The protocols for glucocorticoid replacement in children with salt wasting 21-hydroxylase deficiency are well established; however, the current recommendation for mineralocorticoid replacement is general and suggests individualized dose adjustments. This study aims to retrospectively review the 9-∝-fludrocortisone dose regimen in salt wasting 21-hydroxylase deficient children who have been adequately treated during infancy.
METHODS:Twenty-three salt wasting 21-hydroxylase deficient patients with good anthropometric and hormonal control were followed in our center since diagnosis. The assessments of cortisone acetate and 9-∝-fludrocortisone doses, anthropometric parameters, and biochemical and hormonal levels were rigorously evaluated in pre-determined intervals from diagnosis to two years of age.
RESULTS:The 9-∝-fludrocortisone doses decreased over time during the first and second years of life; the median fludrocortisone doses were 200 μg at 0-6 months, 150 μg at 7-18 months and 125 μg at 19-24 months. The cortisone acetate dose per square meter was stable during follow-up (median = 16.8 mg/m2/day). The serum sodium, potassium and plasma rennin activity levels during treatment were normal, except in the first month of life, when periodic 9-∝-fludrocortisone dose adjustments were made.
CONCLUSIONS:The mineralocorticoid needs of salt wasting 21-hydroxylase deficient patients are greater during early infancy and progressively decrease during the first two years of life, which confirms that a partial aldosterone resistance exists during this time. Our study proposes a safety regiment for mineralocorticoid replacement during this critical developmental period.
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency (21OHD) is a common genetic disorder that accounts for >90% of congenital adrenal hyperplasia cases. Mutations in the CYP21A2 gene, which encodes 21-hydroxylase (an enzyme involved in aldosterone and cortisol biosynthesis), cause 21OHD. ACTH levels rise because of impaired cortisol secretions, thereby stimulating the adrenal cortex and accumulating androgen precursors, which manifest in varying degrees of hyperandrogenism. The severity of clinical symptoms and hormonal abnormalities correlates with the severity of enzymatic impairment manifested through three main phenotypes: the non-classical form, the classical simple virilizing form and the classical salt wasting (SW) form, which is the most severe (1,2).
In SW-21OHD, patients display CYP21A2 mutations that abolish 21-hydroxylase activity and completely disrupt the cortisol and aldosterone biosynthesis. Females present with virilized external genitalia at birth, and both sexes present with adrenal crisis in the first months of life. This adrenal crisis is characterized by hyponatremia, hyperkalemia, hyperreninemia and dehydration and leads to acute circulatory collapse if left untreated (2). SW-21OHD treatment consists of glucocorticoid and mineralocorticoid replacement in conjunction with sodium chloride supplementation during infancy (3).
The guidelines for glucocorticoid replacement in children are well established (3) and include short half-life glucocorticoids to prevent adrenal crises and virilization, along with minimizing the growth suppression associated with longer-acting glucocorticoids (4). The current recommendation for mineralocorticoid replacement in SW-21OHD is based on expert opinions that suggest an initial dose of 100-200 μg/day of 9-α-fludrocortisone, regular dose reassessment and individualized adjustments (3). The dose adjustments should be based on clinical and laboratory parameters, such as blood pressure, sodium, potassium and plasmatic renin activity (PRA) levels (5).
Mineralocorticoid replacement doses vary during infancy primarily because of improvements in mineralocorticoid sensitivity, which tends to increase, particularly during the first year of life (6). The higher mineralocorticoid demand during early infancy is explained by renal function immaturity, the limited sodium content in human breast milk and higher sodium requirements for child development during this period (7, 8).
Despite the importance of mineralocorticoid treatment during infancy in SW-21OHD patients, there is a lack of data in the literature concerning the optimal replacement dosage. The only study that has evaluated 9-α-fludrocortisone replacement doses was the European-wide survey, which included 125 centers that were actively involved in caring for 21OHD patients. The questionnaire results showed a wide range for 9-α-fludrocortisone doses and divided opinions concerning the need for sodium supplementation (9).
To improve mineralocorticoid management during this critical period, we conducted a retrospective analysis of 9-∝-fludrocortisone replacement doses and the clinical and laboratory follow-up parameters during the first and second years of life in our cohort of adequately treated SW-21OHD patients.
PATIENTS AND METHODSThe study protocol was approved by the Institutional Review Board, and informed consent was obtained from all of the patients or their parents. Our cases were selected from a large cohort of 141 SW-21OHD patients who were followed at the Endocrinology Division in our center. The inclusion criteria were regular follow-up in our institution from diagnosis or early age (maximum 90 days) to two years of age and adequate clinical and hormonal control. Our cohort comprised 23 SW-21OHD patients who were homogeneously treated and assisted by the same physicians from 1988 to 2006.
The females had ambiguous genitalia and dehydration with hyponatremia and/or hyperkalemia at diagnosis, while the males were diagnosed based on dehydration with hyponatremia and hyperkalemia or the previous knowledge of an older affected sibling. At the diagnosis, all of the patients had high basal levels of 17-hydroxyprogesterone (17OHP) (>50 ng/mL), and the PRA (when available) was usually >20 ng/mL/h. The SW-21OHD diagnosis was confirmed by identification of homozygous or compound heterozygous CYP21A2 mutations, which predicted total or near total impairment of enzymatic activity (Table 1) (10).
Clinical basal hormone levels and molecular data of the SW-21OHD infants.
| Case | Age at | Clinical | 17OHP | Testo | Andro | Na/K | PRA | CYP21A2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis (days) | Karyotype | Manifestations | (ng/ml) | (ng/dl) | (ng/ml) | (mEq/L) | ng/mL/h | Genotype | ||
| 1∗ | 7 | XY | NBS | 626 | - | - | 129/7.9 | 21 | Del8/Cluster | |
| 2∗ | 1 | XY | NBS/AS | 539 | 1,500 | - | 125/8.4 | >25 | Del8/Cluster | |
| 3 | 1 | XX | NBS/AG | 240 | >1,500 | - | 134/6.2 | 8.7 | I2/I2 | |
| 4 | 2 | XX | AG | 232 | - | - | 133/5.7 | - | I2/I2 | |
| 5 | 91 | XX | AG/D | 236 | 177∗∗ | 9.6 | 117/8.0 | 32 | Conv/I2 | |
| 6 | 30 | XX | AG/D | 656 | 289∗∗ | 14.9 | 114/8.6 | 24.5 | I2/Ins A | |
| 7 | 16 | XX | AG/D | 145 | 1,500 | >10 | 131/6.4 | >25 | - | |
| 8 | 12 | XX | AG/D | 156 | 137 | 27 | 131/7.5 | >30 | I2/I2 | |
| 9 | 18 | XX | AG/D | 338 | 125∗∗ | - | 133/6.4 | >30 | I2/I2 | |
| 10∗ | 6 | XY | D/AS | 134 | 1,260 | >8.5 | 132/7.6 | - | Q318X/I2 | |
| 11∗ | 16 | XX | AG/D | 200 | - | - | 118/6.7 | >25 | Q318X/I2 | |
| 12 | 15 | XX | AG/D | 194 | - | - | 127/9.0 | 19 | Conv/Conv | |
| 13 | 60 | XX | AG/D | 217 | 75 | 4.7 | 125/6.6 | >25 | Conv/I2 | |
| 14 | At birth | XY | AS | 192 | 840 | 8.1 | 136/5.5 | >22 | Conv/I2 | |
| 15 | 6 | XY | D/AS | 110 | 746 | >10 | 133/5.6 | >37 | Q318X/R356W | |
| 16 | 23 | XX | AG/D | 168 | 118∗∗ | 18.3 | 110/8.0 | - | I2/H365Y | |
| 17 | 24 | XX | AG/D | 59 | 910∗∗ | - | 120/5.3 | >19.6 | I2/IVS2+5 G>A | |
| 18 | 20 | XX | AG/D | 76 | - | - | 130.5.2 | - | I2/Q318X | |
| +R356W | ||||||||||
| 19 | 30 | XY | D | 160 | - | - | 110/11.6 | - | Del 8/Del 8 | |
| 20 | 5 | XX | AG | 95 | 772 | - | 135/3.5 | - | I2/I2 | |
| 21 | 30 | XX | AG/D | >20 | 350 | >10 | 22.3 | Q318X/I2 | ||
| 22 | 60 | XY | D | >20 | 200 | 14 | 107/6.5 | >25 | - | |
| 23 | 39 | XY | D/AS | 132 | 244 | - | 110/8.4 | >25 | E351V/I2 | |
NBS: Newborn screening; AG: ambiguous genitalia; D: dehydration; AS: affected sibling; Andro: androstenedione; Testo: testosterone; Del: deletion, I2: intron two splice, Ins: insertion; Conv: Conversion; - : data not available; ∗: Sibling; ∗∗ Radioimmunoassay;
The patients were initially treated with cortisone acetate: ∼18 mg/m2/day divided into three doses and 9-α-fludrocortisone 100-200 μg/day. Two grams/day of sodium chloride was supplemented during exclusive breastfeeding periods. The patients were evaluated weekly during the first two months of life, monthly from three to six months of life and every three months thereafter. Clinical data, such as height, weight, blood pressure and sodium and potassium levels, were measured at every appointment. Basal serum testosterone, androstenedione, 17OHP and PRA levels were measured every three months. Mineralocorticoid treatment was considered adequate if the sodium and potassium levels were maintained within the normal or near normal range and if the PRA levels were within the upper limit of the normal range. Glucocorticoid treatment did not attempt to normalize the 17OHP levels and was considered adequate if at least three of the four assessments (per year) of testosterone and androstenedione levels were also normal.
During the first two years of life, the cortisone acetate and 9-α-fludrocortisone doses and clinical and laboratory data, such as height, weight, blood pressure, sodium levels, potassium levels and PRA levels, were reviewed in our cohort.
Hormonal assays and genetic analysisRadioimmunoassay of 17OHP was measured using commercial reagents (Diagnostic Systems Laboratories Inc., Webster, TX, USA); the intra-assay and inter-assay coefficients of variation were ∼9%. The PRA was measured by radioimmunoassay using commercial reagents (Diasorin, Stillwater, MN, USA); the intra-assay and inter-assay coefficients of variation were ∼8% and ∼7%, respectively. From 1992 to 1997, testosterone was measured with radioimmunoassay (Diagnostic Systems Laboratories Inc., Webster, TX, USA), and the intra-assay and inter-assay coefficients of variation were ∼10-12%. After 1997, testosterone was measured with fluoroimmunoassay (AutoDELFIA, Perkin Elmer Inc., Walthan, MA, USA); the intra-assay and inter-assay coefficients of variation were ∼3% and ∼6%, respectively. Androstenedione was measured with radioimmunoassay (Diagnostic Systems Laboratories Inc., Webster, TX, USA). The intra-assay and inter-assay coefficients were ∼10%.
The genetic analyses of CYP21A2 included Southern blotting, followed by hybridization with a genomic CYP21 probe to investigate large rearrangements (11) and allele-specific PCR to investigate 15 common micro-conversions (10). Direct sequencing was performed when the mutations were not detected in both of the alleles or were not concordant with the phenotype (12).
StatisticsThe results are presented as the median, mean and SD. The group comparisons were performed with a Kruskal-Wallis one-way analysis of variance on rank, followed by Dunn's post test; p<0.05 was considered significant. The graphics were designed using Prism 3 software (GraphPad Software Inc., San Diego, CA, USA).
RESULTSIn the 23 salt wasting 21OHD patients studied, the salt wasting crises occurred at a mean age of 23 days (range 6-90 days). The mean basal sodium level at the time of diagnosis was 128 mEq/L (range 107-136 mEq/L), and the mean potassium level was 6.7 mEq/L (range 4.5-11.6 mEq/L) (Table 1).
The patients were initially treated with the standard dose of cortisone acetate (18 mg/m2/day), 9-α-fludrocortisone (100-200 μg/day) and sodium chloride (2 g/day); the cortisone acetate and 9-α-fludrocortisone doses were adjusted according to the above-mentioned clinical and laboratory parameters.
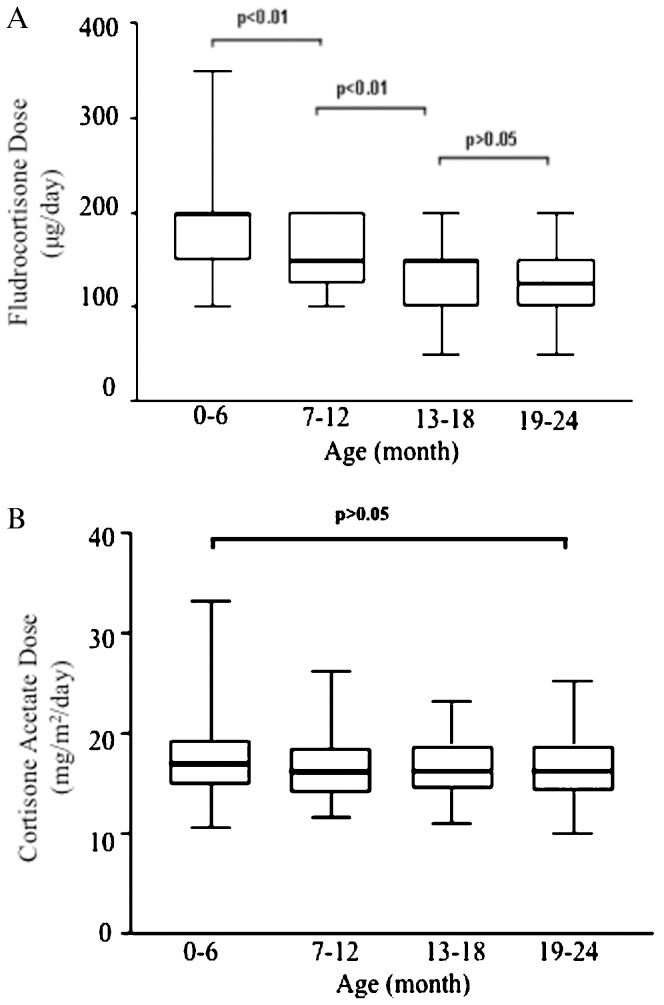
During follow-up, the need for 9-α-fludrocortisone replacement progressively decreased. The 9-α-fludrocortisone dose in the first semester of life was significantly higher than in the second semester, which in turn was significantly higher than in the second year of life (p<0.01) (Figure 1A). The median 9-α-fludrocortisone dose from 0-6 months of life was 200 μg/day (range 100-350 μg), 150 μg/day (range 100-200 μg) from 7-12 months, 150 μg/day (range 50-200 μg) from 13-18 months and 125 μg/day (range 50-200 μg) from 19-24 months (Figure 1A). Note that the range of 9-α-fludrocortisone doses during the first six months of life was larger than the range observed for the following periods (Figure 1A).
A) Box plots describe fludrocortisone dose variability (μg/day) according to age; the dose decreases progressively during the first 2 years of treatment. B) Box plots describe the cortisone acetate dose (mg/m2/day) variability. Cortisone acetate doses per square meter were stable during the first two years of treatment. The boxes at the lower and upper ends represent the lower and upper quartiles, respectively, and the bold horizontal line inside the boxes represents the median dose. The vertical bars reach from the boxes to the non-outlier minimum and maximum values.
In contrast, the cortisone acetate dose (adjusted for body surface area) was constant during the first two years of life (mean dose 16.3 mg/m2, SD±3.4) (Figure 1B).
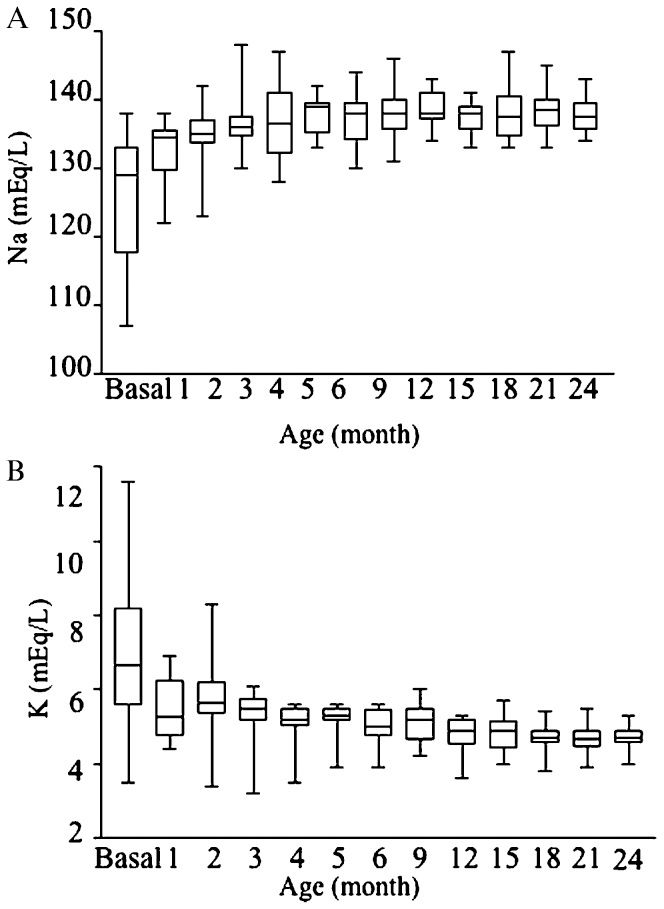
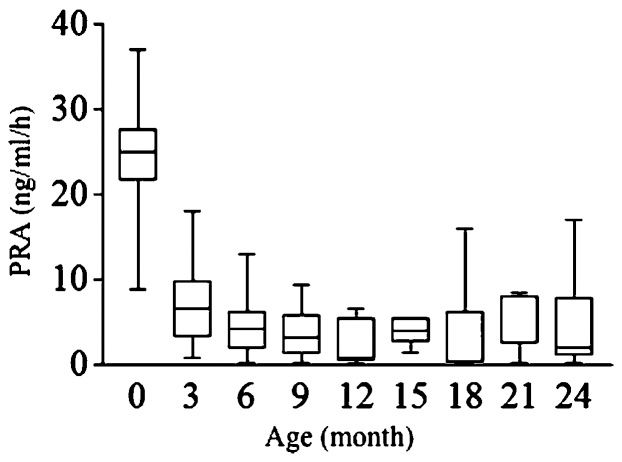
The mineralocorticoid replacement was adequate in these patients, as shown by the normal or near normal serum sodium and potassium levels from the second month to the second year of life (Figures 2A and 2B). Additionally, PRA was maintained in the upper limit of the normal range from three months of age onwards (Figure 3). During the studied period, none of the patients presented with clinical signs of mineralocorticoid overload, such as arterial hypertension, tachycardia or congestive heart failure, or deficiencies (e.g., dehydration and/or arterial hypotension).
A) Box plots show the serum sodium levels in the basal state, monthly during the first 6 month of life and then every three months during the first two years of treatment. B) Box plots show the serum potassium levels in the basal state, monthly during the first six month of life and then every three months during the first two years of treatment.
The patients reached normal height (±1 SD) and weight (±1 SD) after two years of treatment, which assured adequate hormonal replacement. In males, the mean height and weight were 85.8 cm (SD = 0) and 12.6 kg (SD = 0), respectively; in females, the mean height and weight were 81.3 cm (SD = -1) and 11.7 kg (SD = -0.4), respectively.
DISCUSSIONAppropriate glucocorticoid and mineralocorticoid replacement during infancy in SW-21OHD children is critical for maintaining a positive sodium balance that enables adequate body growth and brain development. Studies evaluating the late effect of neonatal sodium deficiency in neurological performance, such as motor function, intelligence (IQ), memory, language and behavior, have shown poor neurodevelopment outcomes in the second decade of life (13). Impaired cognitive function has been observed in 21OHD patients, particularly with SW-21OHD (14), which reflects the adverse effects of hyponatremia episodes and suboptimal hormone replacement therapy. However, the acute consequences of mineralocorticoid excess are volume overload and congestive heart failure, and the chronic consequences include growth impairment caused by its glucocorticoid properties (15).
Therefore, the goal of therapy in SW-21OHD patients is to normalize the androgen levels and total-body sodium depletion to allow normal growth and development and avoid volume overload (16). In our study, the required glucocorticoid dose (adjusted per square meter) was stable during the first two years of life. In contrast, the need for 9-α-fludrocortisone was higher during the first six months and continually decreased during follow-up; at two years of age, the mean dose of 9-α-fludrocortisone was 25-35% lower than the initial dose, which suggested an improvement in the kidney regulation of salt and water balance.
In fact, a relative state of aldosterone resistance has been found in the neonatal period (6). Healthy term neonates exhibit extremely high plasma aldosterone levels (6, 17), which is most likely an adaptive response to kidney tubular immaturity at birth (6, 7, 17-19). It has been proposed that during early infancy, renal proximal tubular cells, which are responsible for the reabsorption of 60-80% of the filtered load of salt and water, N+/K+-ATPase (the main enzyme responsible for active sodium transport), and the mineralocorticoid nuclear receptor (MR, the rate-limiting step for sodium balance in the distal renal tubules) do not reach full maturation (7). Additionally, in 21OHD patients, the accumulated androgen precursors, progesterone and 17OH-progesterone (17OHP) inhibit mineralocorticoid receptor transactivation by aldosterone (20).
The limited sodium content in breast milk most likely also corresponds with greater 9-α-fludrocortisone requirements in the first semester of life. The sodium composition of breast milk ranges from 3-18 mEq/L, and the milk volume production is ∼800 mL/day, which provides sufficient sodium to supply a healthy full-term newborn (i.e., ∼1-2 mEq/kg/day) but not enough to replace the sodium deficit that arises in salt wasting newborns (i.e., ∼4-5 mEq/kg/day) (21-23).
The range of mineralocorticoid doses was wider in the first semester of life, most likely because of the greater inter-individual variability of mineralocorticoid sensitivity and/or greater difficulty in setting up an initial mineralocorticoid dose. Therefore, individual drug adjustments during this period should be assessed weekly rather than monthly.
In conclusion, this retrospective analysis of 9-α-fludrocortisone dose schedules in a group of 21OHD children during the first two years of life suggests a 9-α-fludrocortisone-dose regimen that closely monitors the anthropometric, clinical and biochemical parameters to ensure adequate levels and avoid complications related to mineralocorticoid under- or overtreatment.
This study was supported by FAPESP #2008/57616-5, Bachega TASS and Mendonca BB by CNPq #305117/2009-2 and #305743/2011-2.
No potential conflict of interest was reported.
Gomes LG and Madureira G wrote the paper. Mendonca BB performed the statistical analysis. Bachega TA was the mentor and supervisor of this work. The four co-authors performed follow-ups of the patients in this study.