Scar formation is a consequence of the wound healing process that occurs when body tissues are damaged by a physical injury. Hypertrophic scars and keloids are pathological scars resulting from abnormal responses to trauma and can be itchy and painful, causing serious functional and cosmetic disability. The current review will focus on the definition of hypertrophic scars, distinguishing them from keloids and on the various methods for treating hypertrophic scarring that have been described in the literature, including treatments with clearly proven efficiency and therapies with doubtful benefits. Numerous methods have been described for the treatment of abnormal scars, but to date, the optimal treatment method has not been established. This review will explore the differences between different types of nonsurgical management of hypertrophic scars, focusing on the indications, uses, mechanisms of action, associations and efficacies of the following therapies: silicone, pressure garments, onion extract, intralesional corticoid injections and bleomycin.
Hypertrophic scars (HTSs) are defined as visible and elevated scars that do not spread into surrounding tissues and that often regress spontaneously (1). These scars are characterized by proliferation of the dermal tissue, with excessive deposition of fibroblast-derived extracellular matrix (ECM) proteins and especially collagen, over long periods and by persistent inflammation and fibrosis (2).
Numerous methods have been described for the treatment of abnormal scars, but to date, the optimal treatment method has not been established. A wide variety of treatments have been advocated for HTSs. Among these treatments are surgical excision with or without grafting (1), pressure therapy (3), intralesional interferon (4), topical and intralesional corticosteroids (5), intralesional bleomycin (6), laser therapy (7), silicone gel sheeting (8), onion extract gel and other therapies directed at collagen synthesis (9).
Distinguishing hypertrophic scars from keloidsWhen faced with patients seeking treatment for pathological scars, many physicians have difficulty in differentiating HTSs from keloids; therefore, it is crucial to establish criteria to distinguish them.
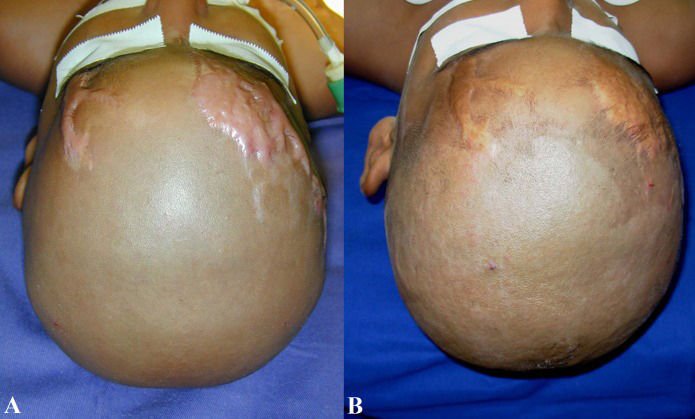
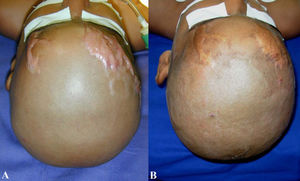
HTSs are usually raised, although rarely elevated more than 4 mm above the skin; red or pink in color; hard; and pruritic. Additionally, these scars do not extend beyond the general geographic margins of the wound and tend to regress over time (10) (Figure1). HTSs primarily contain type III collagen, oriented parallel to the epidermal surface and with abundant nodules and large extracellular collagen filaments (11).
Hypertrophic scar regression in a burned child after four years (A and B). Hypertrophic scars are usually raised, although rarely elevated more than 4 mm above the skin; red or pink in color; hard; and pruritic. Additionally, these scars do not extend beyond the general geographic margins of the wound and tend to regress over time.
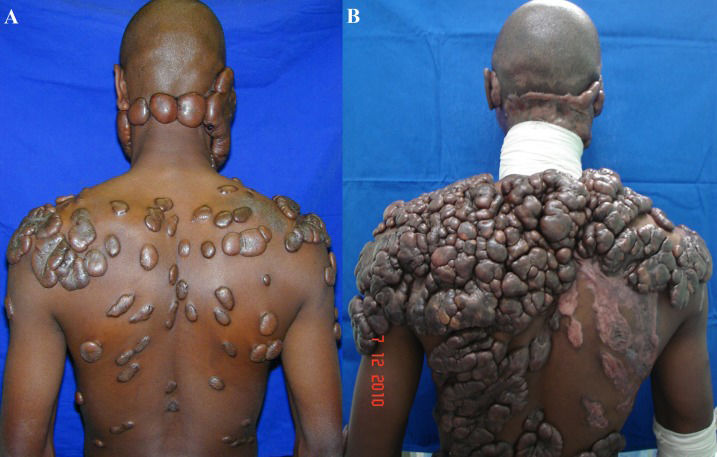
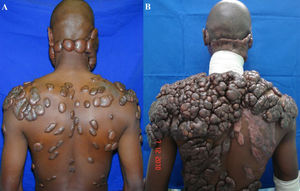
In contrast, keloids continue to evolve over time, without a quiescent or regressive phase and do infiltrate the surrounding tissue (Figure2). Keloids appear as firm, mildly tender, bosselated tumors with a shiny surface and occasional telangiectasia. The epithelium is thinned and there may be focal areas of ulceration. The color is pink to purple and may be accompanied by hyperpigmentation (12). The initial lesions are erythematous and become brownish red, followed by paling as they age. The lesions preferentially develop on the earlobes, shoulders and presternal skin; are void of hair follicles and other glands; and usually project above the level of the surrounding skin (13). Keloids are primarily composed of abnormally thick, irregularly branched and septal disorganized type I and III collagen bundles without nodules and with excess myofibroblasts (11) and overproduction of multiple fibroblast proteins, indicating the persistence of wound healing or even a failure to downregulate wound-healing cells. In addition, keloids are not triggered to enter the final phase of wound healing, or the “remodeling” phase, whereas HTSs will eventually do so (14).
Distinguishing HTSs from keloids histopathologically is occasionally difficult because thickened hyalinized collagen (keloidal collagen), the hallmark of keloids, is not always detectable and because α-smooth muscle actin (α-SMA), a differentiating marker of HTSs, is variably expressed in both types of scars (15).
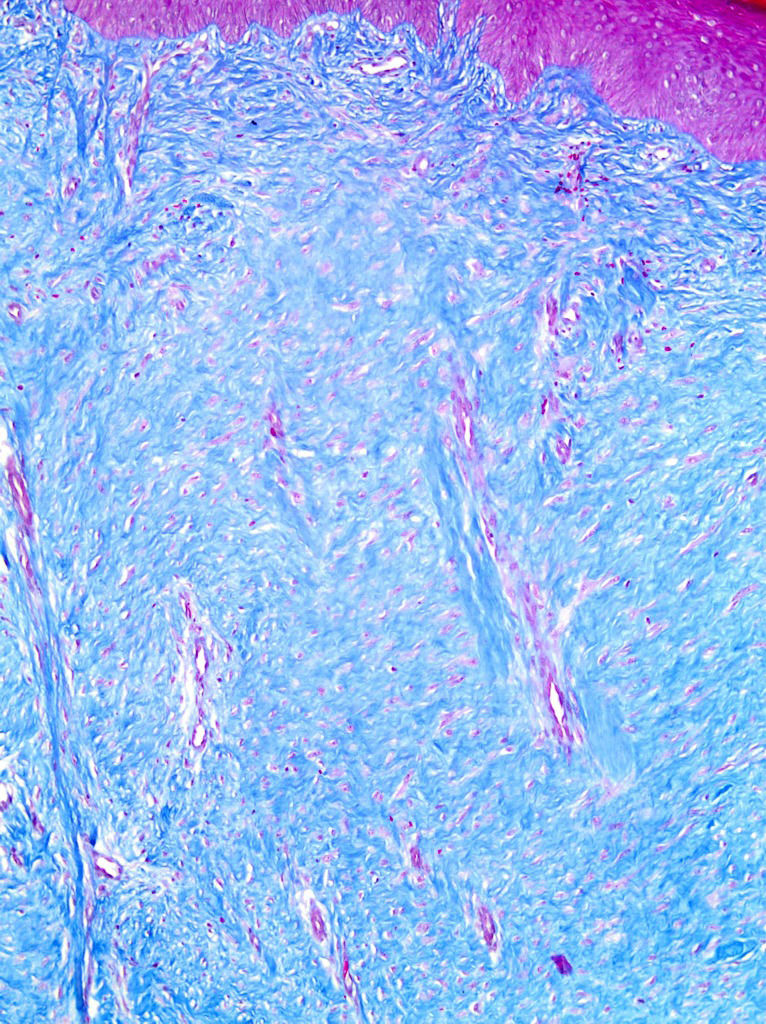
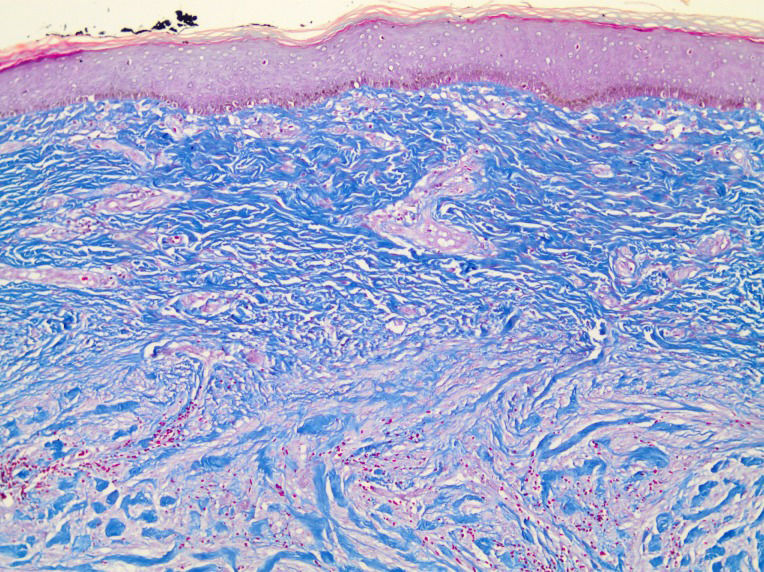
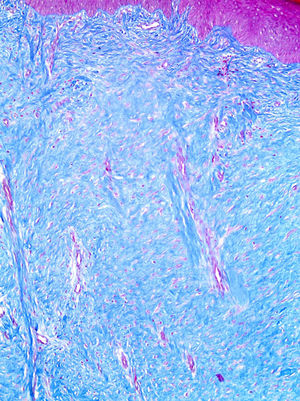
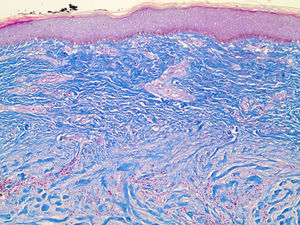
The histopathological findings most commonly observed in HTSs are flattening of the epidermis and replacement of the papillary and reticular dermis by scar tissue with prominent vertically oriented blood vessels (Figure3). In keloids, there is no flattening of the overlying epidermis, no scarring of the papillary dermis, the presence of a significant amount of keloidal collagen, an absence of prominent vertically oriented blood vessels and the presence of a significant disarray of fibrocollagenous fascicles (Figure4) (15).
The differential and exclusive diagnosis of diseases that are similar to keloids and HTSs is important because various types of malignant tumors resemble these scars (16)–(19). For example, malignant dermatofibrosarcoma protuberans (DFSP) tumors have been mistaken for keloids or HTSs (16,17).
Nodular scleroderma and keloidal scleroderma are rare benign tumors with lesions that clinically resemble keloids (20). The skin lesions are characteristically nodules or plaques, hard in consistency, and nontender, with a predilection for the superior portions of the thorax and sparing of the face and hands (21).
Gonzalez-Vela et al. (22) described keloids and HTSs as differential diagnoses of sclerotic neurofibroma. Sclerotic neurofibroma is differentiated from a keloid by an absence of previous surgical excision and by the positivity of the sclerotic neurofibroma cells for the protein S100 (22).
Other rare, benign keloid and HTS-like diseases include keloidal granuloma (23), erythema elevatum diutinum (24), infantile digital fibromatosis (25), dermatofibroma,(26) penile edema (27), pseudoangiomatous hyperplasia (28) and lichen sclerosus (29).
Hair folliculitis occasionally leads to hypertrophic scarring (30) but more often leads to progressive folliculitis caused by bacterial or fungal infection. The nape is an area of predilection for hair folliculitis. Treatment of any infection should be primary and steroid injection is contraindicated for infection. Moreover, a fungus may cause a skin nodule that mimics a keloid (31). Thus, examination for fungal infection should be conducted in cases of nape hair folliculitis, even if the nodule appears to be a keloid or HTS.
In conclusion, the following issues are considered important in the examination of a keloid or HTS: a biopsy should be conducted in anomalous cases because malignant disease may be the original or secondary problem, steroid injection should be performed only after careful consideration because malignancy or infections may be present, making a careful differential diagnosis is particularly challenging in African-Americans because the skin and the tumor colors are often similar and the presence of bacterial or fungal infection should be investigated (32).
DemographicsThe majority of individuals who develop HTSs and keloids are young, with ages ranging from 10 to 30 years old. The elderly rarely develop these lesions (33). This observation is partly attributed to the following facts: young individuals are more prone to trauma; their skin generally possesses more elastic fibers, resulting in greater tension; and the rate of collagen synthesis is greater in younger individuals (34). Keloids are more common in patients with darker skin, with an incidence of 4.5% to 16% in the black and Hispanic populations (34).
HTSs are a common complication of burn injury. In the developed world, approximately four million patients acquire scars due to burns each year and the incidence is even greater in developing countries (4). Previous studies have reported diverging incidences of hypertrophic scarring, with incidence rates varying from 40% to 94% following surgery and from 30% to 91% following burns (35,36).
EtiologyWound healing is classically divided into four stages: hemostasis, inflammation, proliferation and tissue remodeling. In these four stages, there are complicated interactions within a complex network of profibrotic and antifibrotic molecules, such as growth factors, proteolytic enzymes and ECM proteins (37). Each molecule has its own function in the different phases of the wound healing process. As soon as an injury occurs, the process of hemostasis begins and the bleeding is controlled by the aggregation of platelets at the site of injury. The subsequent formation of a fibrin clot helps to stop the bleeding and provides a scaffold for the attachment and proliferation of cells. Growth factors and cytokines are mainly secreted by inflammatory cells and contribute to the initiation of the proliferative phase of wound healing. Later, angiogenesis and collagen synthesis, followed by tissue remodeling complete the stages of the wound healing process.
The delicate balance of the deposition and degradation of ECM proteins is disrupted when either excessive production of collagen, proteoglycans and fibronectin by fibroblasts or deficient degradation and remodeling of the ECM occur (38). HTSs occur when the inflammatory response to injury is prolonged, leading to the pathological characteristics of HTSs, including increased vascularization, hypercellularity, excessive collagen deposition and a decrease in small leucine-rich proteoglycans (SLRPs) (39,40).
HTS tissue contains enhanced amounts of fibroblasts that exhibit an altered phenotype and higher expression of transforming growth factor beta-1 (TGF-β1) than normal fibroblasts do (41). An increase in or prolonged activity of TGF-β1 leads to overproduction and excess deposition of collagen by fibroblasts, often resulting in HTS formation (42). Fibroblasts in HTSs may differentiate into myofibroblasts and account for increased ECM synthesis and contraction of tissue. These cells have a particular phenotype that differs from that of fibroblasts based on their expression of α-SMA (43). Myofibroblasts in HTSs are less sensitive to apoptotic signals, coupled with an ability to produce more collagen and play an important role in HTS formation (43).
Clinical manifestationsThe clinical manifestations of HTSs are variable and correlate with a variety of causes that initiate HTS formation. HTSs can develop anywhere on the body. In contrast, keloids preferentially develop on the earlobes, shoulders and presternal skin; are void of hair follicles and other glands; and usually project above the level of the surrounding skin (13). Curiously, pathological scars are rare on the scalp. Farina Jr et al. recently described a casuistic study of 413 surgical procedures involving collection of thin skin grafts from the scalp, without development of HTSs or keloids among 295 cases over a period of ten years (44).
Hypertrophic scarring following surgical procedures, trauma and especially burns is a significant concern for patients and a challenging problem for clinicians because it can be painful, pruritic, erythematous, raised and cosmetically unacceptable. A previous study reported that the most common and distressing complications in burn patients who developed HTSs were abnormal appearance (75.2%), pruritus (73.3%) and pain (67.6%) (45). The cause of pruritus in HTSs and keloid scars is not yet well characterized, but recent studies have indicated the probable involvement of direct activation of opioid receptors identified in the skin (46).
Prevention and non-surgical treatmentThere is evidence suggesting that increased mechanical tension can initiate HTS formation (47). Based on this hypothesis, it makes sense to minimize mechanical forces after surgery. Surgical excision scars should be positioned along, rather than across, relaxed skin tension lines whenever possible. An appropriate strength, depth and number of sutures should ensure that the risk of dehiscence is minimized.
Inflammation is also known to contribute to hypertrophic scarring, (49) and every attempt to minimize the inflammatory response should be made by ascertaining clean surgery and good wound care to prevent infection thereafter. Using inert suture materials is also important in this context (48).
In patient candidates for skin grafts, the donor site must be well chosen by the surgeon in consultation with the patient to try to hide or avoid HTSs or keloids. In burn patients, the corresponding author believes that using the scalp as a source of thin skin grafts can reduce the level of visible aesthetic deformities at donor sites in patients who have already suffered the immense trauma that being a burn victim entails.
Conceptually and practically, treatment and prevention regimens can be similar and the following section presents the clinical data for both. Early diagnosis can considerably affect the outcome. There is evidence that the most successful non-surgical treatment of an HTS or keloid is achieved when the scar is immature and the overlying epithelium is intact, although further studies are necessary to confirm this concept (50).
SiliconeSilicone gel sheeting (SGS) has been widely used in clinical practice for the treatment of HTSs since the early 1980s. There is good evidence of the efficacy of the SGS, which has become standard practice among plastic surgeons (51).
Although gel sheeting is effective for HTS treatment, patient compliance may not be satisfactory for the following reasons: skin reaction to the tape used for fixation; excessive sweating; difficulty in its application; and the visibility of the treatment in the case of scars located in visible areas, such as the face (52).
In contrast, silicone gel does not require fixation and is nearly invisible when dry, suggesting that it could be especially useful in visible areas (52). However, there are certain problems in its application. For example, silicone gel requires multiple applications in a day and one must wait long enough for drying because the dressing may be smudged. Friction by clothes may also contribute to early removal of the silicone film (51). Because of these problems, silicone gel use may not always be practical.
Karagoz et al. found no statistically significant difference between silicone gel and silicone gel sheet groups when their scores on the Vancouver Scar Scale after treatment were compared. This finding suggests that silicone gel is most likely as effective as SGS for the treatment of HTSs (52).
The mechanism of action of topical silicone materials in the treatment of HTSs is not well understood and various mechanisms of action have been proposed. It has been suggested that the materials' therapeutic effect is not due to pressure, but rather to decreased scarring via wound hydration. There is evidence that SGS affects the hydration status of the scar by decreasing the water vapor transmission rate to nearly half that of normal skin, causing a buildup of moisture on the skin surface under the silicone sheet (53). This evidence suggests that the stratum corneum acts as a water reservoir, with fluid accumulating below the gel, although when visualized directly, this phenomenon is not evident (54). Hydration and occlusion therefore seem to be the principal modes of SGS action and increased skin hydration is most likely responsible for decreased capillary activity, reduced hyperemia and reduced collagen deposition (55). Furthermore, altered hydration is thought to cause electrostatic changes that influence collagen deposition and remodeling within the scar (56). The static electricity generated by friction has also been proposed as a plausible reason for silicone's anti-scarring effects (57).
The lower water vapor transmission rate and the accumulation of water below the material can cause skin maceration (58). Other common side effects associated with gel sheeting include persistent pruritus, skin breakdown, skin rash, foul smell from the gel, poor durability of the sheet, failure of the sheet to improve the hydration of dry scars, a poor response of the scar to treatment and poor patient compliance (59,60). Similar to pressure therapy, detailed multimedia-based patient education improves compliance with SGS, resulting in a better scar outcome (60). Obviously, the key to the success of this therapy is to ensure that hygienic precautions are taken, particularly when it is used in combination with pressure in children or in warm weather or climates (60). It must be noted, however, that complications increase with the use of combined pressure and SGS therapy (60).
Applying silicone gel twice daily or wearing SGS 12 to 24 h per day for 6 to 12 months, with temporary interruption when adverse effects appear, is recommended (61).
Pressure garmentsUsing mechanical compressive force exerted by pressure garments to treat HTSs in burn patients was first described in 1860 (62). It was only in the 1960s that this treatment became standard in several burn centers to accelerate the remodeling phase of wound healing (62). Prophylactic pressure is recommended in burn patients if spontaneous closure of the wound takes longer than 10 to 14 days and in those requiring grafting (2).
Currently, elastic compression using elastic garments is the predominant means of both prophylaxis and treatment for HTSs (63), despite controversial evidence-based data about their value in reducing the prevalence or magnitude of scarring (63). In fact, studies investigating pressure garments have found no significant difference between treatments involving the use of high-pressure garments, lower-pressure garments, or no pressure at all (64). Others, however, claim that pressure therapy achieves HTS regression success rates of 60% to 85% (63), without any conclusive evidence.
To date, the working mechanism of pressure and the way that pressure positively influences the development and maturation of HTSs are not fully understood and explanations remain hypothetical. However, many studies have been performed to try to explain the possible mechanisms of action, exploring theories based on hypoxia, biochemical changes, and cellular and collagenous influences. Certain valuable evidence suggests that pressure controls collagen synthesis by limiting the supply of blood, oxygen, and nutrients to the scar tissue (65); reduces collagen production to the levels found in normal scar tissue more rapidly than the natural maturation process does; encourages the realignment of collagen bundles that are already present (66); partly restores the ECM organization observed in normal scarring; and induces the disappearance of fibrogenic α-SMA-expressing myofibroblasts and vascular cells, most likely by apoptosis (67).
Additionally, certain studies have demonstrated that mechanical compression directly modulates the remodeling phase of wound healing, altering the release and activity of matrix metalloproteinase (MMP)-28 in HTSs and inducing a significant reduction in the protein's presence in keratinocytes in HTSs (68). Moreover, it has been suggested that pressure acts by accelerating the remission phase of the postburn reparative process (66).
Currently, the recommendations for the clinical use of pressure garments are restricted to deep dermal wounds that have healed spontaneously over weeks, grafted wounds surrounded by a deep dermal wound that was permitted to heal spontaneously over weeks, wounds in children and young adults, wounds in individuals with dark skin and wounds in body locations where compression can be applied (69). The amount of effective pressure generated by a given pressure garment is also still unknown and remains controversial (70). Problems with pressure loss from the garments over time and problems with the compliance of the patients using the garments are yet other factors complicating the issue (69,70).
Onion extract and heparin gelOnion extract possesses fibroblast-inhibiting properties that reduce both fibroproliferative activity and the production of ECM, increasing the expression of MMP-1 (71). Heparin strongly interacts with collagen molecules, inducing the formation of the thicker fibrils typical of a mature tissue and also promoting intermolecular bonding in collagen (72). Therefore, heparin and onion extract affect scar development via their inhibitory effects on inflammatory processes, fibroblast proliferation and the synthesizing capacity of fibroblasts (72). Onion extract and heparin exert similar antiproliferative effects that depress fibroblast proliferation and reduce scar size in the case of excessive scar formation in HTSs and keloid scars (73).
The topical gel preparation includes 10% aqueous onion extract, 50 U heparin per gram of gel, and 1% allantoin gel, and this formulation has been used for many years to treat wounds. Despite the gel's popularity, data demonstrating its efficacy are lacking. Certain clinical studies of the efficacy and tolerability of this topical preparation have been conducted. Ho et al. found onion extract, heparin and allantoin gel to be effective, safe and simple to apply for the prevention of scarring in 120 Chinese patients undergoing laser removal of tattoos. The researchers found that the topical gel preparation reduced the risk of scarring significantly, from 23.5% to 11.5% (72). Willital and Heine studied the effect of the same topical gel preparation on fresh scars after thoracic surgery in children and adolescents. The authors randomly assigned 45 young patients with fresh scars after thoracic surgery to the treatment and found that the scars in the treated group were narrower than those in the untreated group after 1 year of the treatment (74). In this study, the benefit of the gel for the treatment of physiologic scars as well as for the treatment of HTSs and keloid scars were described. In a more recent study, the early use of onion extract gel in surgical scars resulted in the improvement of scar height and symptoms, but there was no statistically significant difference in the scars' redness, pliability or overall cosmetic appearance (75). Nevertheless, we have observed that many patients who use a topical preparation containing onion extract, heparin and allantoin gel or another onion extract gel do not notice any significant improvement in their HTSs.
Intralesional corticosteroid injectionsIntralesional corticosteroid injections, used for the treatment of pathological scars since the mid-1960s, continue to play a major role in the regression of HTSs and keloids (76). Steroid injections have been shown to cause HTS and keloid regression in vivo, mainly by decreasing collagen and glycosaminoglycan synthesis, by reducing the inflammatory process in the wound, by decreasing fibroblast proliferation and by increasing hypoxia (77). Insoluble triamcinolone acetonide (TAC; 10 to 40 mg/ml), the most common corticosteroid used for the treatment of scars, may be administered alone or in combination with lidocaine to reduce the pain associated with the injection and several treatments administered once or twice per month are usually required to achieve the desired results (78).
Despite few randomized, prospective studies, there is broad consensus that injected TAC is efficacious and it is the first-line therapy for the treatment of keloids and the second-line therapy for the treatment of HTSs if other, easier treatments have not been efficacious. Response rates vary from 50% to 100%, with a recurrence rate of 9% to 50% (73).
Manuskiatti and Fitzpatrick found clinical improvement of HTSs and keloid scars after treatment with an intralesional injection of TAC combined with Contractubex® gel, which appears to be superior to intralesional TAC administered alone in the treatment of keloids and HTSs, with no significant side effects (78).
Although the use of corticosteroids to suppress abnormal scar formation has been relatively effective for most patients, it has also been a troublesome therapy. Intralesional corticosteroid injection is associated with significant injection pain, even using standard doses of triamcinolone (40 mg/ml), with up to 63% of patients experiencing certain side effects, including hypopigmentation, skin and subcutaneous fat atrophy, telangiectasias, rebound effects and ineffectiveness (79). After intralesional injection, linear hypopigmentation also may develop secondary to lymphogenous uptake of the corticosteroid crystals (80).
BleomycinBleomycin sulfate was introduced by Bodokh and Brun in 1996 as an alternative therapy for keloids and HTSs, based on its action as an inhibitor of the synthesis of deoxyribonucleic acid (DNA) (81). Bleomycin is a secondary metabolite of a strain of Streptomyces obtained from soil and has antitumor, antiviral and antibacterial activity. This compound acts by binding to DNA, whether double stranded and single stranded, causing strand scissions (82). The use of intralesional bleomycin has been documented for the treatment of keloids and HTSs, with promising results (83). Certain studies have investigated the effects of intradermal bleomycin administration into the skin of healthy individuals (84). From a histologic point of view, bleomycin has been found to cause necrosis of keratinocytes and this treatment can also induce inflammatory infiltration, along with expression of various adhesion molecules (84). Furthermore, the presence of apoptotic cells has been noted in common warts treated with bleomycin (85). Despite these findings, the exact mechanism by which bleomycin induces keloid and HTS regression is not entirely clear.
Concerning the side effects of intralesional administration of bleomycin, hyperpigmentation and dermal atrophy have developed in the healthy skin surrounding the lesion in only a few cases (86). The systemic side effects of bleomycin with intradermal/intralesional administration alone are not of concern because the concentration and dosage are not sufficient to incite systemic problems such as hepatotoxicity and pulmonary fibrosis (87).
Certain findings have revealed that bleomycin not only improves cosmetic appearance but also relieves patients' pruritus and pain, symptoms often associated with pathological scars. Although intralesional bleomycin is a promising treatment option for keloids and HTSs, further investigation and efficacy trials are needed before this agent can be included in future treatment protocols (88).
Emerging alternative treatmentsThe use of interferon alpha, beta and gamma increases collagen lysis. In particular, alpha and gamma inhibit the synthesis of collagen types I and III, acting on mRNA in the cell and reducing the levels of TGF-β. However, interferon application is very painful and it is a costly drug (88).
The drug 5-fluorouracil may be used alone or in combination with corticosteroid injections and achieves better results when combined with triamcinolone because monotherapy has limited use due to pain on application (50). The use of a carbon dioxide laser and an argon laser is ineffective due to recurrences, which are treated with steroids. Intense pulsed light therapy has shown satisfactory results, although further studies are needed, especially with later assessment of cases (50).
Drugs such as imiquimod, flurandrenolide, clobetasol, tacrolimus, methotrexate and pentoxifylline are several of the tested agents that have shown a clinical response, an increase in the local production of interferon in particular. However, the results must be considered with skepticism until further studies are conducted (89).
Cryotherapy with liquid nitrogen combined with corticosteroids showed a satisfactory response in the treatment of keloid scars, although its use in HTSs has not been assessed (90).
Botulinum toxin type A stimulates collagen formation and hyperbaric oxygen provides pure oxygen at a pressure slightly higher than atmospheric pressure, leading to decreased growth of atypical fibroblasts and restoration of tissue regeneration. In both cases, use of the treatment does not occur in isolation but rather as a complementary therapy. Still, further studies are necessary (91,92).
Dermal radiofrequency can be another therapeutic option for the treatment of HTSs. This treatment's mechanism of action is based on a slight increase in the temperature of the skin, increasing the extensibility and reducing the density of collagen (by a lifting effect due to the radio frequency) (93).
SurgeryHTSs rapidly increase in size for 3 to 6 months. Then, after a static phase, they begin to regress. The scars mature during a period of at least 1 year and can show decreased contractures, along with flattening, softening and repigmentation without any physical manipulation. For this reason, surgery is usually not necessary. However, surgery is indicated for those cases of HTSs with scar contractures (and especially joint contractures) that could result in loss of function (50).
HTSs may impair movement when they cross joints or exert abnormal forces on surrounding tissues. The shoulder, elbow, and knee are the most common joints affected by contractures after burn injury (94). Certain burn scars can cause enough impairment of function to require surgical intervention. The techniques of releasing burn scar contractures vary significantly, depending on the individual characteristics of the scar in question. In general, local, linear or small planar scars that impair movement may be lengthened by Z or V-Y plasty procedures and skin grafts may be necessary after major contracture release (94). Additionally, the use of a dermal matrix and negative pressure therapy to generate higher-quality skin grafts for the treatment of sequelae and contractures in burns is more and more common (95).
Mental statusIt is important to investigate a patient's current mental status to exclude the possibility of any psychiatric disorder before initiating treatment planning. Patients presenting a history of severe sadness or other depressive symptoms should be diagnosed and followed by a psychologist and psychiatrist and should start treatment only after discharge by the expert. Psychological stress also seems to be related to the recurrence of fibroproliferative scarring (96).
Patients who are dissatisfied with the treatment of their HTSs often cite a pre-existing psychopathological condition in lawsuits filed against their doctors, claiming that they did not have the mental faculties to understand the outcomes and limitations of the proposed treatment. It is necessary to present reports by expert professionals to provide a legal protection measure when prompted in court.
The doctor-patient relationship must include transparency, empathy and trust to reduce the patient's anxiety (97).
CONCLUSIONSIn this review, the authors sought to emphasize the actual need for the correct diagnosis of HTSs, which differ from keloid scarring, the latter being much more difficult to treat. The development of more reliable and objective methods for the diagnosis and the measurement of the severity of HTSs is also essential for further research in the area of prevention and treatment. In the last few years, increased understanding of the molecular and biologic mechanisms involved in HTS formation has allowed the development of more therapeutic options for these lesions. HTSs remain difficult to manage and there is no universally accepted treatment regimen or evidence-based literature to guide their management.
Treatment begins by educating the patient about the etiology of the scarring process and must be individualized, depending on the distribution, size, thickness and consistency of the lesions and the associated inflammation. The physician should select the most appropriate agent according to the patient's needs and the guidelines for these signs. Nonsurgical treatment seems to be the best option most of the time. Currently, silicone gel or a silicone sheet remains the most accepted modality for the treatment and prevention of HTSs, but in many cases, there are specific indications for different types of approaches, such as the use of pressure garments; combinations of corticosteroid injections and onion extract gel; bleomycin and even surgery in cases of contractures associated with the scar, mainly in burns.
Disputes concerning this topic are very far from over. Fibroproliferative disorders represent one of the greatest puzzles in medicine and although there is no consensus, combination therapy has proven to be more effective than monotherapy. It is essential that the physician be aware of the different therapeutic options available and be able to individualize treatment because certain patients may not respond to any single treatment modality. Finally, guidance for patients whose hypertrophic scarring usually regresses completely after six months to three years would appear to be fundamental.
ACKNOWLEDGMENTSThanks to the Department of Pathology and Forensic Medicine of FMRP-USP for providing the histopathological photographs.
AUTHOR CONTRIBUTIONSRabello FB was the text editor. Farina Jr JA was the advisor. Rabello FB, Souza CD and Farina Jr JA provided intellectual contributions to the manuscript preparation and writing.
No potential conflict of interest was reported.