anti-thyroglobulin antibody detectable | 17 (4.36%) | |
| Response to treatment | Excellent | 307 (78.72%) |
| Biochemical incomplete | 20 (5.13%) | |
| Structural incomplete | 32 (8.21%) | |
| Indeterminate | 31 (7.95%) | |
| Tumor outcome | Disease-free status | 307 (78.72%) |
| Persistent disease | 76 (19.49%) | |
| Disease-related death | 7 (1.79%) | |
Values are presented as mean±standard deviation or as count (percentage).
TNM: tumor-nodes-metastasis, ATA: American Thyroid Association, DTC: differentiated thyroid cancer.
Table 2 shows the comparison between NLR, PLR, and MLR with patient's age, histology, PTC variants, tumor size, type of invasion, focal and distant metastasis, and risk stratification. NLR was not statistically significant in any variable analyzed. In contrast, PLR was higher among patients with distant metastasis than in those without it (133.15±43.95 versus 119.24±45.69, p=0.0345). PLR was significantly lower in patients classified as disease-free status than in those with persistent disease or disease-related death (117.72±44.70 versus 131.07±47.85, p=0.0089). Patients aged ≥55 years exhibited a significantly higher MLR than those aged <55 years (0.26±0.10 versus 0.24±0.12, p=0.0379).
Association of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and monocyte-lymphocyte ratio with tumor characteristics, stage, and evolution of differentiated thyroid carcinoma.
| Patients | Neutrophil-lymphocyte ratio | Platelet-lymphocyte ratio | Monocyte-lymphocyte ratio | ||||
|---|---|---|---|---|---|---|---|
| Mean | p-value | Mean | p-value | Mean | p-value | ||
| Thyroid Cancer | |||||||
| Papillary | 357 | 2.05±1.15 | 0.4491 | 120.11±44.66 | 0.7714 | 0.25±0.12 | 0.2949 |
| Follicular | 33 | 2.04±0.73 | 125.45±55.89 | 0.25±0.09 | |||
| Variants | |||||||
| Classic PTC | 144 | 2.04±1.18 | 0.8484 | 118.76±39.65 | 0.9810 | 0.23±0.09 | 0.6133 |
| Follicular | 153 | 2.06±1.22 | 120.98±48.76 | 0.26±0.13 | |||
| Tall cell | 59 | 2.01±0.87 | 120.74±46 | 0.26±0.14 | |||
| Capsular invasion | |||||||
| Yes | 87 | 1.91±0.74 | 0.6555 | 119.16±45.91 | 0.7353 | 0.25±0.12 | 0.8818 |
| No | 301 | 2.08±1.20 | 120.81±45.76 | 0.25±0.11 | |||
| Angiolymphatic invasion | |||||||
| Yes | 83 | 2.06±1.15 | 0.7962 | 120.39±41.86 | 0.6194 | 0.25±0.12 | 0.5674 |
| No | 305 | 2.04±1.11 | 120.45±46.81 | 0.25±0.11 | |||
| Extrathyroidal invasion | |||||||
| Yes | 92 | 2.06±1.19 | 0.8269 | 118.81±41.33 | 0.9478 | 0.25±0.13 | 0.9550 |
| No | 296 | 2.04±1.10 | 120.95±47.08 | 0.25±0.11 | |||
| Lymph node metastasis | |||||||
| Yes | 126 | 2.04±1.08 | 0.9334 | 115.58±38.14 | 0.3197 | 0.24±0.11 | 0.7524 |
| No | 264 | 2.05±1.14 | 122.94±48.72 | 0.25±0.12 | |||
| Distant metastasis | |||||||
| Yes | 37 | 2.15±0.91 | 0.1786 | 133.15±43.95 | 0.0345 | 0.24±0.08 | 0.4285 |
| No | 353 | 2.03±1.14 | 119.24±45.69 | 0.25±0.12 | |||
| Tumor Size | |||||||
| <1 cm | 288 | 2.03±1.04 | 0.9992 | 118.38±41.74 | 0.7888 | 0.25±0.10 | 0.4843 |
| ≥1 cm | 101 | 2.05±1.15 | 121.25±47.05 | 0.25±0.12 | |||
| Age | |||||||
| <55 years | 262 | 2.04±1.17 | 0.3177 | 121.17±44.69 | 0.3688 | 0.24 ± 0.12 | 0.0379 |
| ≥55 years | 128 | 2.06±1.00 | 119.31±47.72 | 0.26 ± 0.10 | |||
| TNM staging (eighth edition) | |||||||
| Stages I and II | 354 | 2.04±1.15 | 0.4112 | 119.20±45 | 0.1004 | 0.25±0.12 | 0.0986 |
| Stages III and IV | 35 | 2.06±0.8 | 132.64±50.53 | 0.26±0.10 | |||
| ATA risk | |||||||
| Low | 195 | 2.09±1.22 | 0.8286 | 121.85±48.66 | 0.4852 | 0.25±0.12 | 0.4317 |
| Intermediate | 101 | 1.98±0.96 | 115.44±40.44 | 0.24±0.13 | |||
| High | 94 | 2.09±1.22 | 121.85±48.66 | 0.25±0.12 | |||
| Radioiodine therapy | |||||||
| Yes | 361 | 2.06±1.13 | 0.3018 | 120.46±45.50 | 0.8120 | 0.25±0.12 | 0.8856 |
| No | 32 | 1.80±0.84 | 118.47±49.47 | 0.24±0.08 | |||
| Response to treatment | |||||||
| Excellent | 307 | 2.01±1.11 | 0.3453 | 117.72±44.70 | 0.0717 | 0.24±0.11 | 0.4423 |
| Biochemical incomplete | 20 | 2.27±1.30 | 128.54±44.41 | 0.27±0.12 | |||
| Structural incomplete | 32 | 2.05±0.77 | 130.46±49.54 | 0.25±0.10 | |||
| Indeterminate | 31 | 2.25±1.36 | 133.34±49.64 | 0.27±0.16 | |||
| Disease outcome | |||||||
| Disease-free status | 307 | 2.01±1.11 | 0.0812 | 117.72±44.70 | 0.0089 | 0.24±0.11 | 0.1219 |
| Persistent disease and disease-related death | 83 | 2.18±1.14 | 131.07±47.85 | 0.26±0.13 | |||
Values are presented as mean±standard deviation. Statistical significance was set at p<0.05.
PTC: papillary thyroid cancer, TNM: tumor-node-metastasis, ATA: American Thyroid Association.
In the univariate logistic regression analysis, a higher PLR (odds ratio [OR]: 1.004, 95% confidence interval [CI]: 1.001-1.008, p=0.0247), tumor size of ≥1 cm (OR: 2.942, 95% CI: 1.416-6.113, p=0.0038), age greater than 55 years (OR: 1.701, 95% CI: 1.096-2.640, p=0.0179), TNM stages III and IVb versus stages I and II (OR: 3.268, 95% CI: 1.982-5.389, p≤0.0001), tall cell variant versus follicular variant (OR: 2.016, 95% CI: 1.106-3.676, p=0.0221), intermediate (OR: 4.916, 95% CI: 2.509-9.631, p≤0.0001), and high ATA risk versus low risk (OR: 6.652, 95% CI: 3.583-12.352, p≤0.0001) were factors significantly associated with a low rate of disease-free status (Table 3).
Factors associated with disease-free status in patients with differentiated thyroid cancer.
| Variables | p-value | HR | 95% CI |
|---|---|---|---|
| Cox univariate analysis | |||
| NLR | 0.4074 | 1.072 | 0.909-1.265 |
| PLR | 0.0247 | 1.004 | 1.001-1.008 |
| MLR | 0.0904 | 4.272 | 0.796-22.944 |
| Tumor size of ≥1 cm versus <1 cm | 0.0038 | 2.942 | 1.416-6.113 |
| Age≥55 years versus <55 years | 0.0179 | 1.701 | 1.096-2.640 |
| TNM stages III and IV versus I and II | <0.0001 | 3.268 | 1.982-5.389 |
| Papillary variant | |||
| Tall cell versus follicular | 0.0221 | 2.016 | 1.106-3.676 |
| Classic versus follicular | 0.9494 | 1.018 | 0.590-1.756 |
| High versus low ATA risk | <0.0001 | 6.652 | 3.583-12.352 |
| Intermediate versus low ATA risk | <0.0001 | 4.916 | 2.509-9.631 |
| Cox multivariate analysis | |||
| MLR | 0.0147 | 8.775 | 1.532-50.273 |
| High versus low ATA risk | <0.0001 | 5.998 | 3.126-11.505 |
| Intermediate versus low ATA risk | <0.0001 | 4.892 | 2.492-9.605 |
Statistical significance was set at p<0.05.
NLR: neutrophil-lymphocyte ratio, PLR: platelet-lymphocyte ratio, MLR: monocyte-lymphocyte ratio, TNM: tumor-node-metastasis, ATA: American Thyroid Association, HR: hazard ratio, CI: confidence interval.
A multiple logistic regression analysis was performed with a model including the following variables used in the univariate analysis: age≥55 years versus <55 years, NLR, MLR, PLR, tumor size of ≥1 cm versus <1 cm, TNM stages III and IV versus stages I and II, papillary carcinoma variant (tall cells versus follicular, classic versus follicular), and ATA risk (high versus low, intermediate versus low). A higher MLR (OR: 8.775, 95% CI: 1.532-50.273, p=0.0147), intermediate ATA risk (OR: 4.892, 95% CI: 2.492-9.605, p≤0.0001), and high ATA risk (OR: 5.998, 95% CI: 3.126-11.505, p≤0.0001) were factors associated with a lower disease-free status (Table 3).
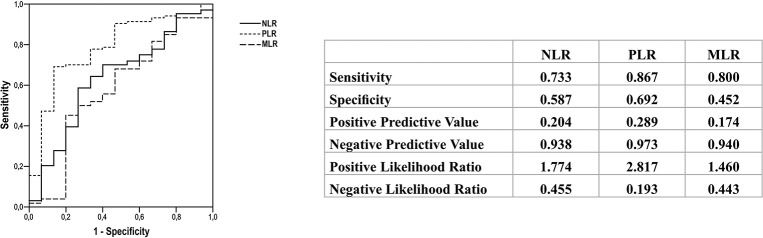
ROC curve analysisROC curves allowed the identification of discriminatory cut-off values for each analysis. Values above the cut-off were observed among patients with distant metastasis and not in those with lymph node metastasis. The best cut-off values to differentiate lymph node metastasis from distant metastasis were as follows: NLR>1.93 (sensitivity of 73.3%, specificity of 58.7%, accuracy of 63.5%, positive predictive value of 20.4%, and negative predictive value of 93.8%), PLR>124.34 (sensitivity of 86.7%, specificity of 69.2%, accuracy of 78.7%, positive predictive value of 28.9%, and negative predictive value of 97.3%), and MLR>0.21 (sensitivity of 80%, specificity of 45.2%, accuracy of 57.9%, positive predictive value of 17.4%, and negative predictive value of 94%) (Figure 2). These parameters did not allow discrimination between distant metastasis and disease-free status (AUC: NLR, 0.574; PLR, 0.575; and MLR, 0.550), between lymph node metastasis and disease-free status (AUC: NLR, 0.540; PLR, 0.544; and MLR, 0.540), between metastasis (lymph node and distant) and no metastasis (AUC: NLR, 0.524; PLR, 0.514; and MLR, 0.502), and between metastasis (lymph node and distant) and disease-free status (AUC: NLR, 0.552; PLR, 0.581; and MLR, 0.550) (data not shown).
DISCUSSIONThis study evaluated the role of NLR, PLR, and MLR as predictive tools for disease status in patients with DTC. The cut-off values of NLR, PLR, and MLR enabled the discrimination of distant metastasis from lymph node metastasis with good sensitivity and accuracy. In addition, we demonstrated that MLR associated with a low ATA risk in patients with DTC was a predictive factor of disease-free status. Furthermore, PLR was related to disease-free status, and it was comparatively higher in DTC patients with distant metastasis, persistent disease, and disease-related death than in patients free of disease. However, we did not find any association between NLR and disease outcome in the same population.
NLR, PLR, and MLR may reflect two connected processes, namely systemic inflammatory response and immune system status. The exact pathways between these ratios and tumor behavior remain unclear. However, it is well known that cancer cells can secrete inflammatory factors such as interleukin (IL)-1, IL-2, IL-6, IL-8, IL-12, IL-17, granulocyte colony-stimulating factor, and tumor necrosis factor-alpha, which may contribute to the tumor microenvironment, leading to tumor invasion, leukocytosis, and thrombocytosis (4,21). Neutrophilia, thrombocytosis, and increased monocytes may be observed in tissue damage and inflammation, playing an essential role in tumor proliferation, invasion, metastasis, and recurrence. Lymphocytes are the leading components of immunity against tumors, stimulating the release of cytokines such as interferons and tumor necrosis factor-alpha, which results in subsequent tumor reduction. Decreased lymphocyte count is associated with an inadequate immunologic reaction that increases the susceptibility to tumor progression and metastasis (1,2,4,22).
NLR is the most widely investigated marker in thyroid cancer as well as in several other malignancies including gastrointestinal tumors and breast, lung, prostate, and ovarian cancers (4). Most studies that analyzed NLR in patients with DTC have shown that a high NLR was associated with poor prognosis and survival, and it was primarily related to TNM classification, patients' age, tumor size, multifocality, lymph node metastasis, extrathyroidal invasion, and risk of recurrence (8-13). However, two meta-analyses differ in their results regarding the utility of NLR (23,24). Feng et al. (23) suggested that NLR may serve as a biomarker to predict tumor growth, metastasis, and prognosis, whereas Liu et al. (24) stated that NLR is not a reliable indicator of prognosis in patients with DTC. Consistent with the latter, our study could not confirm the usefulness of NLR since no association was found between NLR and prognosis or tumor features in the included patients. In contrast, we could determine a cut-off value of NLR through the ROC curve wherein NLR>1.93 discriminated distant metastasis from lymph node metastasis (sensitivity of 73.3% and accuracy of 63.5%).
Although PLR was assessed less frequently than NLR in previous studies, some authors have found a link between PLR and poor prognosis among patients with DTC (13,16,21). We showed that patients with distant metastasis presented a higher PLR, as well as a lower PLR was found in patients classified as disease-free status. Besides that, PLR>124 helped differentiate distant metastasis from lymph node metastasis with high sensitivity (86.7%) and accuracy (78.7%). The majority of the studies have analyzed patients with DTC, however, two Chinese studies suggested that a high PLR was associated with poor prognosis in medullary thyroid cancer, while NLR did not exhibit such an association (14,15). Jiang et al. (14) demonstrated an association of high PLR with lymph node metastasis and recurrence. A year later, the same group reported that increased PLR was also predictive of lymph node metastasis, capsular invasion, advanced tumor stages, and recurrence (15).
Very few studies have evaluated MLR or LMR in patients with thyroid cancer (17-20,25). Ahn et al. (19) performed a retrospective study including 35 patients with anaplastic thyroid cancer and found an association between low LMR and poor survival. In contrast, Yamazaki et al. (25) evaluated a greater number of patients with anaplastic thyroid cancer and did not find a similar association. Three other authors (17,18,20) assessed LMR in patients with PTC and found that a low preoperative LMR could predict recurrence and overall survival. Although we assessed the inverse ratio (MLR), a similar relationship was seen in our study. We observed a higher MLR among older patients (≥55 years old) and it was a predictive factor for disease-free status. Additionally, we found that an MLR of <0.21 could discriminate distant metastasis from lymph node metastasis, with a sensibility of 80% and an accuracy of 58%.
A small number of studies have demonstrated the relationship between these ratios and benign thyroid diseases such as Graves' disease (GD), Hashimoto's thyroiditis (HT), toxic adenoma (TA), and subacute thyroiditis (SAT) (26-30). Hu et al. (28) proposed combining thyroid hormones (free thyroxine and triiodothyronine) and the eosinophil-to-monocyte ratio to distinguish GD from SAT. Taskaldiran et al. (30) retrospectively analyzed NLR and PLR in patients with GD, SAT, and TA and suggested that high PLR and NLR may be useful in differentiating SAT from GD and TA. Another interesting retrospective study performed by Kim et al. (29) reported that elevated NLR was an independent prognostic factor for relapse in patients with GD. Aktas et al. (26) and Bilge et al. (27) found a significantly higher NLR in patients with HT than in healthy controls.
This study had some limitations including those inherent to a retrospective and single-institution study. Moreover, the total number of subjects included in this study was small compared to other studies; however, our analysis was able to present robust results. Despite these limitations, the present study is the first to analyze three different ratios covering the low, intermediate, and high risk of metastasis among patients with DTC.
CONCLUSIONWe found that cut-off values of NLR, PLR, and MLR were able to discriminate distant metastasis from lymph node metastasis with good sensitivity and accuracy. High PLR and MLR were associated with active disease status. Lower PLR was associated with disease-free status, while a higher PLR was observed in DTC patients with distant metastasis, persistent disease, and disease-related death. Our study demonstrated that PLR, NLR, and MLR could be considered useful, readily available, and low-cost tools during clinical follow-up of patients with DTC. However, a large-scale study is required to confirm our results.
AUTHOR CONTRIBUTIONSRiguetto CM, Barreto IS, Maia FFR, Assumpção LVM and Zantut-Wittmann DE participated in the data acquisition, analysis and interpretation. Riguetto CM and Wittmann DEZ participated in the conception and design of the study, interpretation of results, and manuscript drafting.
Zantut-Wittmann DE has a research grant from CNPq (National Council of Research) (proc 302827/2018-8).
No potential conflict of interest was reported.