To determine the role of RNA-binding protein with serine-rich domain 1 (RNPS1) in uterine corpus endometrial carcinoma (UCEC), the role of RNPS1 knockdown in UCEC development in vitro and in vivo, and the relationship between RNPS1 and mismatch repair (MMR) in UCEC.
METHODS:We predicted the potential function of RNPS1 using bioinformatics systems. The expression of RNPS1 in tissues and cell lines was analyzed by western blotting and immunohistochemistry. The expression of RNPS1 in MMR was assessed using bioinformatics and western blotting. The proliferation and apoptosis of UCEC cells were assessed under RNPS1 knockdown conditions, and RNPS1 regulation in MMR was detected by suppressing Notch signaling. Associations between RNPS1 and gene mutations in UCEC and prognosis were analyzed.
RESULTS:The RNPS1 level was higher in UCEC tumors than in normal tissues and tumors or RL952 cells. Prognostic outcomes were worse when UCEC showed abundant RNPS1 expression. Lentiviral RNPS1 knockdown weakened tumor cell proliferation and suppressed biomarker expression, reduced the tumor volume, promoted apoptosis in vitro and in vivo, and inhibited UCEC development. Increased MutS homolog 2 (MSH2) and MutS homolog 6 (MSH6) levels in MMR after RNPS1 knockdown were reversed by inhibiting Notch signaling. Furthermore, RNPS1 was associated with mutations in NAA11, C2orf57, NUPR1, and other genes involved in UCEC prognosis.
CONCLUSION:RNPS1 may regulate the expression levels of MSH2 and MSH6 in MMR, enhancing the proliferation, development, and prognosis of UCEC through a Notch signaling pathway in UCEC. Our study offers a new method and strategy for delaying UCEC development through modulating MMR.
Uterine corpus endometrial carcinoma (UCEC) is a common gynecological carcinoma with a high recurrence rate (1). Endometrioid adenocarcinoma (EC) is the most common pathological manifestation of UCEC, with proliferative endometrial tumor cells showing glandular complexity and cellular pleomorphism (2). The risk of recurrence is intermediate or high in patients with EC after radical hysterectomy combined with pelvic and para-aortic lymphadenectomy, a considerable challenge for clinics (3). Although evolving medical technologies have led to long-term declines in mortality due to EC, the mechanisms and treatment of EC require further exploration.
DNA mismatch repair (MMR) protein deficiency is a result of microsatellite instability (MSI), a hallmark of EC (4,5), providing the biological relevance and potential utility of the modal classification of EC and increasing the complexity of the genomic instability underlying tumorigenesis (6). As MMR regulation may be a prognostic factor for patients with advanced UCEC (7,8), an MMR regulator for treating UCEC needs to be identified.
RNA-binding protein with serine-rich domain 1 (RNPS1) belongs to the mRNA nuclear export and mRNA surveillance post-splicing multiprotein complex (9) involved in lung squamous cell and ovarian adenocarcinoma (10,11). However, the mechanisms underlying the role of RNPS1 in MMR in UCEC remain unclear.
The Notch signaling pathway is implicated in many cancers, including in the maintenance of cancer stem cells, metabolism, survival, drug resistance, epithelial-mesenchymal transition, and genomic instability (12). The Notch signaling pathway may enhance the invasive properties of EC, indicating that it may serve as a promising target for the treatment of this malignancy (13).
The present study aimed to determine the role of RNPS1 in UCEC. We used an RNPS1 knockdown lentivirus (sh-RNPS1) to regulate MMR progression through the Notch signaling pathway and tumor progression in UCEC. The present findings indicate that RNPS1 regulates MMR in UCEC. These results may provide novel strategies for UCEC therapy via MMR-associated mechanisms.
MATERIALS AND METHODSUCEC specimens from patientsSixteen patients pathologically diagnosed with moderately differentiated (G2), type I, MMR-deficient EC were selected at the Department of Pathology of the First Affiliated Hospital of Hebei North University from May 2019 to May 2021, with adjacent para-carcinoma tissues serving as controls. All patients provided written informed consent to participate and the use of their samples was approved by the ethics committee of this study.
Bioinformatics analysisThe expression of RNPS1 in various types of cancers was analyzed using TIMER2.0 (http://timer.cistrome.org/). Gene set enrichment analysis (GSEA) data were analyzed as previously described (14). The survival of patients with UCEC showing low and high RNPS1 expression was assayed using the Kaplan-Meier plotter (http://kmplot.com/analysis/). Pan-cancer was analyzed as previously described (15). Genes related to mutation and prognosis were analyzed using target gene assays (https://www.mutarget.com/analysis?type=target).
Cell lines and cell cultureNormal endometrial (NECs; CP-H058), KLE (CL-0133), RL952 (CL-0197), and Ishikawa (CL-0283) cells (all from Procell, Wuhan, China) and ECC-1 cells (BS-C163325, BinSuiBio, Shanghai, China) were maintained and cultured as previously described (16).
AnimalsHealthy male Balb/c mice (Chongqing Tengxin Biotechnology Co., Ltd., Chongqing, China) were housed under specific pathogen-free conditions. The mice were managed by the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996), and the Ethics Committee of the First Affiliated Hospital of the Hebei North University approved the experimental design (protocol No: 2019ECHNU033).
AntibodiesThe following antibodies were purchased from the respective suppliers: anti-RNPS1 (ab79233), anti-Ki-67 (ab15580), anti-CEA (ab207718), anti-CA199 (ab3982), anti-CA153 (ab109185), anti-HE4 (ab200828), anti-Bcl-2 (ab182858), anti-Bax (ab182733), anti-MLH1 (ab92312), anti-cleaved caspase-3 (ab2302), anti-MSH2 (ab212188), anti-MSH6 (ab92471), and anti-PMS2 (ab110638) (all from Abcam, Cambridge, USA) and anti-β-actin (M01263-2; Boster, Wuhan, China). The secondary antibodies used were anti-rabbit IgG (AS014) and anti-mouse IgG (H+L) (AS003), both from ABclonal (Wuhan, China), and IMR-1A (HY-100431A; MedChemExpress, Dallas, TX, USA).
RNPS1-knockdown lentivirus administrationAn RNPS1-knockdown (sh-RNPS1) lentivirus and a control lentivirus (Table 1) were designed and chemically synthesized (GenePharma Corporation, Shanghai, China) and stored at -80°C. The cells were transduced with the lentiviruses, as previously described (17).
The mice were randomly assigned to the following groups (n=6 per group): control (con), untreated RL952 cells, or mice injected with RL952 cells; sh-RNPS1, RL952 cells transduced with sh-RNPS1 lentivirus or mice injected with RL952 cells infected with sh-RNPS1; lentivirus control (LC)-shRNPS1, lentivirus cells treated with RNPS1 control lentivirus, or mice injected with lentivirus cells incubated with RNPS1 control lentivirus; and IMR-1A+sh-RNPS1, RL952 cells incubated with sh-RNPS1 lentivirus, or mice injected with RL952 cells incubated with sh-RNPS1 lentivirus or IMR-1A (10 μg in vitro or 10 mg/kg in vivo).
UCEC mouse modelThe mice were subcutaneously injected with RL952 cells 5 days later, as previously described (18). The mice were sacrificed when they rapidly lost >20% of their weight and showed signs of deteriorating health, such as hunching, dehydration, and labored breathing due to the metastatic burden.
Western blottingProteins (50 μg) from each sample were resolved by 12% SDS-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes as previously described (19).
Immunohistochemistry (IHC)Tissue sections (4-μm-thick) were incubated with primary antibodies against RNPS1 and Ki-67 at 4°C overnight, followed by incubation with a secondary antibody, as previously described (20).
MTT assayAfter stirring the samples for 30 min, they were passed through a filter with pores (diameter, 0.22 µm), and then stored at 4°C, as previously described (21).
Statistical analysisData are expressed as the means±standard deviations and were statistically analyzed by one-way or two-way ANOVA using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). The Holm-Sidak test was used for multiple comparisons. Statistical significance was set at p<0.05.
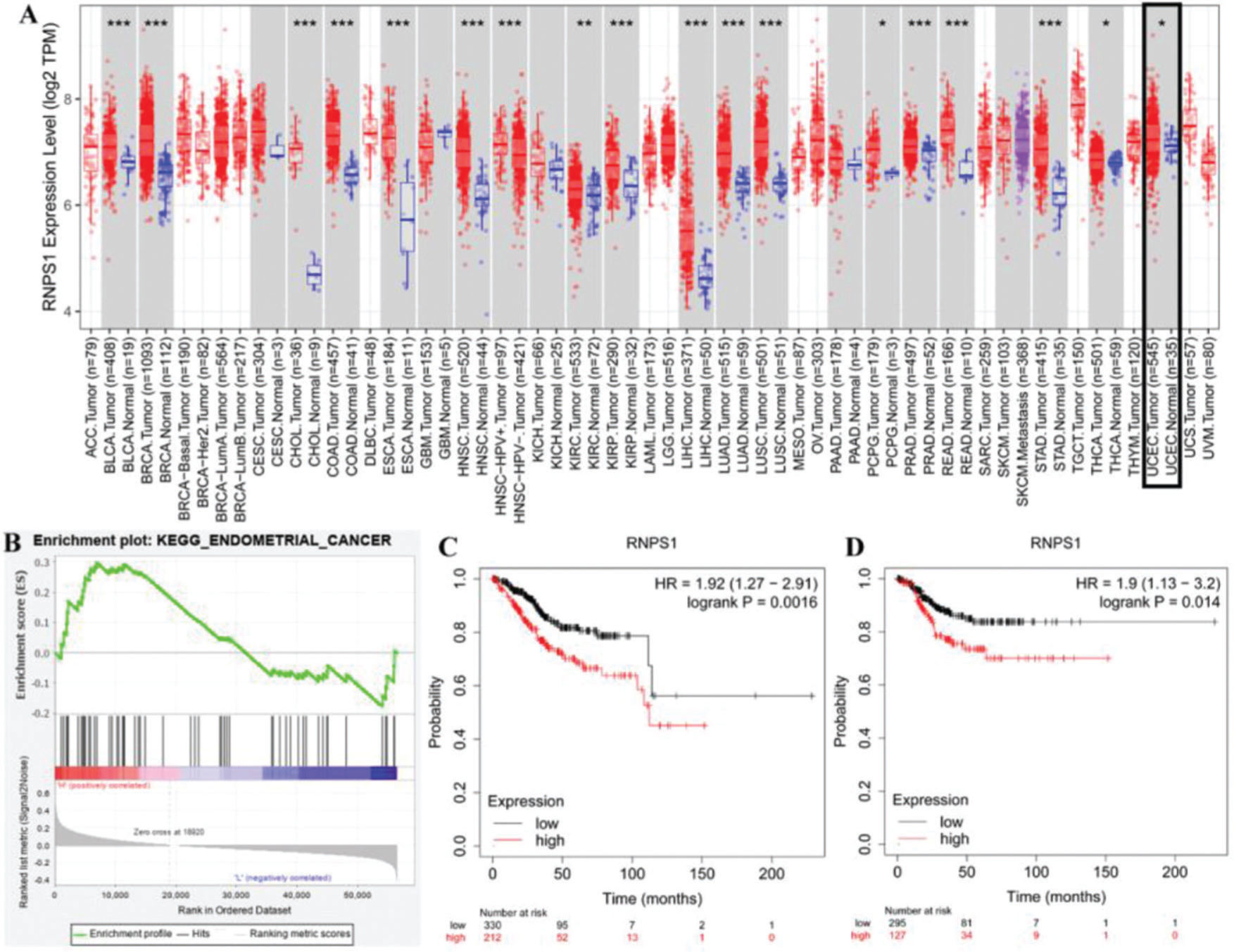
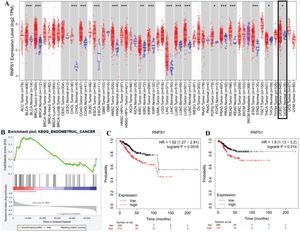
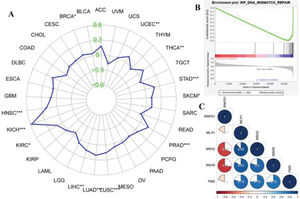
RESULTSIdentifying the potential function of RNPS1 in UCEC using bioinformatics analysisFigure 1 shows the predicted role of RNPS1 in UCEC detected by TIMER2.0, GSEA, and a Kaplan-Meier plotter. Figure 1A shows that the expression of RNPS1, as determined by TIMER2.0, was higher in UCEC tumors than in normal tissues (p<0.05). The GSEA findings (Figure 1B) showed that RNPS1 was positively correlated with UCEC progression (p<0.05). Analysis of the overall survival (OS) and recurrence-free survival (RFS) indicated that the prognostic outcomes of patients with UCEC and high RNPS1 expression (Figure 1C and D) were worse (p<0.05).
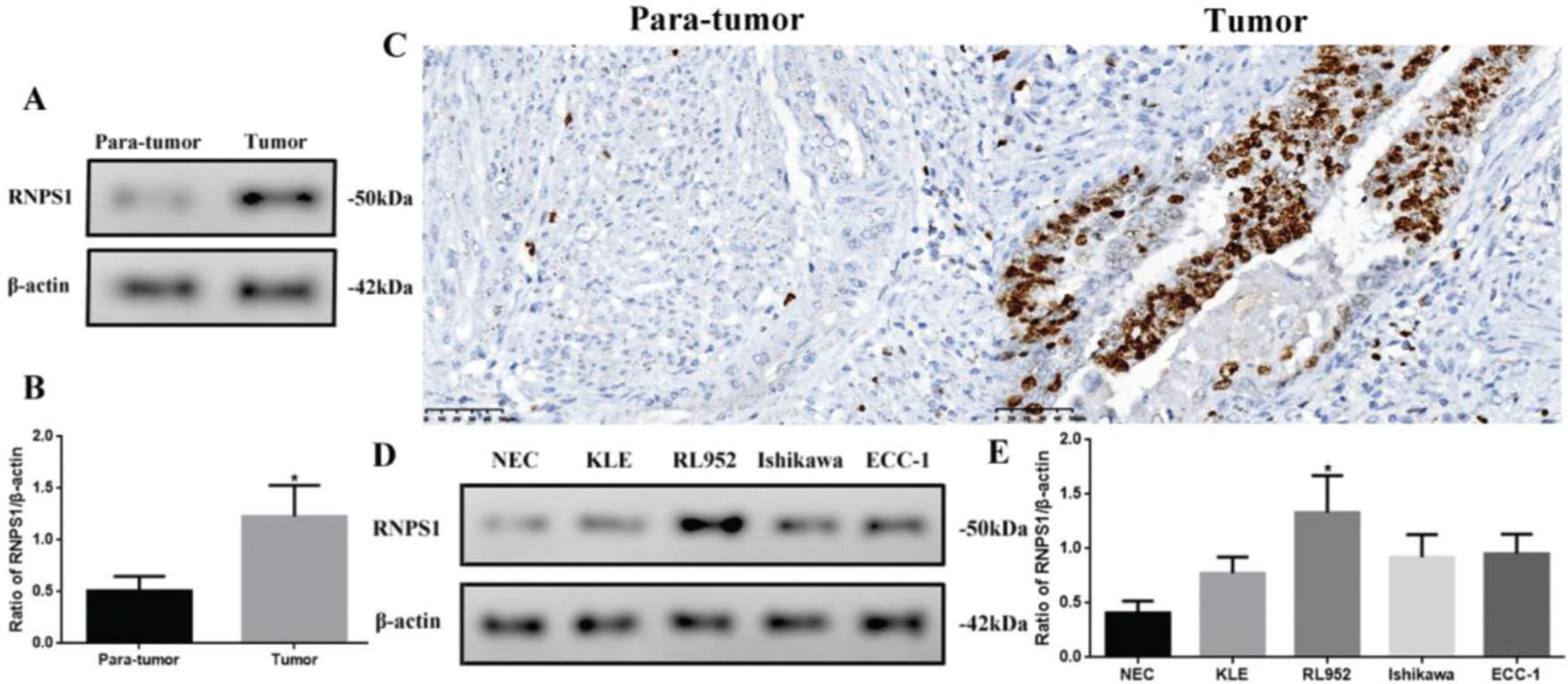
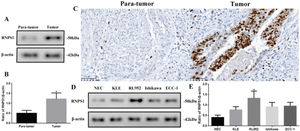
Different levels of RNPS1 in tissues or cell lines of UCECAs RNPS1 might be involved in UCEC, we analyzed the differences in RNPS1 expression among UCEC tissues or cell lines (Figure 2). The RNPS1 level was significantly higher in tumors than in para-tumor tissues (p<0.05, Figure 2A and B). Similar to the IHC results (Figure 2C), RNPS1 was localized in the nucleus. In addition, RNPS1 expression was significantly higher in RL952 cells than in other cells (p<0.05; Figure 2D and E). Therefore, we used RL952 as the UCEC model cell line associated with RNPS1 for further investigation.
(A) RNPS1 expression, (B) quantitation of the RNPS1 expression levels from the western blots, and (C) IHC staining analysis for RNPS1 in para-tumor and tumor tissues (×400). (D) RNPS1 expression in NEC, KLE, RL952, Ishikawa, and ECC-1 cells. (E) Quantitation of the RNPS1 expression levels in cells. Protein levels were normalized to those of β-actin. (n=6, *p<0.05: tumor vs. para-tumor or RL952 vs. other cells).
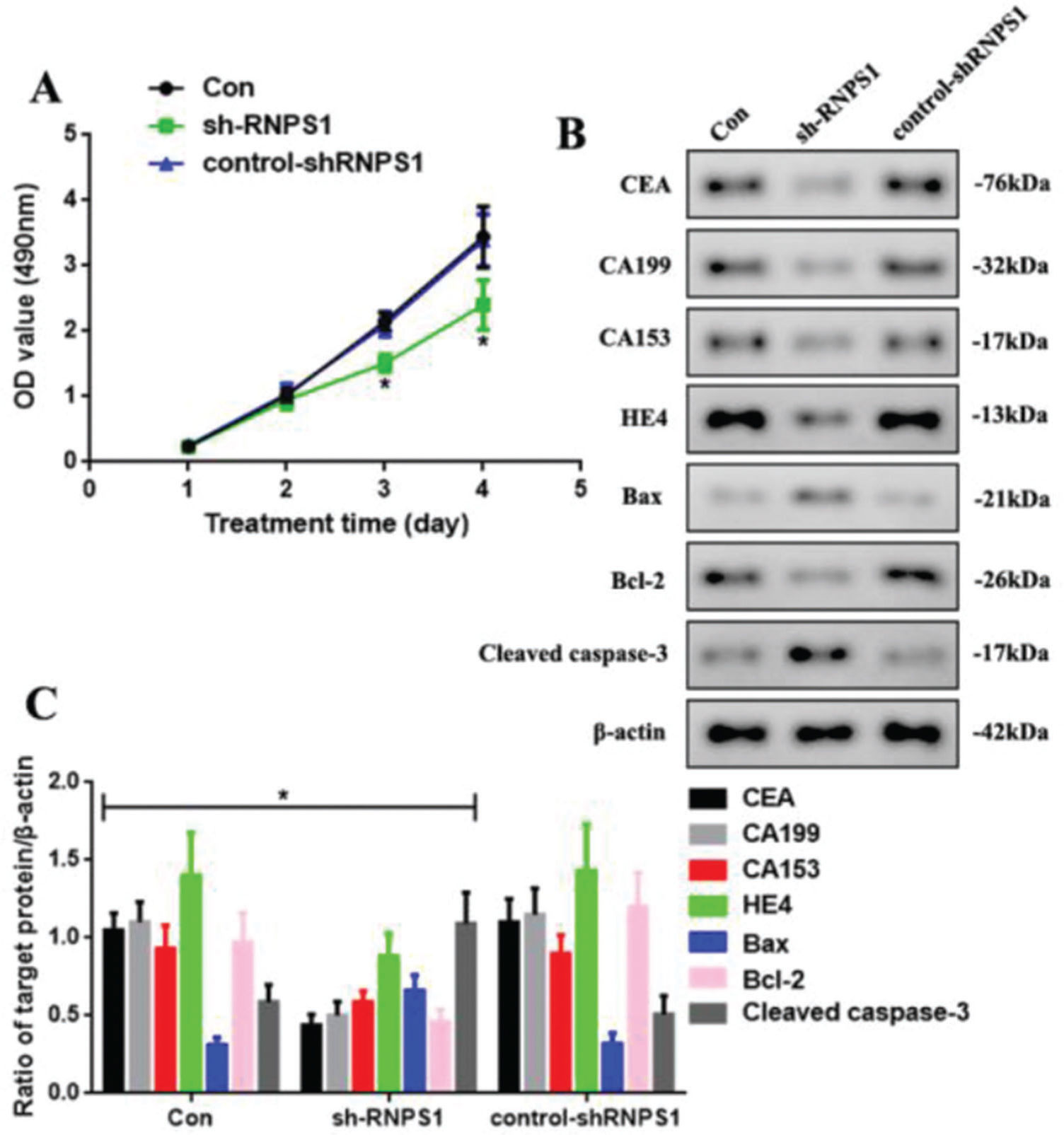
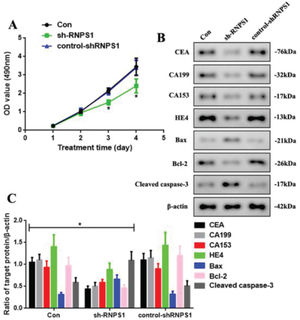
We knocked down RNPS1 using the lentivirus sh-RNPS1 to verify the role of RNPS1 in UCEC and its correlation with the previously obtained results (Figure 3). The control, sh-RNPS1, and control-shRNPS1 RL952 cells proliferated (Figure 3A). However, the optical density of the samples, as detected by MTT, decreased at 3 and 4 days in the sh-RNPS1 group (p<0.05). We measured the levels of apoptotic and tumor biomarkers of UCEC to determine their regulation after RNPS1 knockdown (Figure 3B). The levels of CEA, CA199, CA153, HE4, and Bcl-2 were reduced, whereas the activities of Bax and cleaved caspase-3 were induced in the sh-RNPS1 group (p<0.05, Figure 3C).
(A) Proliferation assays of cells from the Con, sh-RNPS1. and control-shRNPS1 groups at 4 days. (B) Western blots for the analysis of the levels of CEA, CA199, CA153, HE4, Bax, Bcl-2. and cleaved caspase-3. (C) Quantitation of the levels of CEA, CA199, CA153, HE4, Bcl-2, Bax, and cleaved caspase-3. (n=6, *p<0.05: sh-RNPS1 vs. other groups).
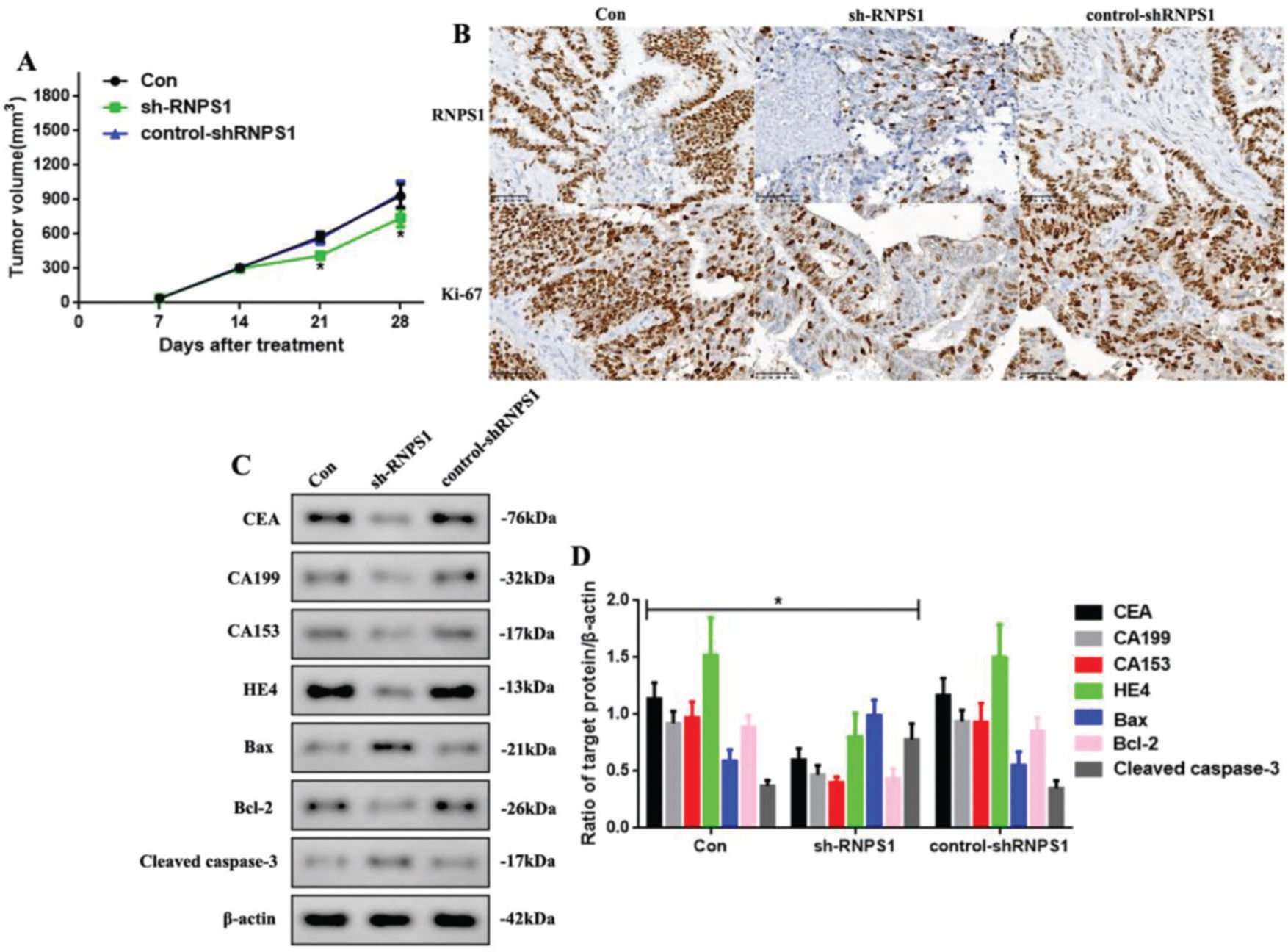
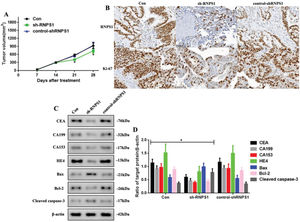
As RNPS1 knockdown regulated the progress of UCEC in vitro, we examined whether this also occurred in vivo (Figure 4). We found that the tumor volumes of the sh-RNPS1 mice were significantly decreased at 21 and 28 days (p<0.05, Figure 4A), and the level of RNPS1 and the proliferation index, Ki-67, were reduced (Figure 4B). We assessed the levels of apoptotic and tumor biomarkers in UCECs (Figure 4C). The levels of CEA, CA199, CA153, HE4, and Bcl-2 decreased, while those of Bax and cleaved caspase-3 increased (p<0.05, Figure 4F).
(A) Tumor volume in mice from the Con, sh-RNPS1, and control-shRNPS1 groups at 28 days. (B) IHC analysis for detecting the RNPS1 levels and Ki-67 index. (C) Western blots for the analysis of the levels of CEA, CA199, CA153, HE4, Bcl-2, Bax, and cleaved caspase-3, (D) Quantitation of the levels of CEA, CA199, CA153, HE4, Bax, Bcl-2, and cleaved caspase-3. (n=6, *p<0.05: sh-RNPS1 vs. other groups).
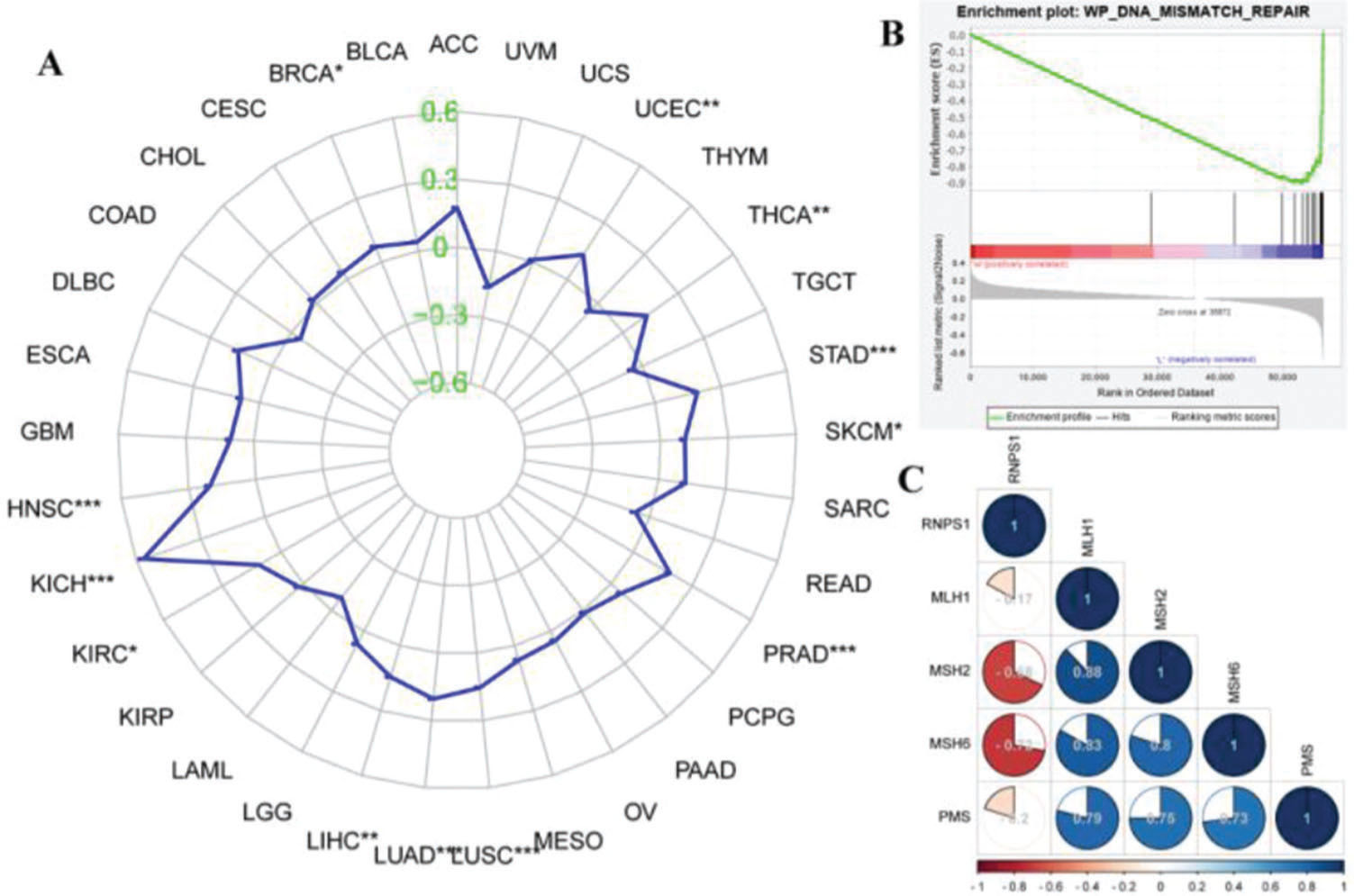
Figure 5A shows the functions of RNPS1 and MSI in different types of cancer. We found that RNPS1 was positively associated with MSI in the UCEC. The GSEA results showed that RNPS1 was negatively for MMR (Figure 5B). Correlation analysis verified that RNPS1 was negatively correlated with the MMR markers MSH2 and MSH6 but positively correlated with MSH1 and PMS2 in UCEC (Figure 5B).
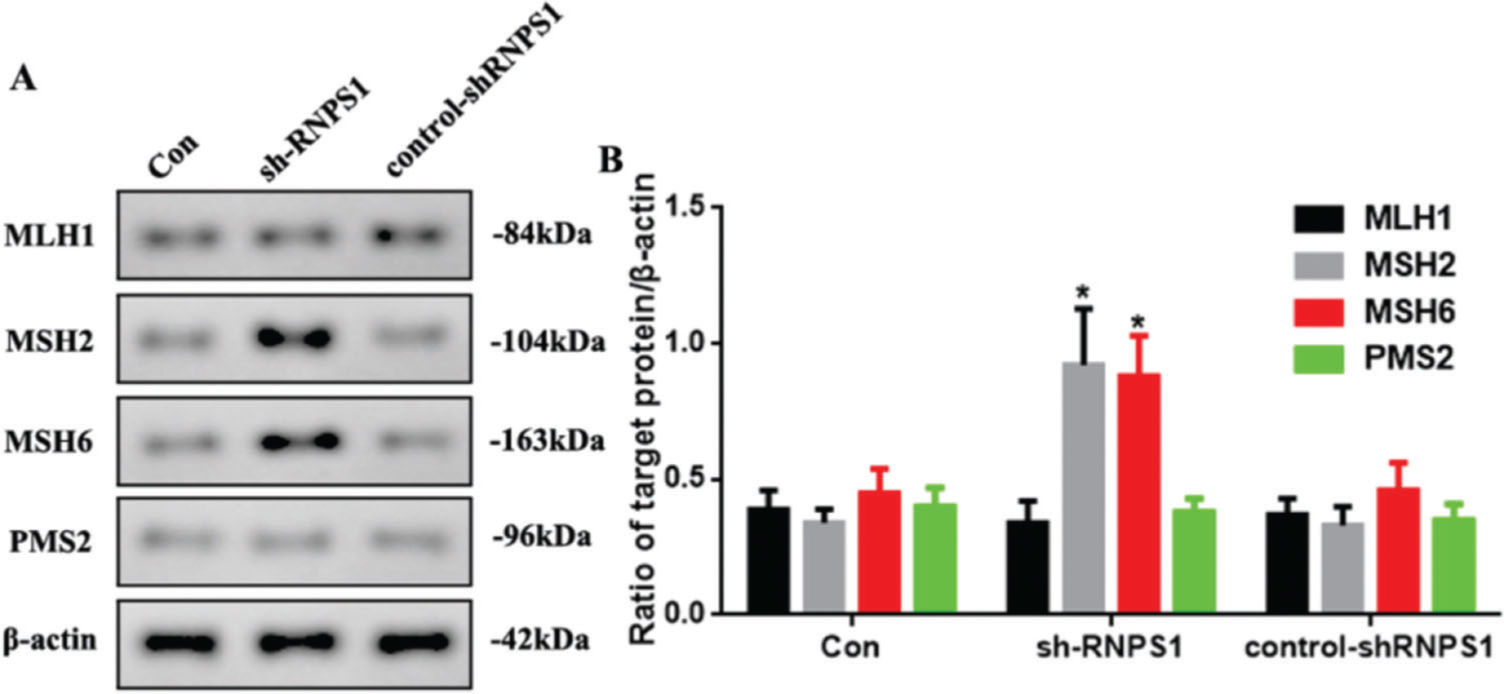
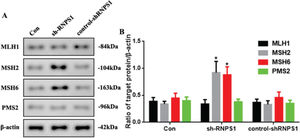
MMR marker levels were increased by RNPS1 knockdown in vivoAs previous predictions have indicated that the RNPS1 gene is associated with MSI and MMR in UCEC, we examined MMR marker levels in vivo. Figure 6A shows the increased expression of MSH2 and MSH6 in the sh-RNPS1 group (p<0.05) compared with the other groups (Figure 6B).
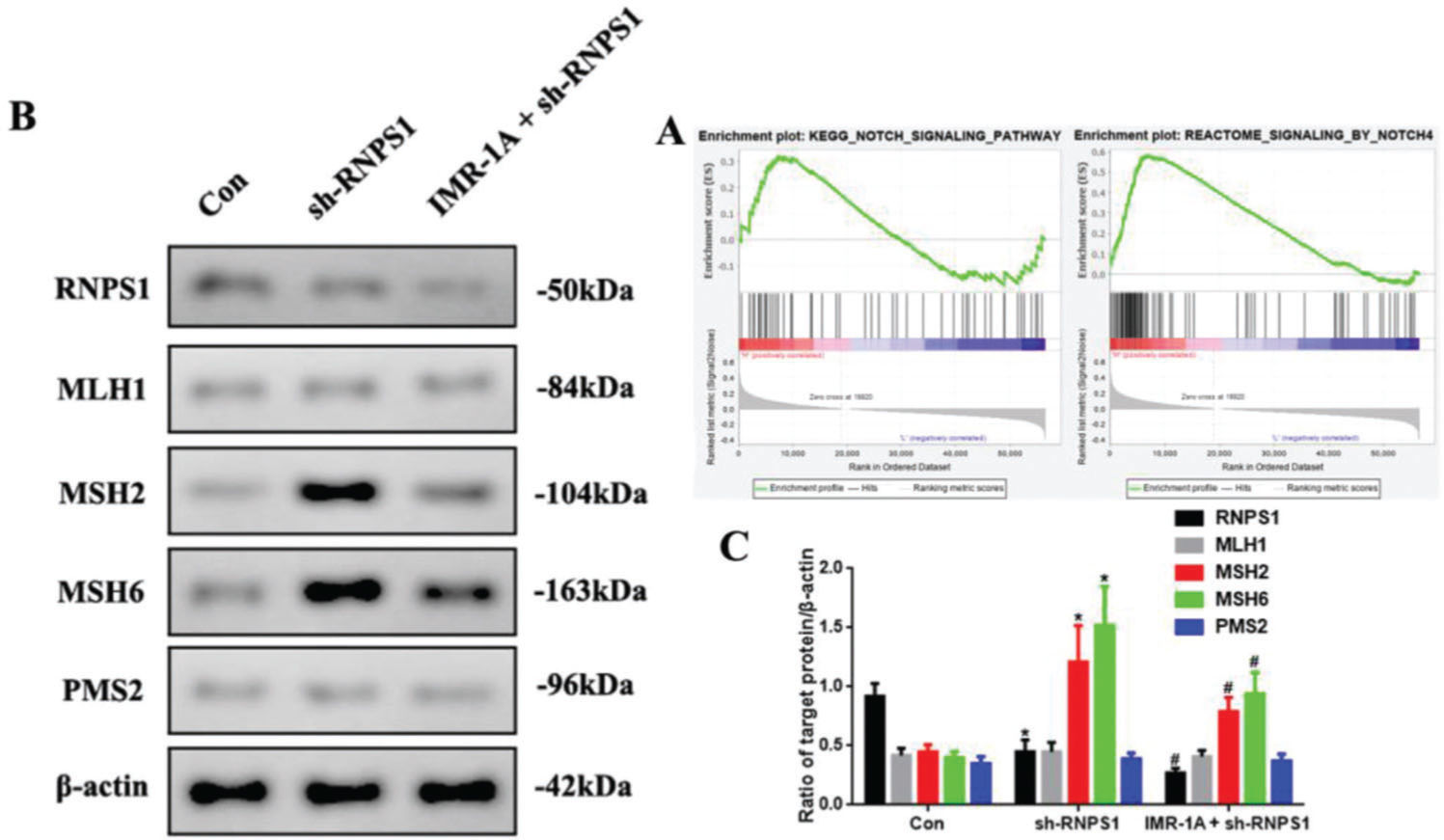
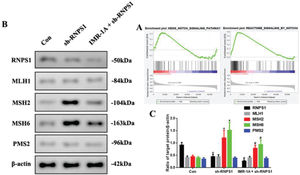
Mismatch repair was regulated by RNPS1 knockdown through Notch signaling pathway in vivoThe GSEA findings showed that RNPS1 was positively for Notch, especially, Notch4 signaling (Figure 7A). To confirm this, we blocked Notch expression using an inhibitor of Mastermind Recruitment-1A (IMR-1A). Figure 7B shows that IMR-1A decreased the levels of MSH2 and MSH6 after RNPS1 knockdown (p<0.05) compared with the case in the sh-RNPS1 group (Figure 7C); notably, IMR-1A reduced the RNPS1 levels.
(A) GSEA of the correlation between RNPS1 and Notch or Notch4 signaling pathway in UCEC. (B) Western blot assay for the analysis of the RNPS1, MSH1, MSH2, MSH6, and PMS2 expression levels in vivo. (B) Quantitation of RNPS1, MSH1, MSH2, MSH6, and PMS2 expression levels. (n=6, *p<0.05: sh-RNPS1 vs. Con; #p<0.05: IMR-1A+sh-RNPS1 vs. sh-RNPS1).
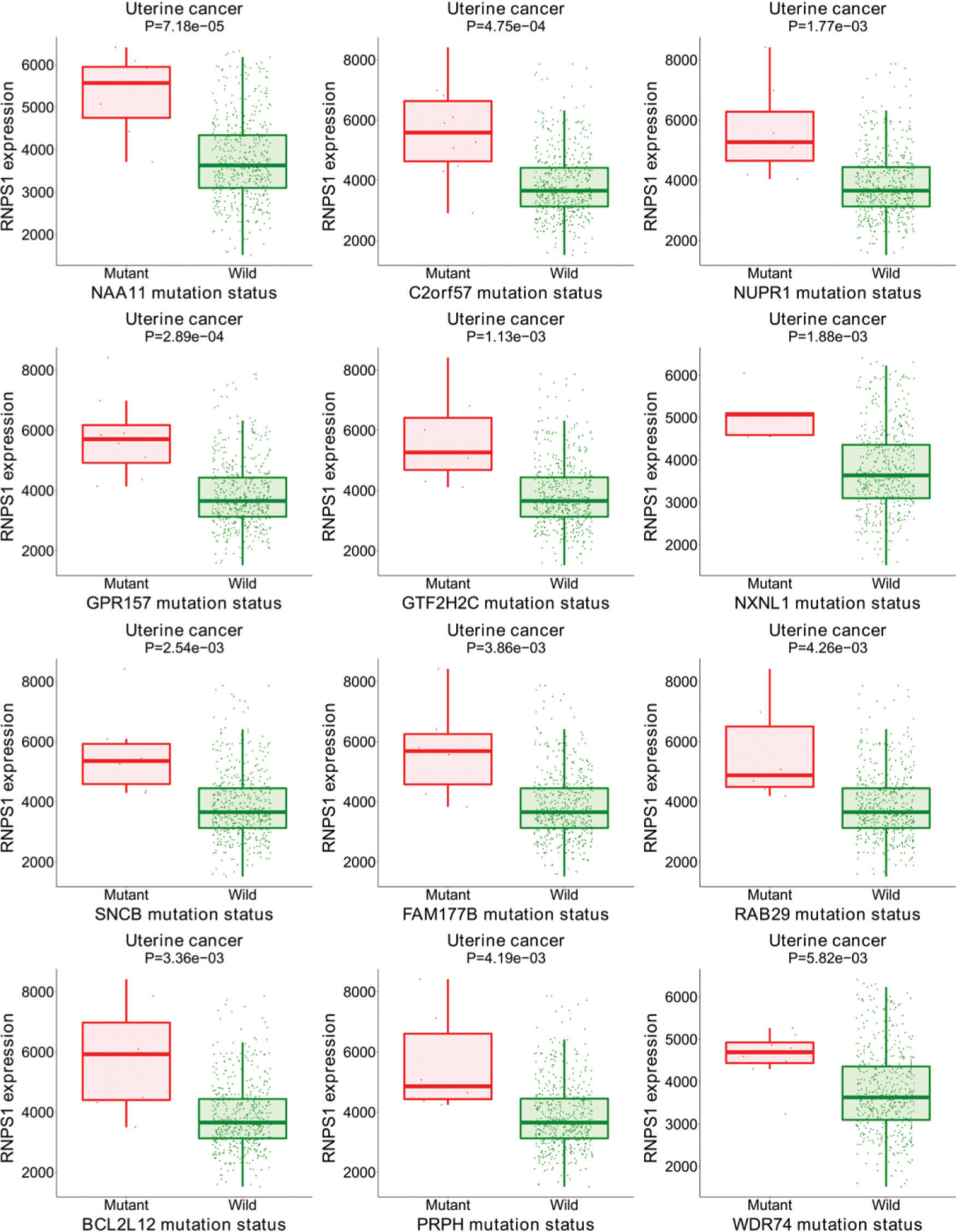
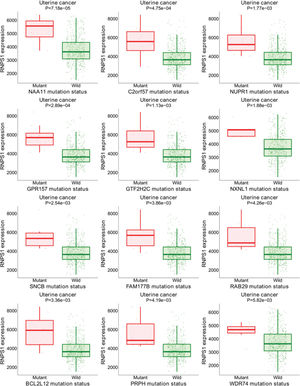
Figure S1 shows the results of the target gene assays of RNPS1 in UCEC tissues. Mutations were found in the group with higher levels of RNPS1, NAA11, C2orf57, NUPR1, GPR157, GTF2H2C, NXNL1, SNCB, FAM177B, RAB29, BCL2L12, PRPH, and WDR74. These results suggest that RNPS1 is associated with these gene mutations and participates in the prognosis of UCEC.
DISCUSSIONEndometrioid EC is the most common uterine malignancy, with a mortality rate of approximately 20%; it is the most prevalent gynecological malignancy in western and developed countries (22,23). The mortality rates of endometrioid EC are increasing, indicating the need for more effective diagnostic and treatment strategies (24). MMR deficiency due to the loss of MMR protein expression, a hotspot mutation in the POLE exonuclease domain, and a nonspecific molecular profile may be prognostic factors for patients with advanced UCEC (7,8,25).
A recent whole-genome analysis found that RNPS1 regulates carcinogenesis, especially, in EC (26). As little is known about RNPS1, it should be a hot topic in oncological research. In the present study, bioinformatics and molecular biological methods were applied to determine the functions of RNPS1 in UCEC. Higher levels of RNPS1 are expressed in UCEC tumors than in normal tissues; the prognostic outcomes of patients with UCEC were poor, and abundant expression of the RNPS1 isoform were observed. These results showed that RNPS1 may be an oncogene involved in the prognosis of UCEC. To verify this issue, we compared the levels of the RNPS1 protein in various tissues and cell lines. The results showed that RNPS1 expression was increased in EC tissue and RL952 cells, consistent with the findings of the bioinformatics analyses.
Apoptosis is a programmed cell death that does not elicit an inflammatory response (27). Knocking down RNPS1 weakened tumor cell proliferation and biomarkers, reduced tumor volume, increased apoptosis in vitro and in vivo, and inhibited UCEC development in our study. These results showed that RNPS1 could regulate apoptosis and tumor progression in UCEC and were consistent with the findings of the bioinformatics analyses.
We further explored the mechanism underlying the action of RNPS1 in EC using bioinformatics methods and found that RNPS1 correlated positively with MSI and negatively with MMR proteins, especially, MSH2 and MSH6. Western blotting confirmed the bioinformatic findings of a relationship between RNPS1 and MMR, as the RNPS1 knockdown lentivirus improved the expression of MSH2 and MSH6 but not MSH1 and PMS2. We plan to report this issue, but some methodological deficiencies resulting from limited laboratory conditions prevent more rigorous and advanced experimentation. Other scholars should investigate these findings further.
Clonal diversity was due to the accumulation of mutations in genes of diverse pathways, such as Notch and MMR pathways (28). Notch signaling can provide the first mechanistic example of altered glycosylation in tumor cells with MSI (29). Our GSEA findings showed that RNPS1 correlated positively with Notch signaling, especially, the Notch4 signaling pathway in UCEC. We investigated this aspect by blocking Notch signaling using IMR-1A. The results showed that IMR-1A decreased MSH2 and MSH6 levels after lentivirus-based RNPS1 knockdown. Furthermore, IMR-1A reduced the RNPS1 levels, suggesting that Notch is an upstream factor for RNPS1. However, as a specific Notch4 inhibitor is not yet available, we could only inhibit Notch signaling. The present results showed that RNPS1 may be a downstream target factor of Notch; therefore, the Notch4 signaling pathway may regulate MMR in UCEC.
Bioinformatic analyses of RNPS1 in patients with UCEC using the target gene system showed that RNPS1 may be associated with mutations in NAA11, C2orf57, NUPR1, GPR157, GTF2H2C, NXNL1, SNCB, FAM177B, RAB29, BCL2L12, PRPH, and WDR74, participating in UCEC prognosis. These data await confirmation in future investigations and will be the focus of our subsequent studies.
We found that RNPS1 knockdown significantly activated apoptosis and inhibited EC development and MSH2 and MSH6 expression via the Notch signaling pathway. Overall, our findings offer a new strategy for treating UCEC.
AUTHOR CONTRIBUTIONSLiu X designed the study and manuscript drafting. Ma H and Ma L analyzed the data. Li K wrote and revised the manuscript. Kang Y polished the first manuscript drafting and designed the methodology.
No potential conflict of interest was reported.