The aim of the present study was to investigate the relationship between pericoronary fat and the severity and extent of atherosclerosis, quantified using 64-multidetector computed tomography, in patients with suspected coronary artery disease.
METHODS:The study population consisted of 131 patients who were clinically referred for noninvasive multislice computed tomography coronary angiography for the evaluation of coronary artery disease. Patients were classified as follows: no atherosclerosis, Group 1; nonobstructive atherosclerosis (luminal narrowing <50% in diameter), Group 2; and obstructive atherosclerosis (luminal narrowing ≥50%) in a single vessel or obstructive atherosclerosis in the left main coronary artery and/or multiple vessels, Group 3. Epicardial adipose tissue was defined as the adipose tissue between the surface of the heart and the visceral layer of the pericardium (visceral epicardium). Epicardial adipose tissue thickness (mm) was determined in the right ventricular anterior free wall. The mean thickness of the pericoronary fat surrounding the three coronary arteries was used for the analyses.
RESULTS:The average thickness over all three regions was 13.2 ± 2.1 mm. The pericoronary fat thickness was significantly increased in Group 3 compared with Groups 2 and 1. The epicardial adipose tissue thickness was significantly increased in Group 3 compared with Groups 2 and 1. A receiver operating characteristic curve for obstructive coronary artery disease was assessed to verify the optimum cut-off point for pericoronary fat thickness, which was 13.8 mm. A receiver operating characteristic curve for obstructive coronary artery disease was also assessed to verify the optimum cut-off point for epicardial adipose tissue, which was 6.8 cm.
CONCLUSION:We showed that the epicardial adipose tissue and pericoronary fat thickness scores were higher in patients with obstructive coronary artery diseases.
Visceral adipose tissue is an important indicator of cardiovascular risk (1). Abdominal obesity has been shown to be a stronger predictor of cardiovascular risk than increased body mass index (2). Abdominal adipose tissue is able to produce large quantities of tumor necrosis factor-alpha, interleukin-6, free fatty acids, and plasminogen activator inhibitor-1, which are involved in accelerated atherosclerosis, plaque instability, and arterial thrombosis (3-5).
Adipose tissue surrounding the coronary arteries, often called epicardial adipose tissue (EAT), may also act as an endocrine organ due to their comparable patterns of adipocytokine production (6,7). Evidence is accumulating that EAT may be linked to the development of coronary atherosclerosis through several paracrine mechanisms, such as local inflammatory mediators that trigger the atherosclerotic process, and other systemic effects (8).
Previous studies have demonstrated an association between EAT and the presence and extent of coronary artery disease (CAD). The association between EAT thickness and CAD has been previously evaluated using echocardiography. However, computed tomography (CT) is more accurate in quantifying adipose tissue accumulation due to its higher spatial resolution. The aim of the present study was to investigate the relationship between pericoronary fat and the severity and extent of atherosclerosis, quantified using 64-multidetector CT, in patients with suspected CAD.
METHODSThe study population consisted of 131 patients who were clinically referred for noninvasive multislice computed tomography (MSCT) coronary angiography for CAD evaluation. The exclusion criteria for MSCT coronary angiography were a history of percutaneous coronary intervention, previous coronary artery by-pass surgery, or known cardiomyopathy. End-stage renal disease, liver failure, and triglyceride levels >400 mg/dl were also exclusion criteria for the study. The study protocol was approved by our internal institutional review board.
Demographic data were collected on all patients through a retrospective chart review. Height and weight measured at the time of imaging were used to calculate body mass index (BMI). Hypertension was defined as a systolic blood pressure ≥14 mmHg and/or a diastolic blood pressure ≥90 mmHg or current antihypertensive treatment. Dyslipidemia was defined as total cholesterol ≥200 mg/dl, low-density lipoprotein (LDL) cholesterol ≥130 mg/dl, high-density lipoprotein (HDL) cholesterol <30 mg/dl, or current lipid-modifying agent treatment. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dl or current hypoglycemic treatment. Smoking was classified as current smoking if the patient smoked or quit within the last 30 days and not smoking if the patient never smoked or smoked in the remote past.
Patients were classified as follows: no atherosclerosis, Group 1 (n = 36); nonobstructive atherosclerosis (luminal narrowing <50% in diameter), Group 2 (n = 42); and obstructive atherosclerosis (luminal narrowing ≥50%) in a single vessel or obstructive atherosclerosis in the left main coronary artery and/or multiple vessels, Group 3 (n = 53). EAT and pericoronary fat were quantified using electrocardiogram (ECG)-gated diagnostic cardiac CT scans. CT studies were performed on a 64 detector-row CT scanner (Brilliance 64, Philips Medical Systems, Cleveland, OH, USA). The scan duration time was 7-10 s, and the average heart rate during image acquisition was 58±9 beats/min. Standard coronary imaging protocols were applied, including the use of intra-venous beta blockers for patients with heart rates >65 beats/min (unless contraindicated), and image acquisitions were performed during an inspiratory breath-hold. The imaging parameters were a slice collimation of 64 mm×0.625 mm, gantry rotation time of 420 ms, tube voltage of 120 kV, and tube current of 900 mA. The contrast agent used was iopromide (Schering AG, Berlin, Germany), which was injected intravenously (1.6-2.0 g iodine/s depending on the patient's body weight).
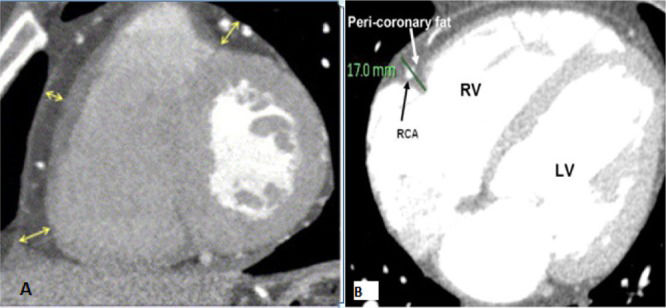
Measurements were performed in the most motionless phase of the cardiac cycle, which was most frequently the mid-diastolic phase, with retrospective cardiac gating at 70-80% of the R-R interval. The window settings were adjusted to enable adequate visualization of the adipose tissue and the pericardium (Figure1). Coronary artery calcification (CAC) was quantified based on the Agatston score, which is a measure of the area of a calcified lesion multiplied by a factor based on the maximum pixel density. The computer system provided the Agatston score for each of the following locations: left main coronary artery, left anterior descending artery, circumflex artery, right coronary artery, aortic annulus and root, ascending aortic wall, and mitral annulus. A total score representing the cumulative calcium burden in all of the above locations was then calculated for each patient as the final Agatston score, which is reported in the results. EAT was defined as the adipose tissue between the surface of the heart and the visceral layer of the pericardium (visceral epicardium). EAT thickness (mm) was measured on the right ventricular (RV) anterior free wall. Measurements were performed at the base (basal level) of the ventricles on short-axis views of a regular Philips CT workstation. The basal level was defined as the level at the base of the ventricles. Three measurements of EAT thickness were made, namely, inferior, center, and superior, corresponding to measurements at the 25%, 50% and 75% level of the RV wall, respectively, from the visceral epicardium to the outside of the myocardium and perpendicular to the surface of the heart. The mean of the three measurements (referred to as ‘EAT‘) was used for the analyses. Pericoronary fat was defined as the adipose tissue between the surface of the heart and the visceral epicardium directly surrounding the main coronary arteries. Pericoronary fat thickness (mm) was quantified on axial views of a regular Philips CT workstation. To avoid overestimating the pericoronary fat due to obliquity, thickness measurements were performed on images in which the axial sections were perpendicular to the surface of the heart. In each of the regions of the right coronary artery (RCA), left coronary artery (LCA), and left circumflex (LCX), the maximum fat thickness, assessed as the largest distance from the myocardium to the visceral epicardium, was determined. The mean thickness of the pericoronary fat surrounding the three coronary arteries was used to analyze the pericoronary fat thicknesses (PCFT).
Statistical analysis of the data was performed using SPSS (Statistical Package for Social Science, Inc., Chicago, Illinois, EUA) for Windows 11.5. Whether the continuous variables were normally distributed was investigated using the Shapiro-Wilk test. Descriptive statistics were expressed as the mean ± standard deviation for continuous variables and as the number of cases and percentage for categorical variables. The difference between groups with respect to normally distributed continuous variables was analyzed by analysis of variance (ANOVA), and the significance of the difference between non-normally distributed continuous variables was analyzed with the Mann-Whitney U test. Categorical variables were evaluated with Pearson's chi-square or Fisher's exact test. The significance of the linear correlation between the three groups and EAT and PCFT measurements was evaluated with Spearman's correlation analysis. A p value of < 0.05 was considered statistically significant.
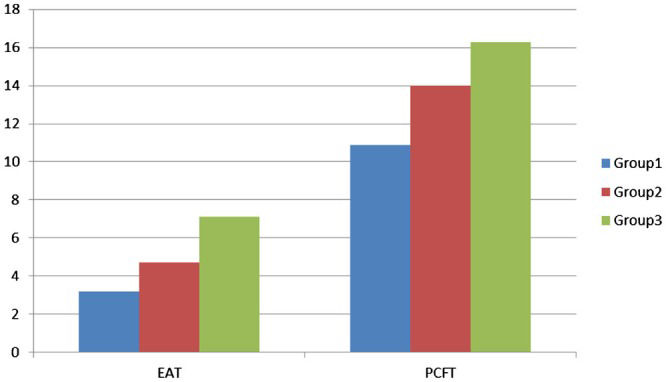
RESULTSThe patient demographics and clinical characteristics are shown in Table1. There was no significant difference between the 3 groups except for smoking and diabetes status. The incidences of smoking and diabetes were significantly higher in Group 3. Patients were divided into 3 groups, and the subjects in all groups were predominantly male. The mean age of the study population was 54 ± 11 years. CAC was significantly higher in Group 3. The average thickness of the layer of PCFT surrounding the coronary arteries was 15.5 ± 3.3 mm (range 6.9-37.6 mm) in the RCA area, 6.2 ± 2.0 mm (range 1.9-15.0 mm) in the LAD area, and 11.8 ± 3.4 mm (range 2.6-30.1 mm) in the LCX area. The average thickness over all three regions was 13.2 ± 2.1 mm. PCFT was significantly increased in Group 3 compared with Groups 2 and 1 (R = -0.240, R = -0.233, R = -0.283) (Tables2-3). No obstructive CAD was present in 36 patients (27%), whereas non-obstructive atherosclerotic CAD and obstructive atherosclerosis were present in 42 (32%) and 53 (41%) patients, respectively. The EAT thickness was significantly increased in Group 3 compared with Groups 2 and 1 (7.1 ± 2.7 cm, 4.7 ± 1.9 cm and 3.2 ± 1.1 cm, respectively, and R = -0.438, R = -0.328, and R = -0.447, respectively) (Figure2 and Tables2-3).
Baseline demographic and laboratory data characteristics.
| Group 1(n = 36) | Group 2(n = 42) | Group 3(n = 53) | p | |
|---|---|---|---|---|
| Ages (years) | 52 ± 10 | 51± 8 | 56 ± 12 | NS |
| Gender (male %) | 63 | 61 | 67 | NS |
| Urea (mg/dl) | 32 ± 8 | 30 ± 8 | 31 ± 9 | NS |
| Creatinine (mg/dl) | 0.8 ± 0.5 | 0.9 ± 0.3 | 0.7 ± 0.6 | NS |
| FPG (mg/dl) | 93 ± 30 | 94 ± 27 | 94 ± 23 | NS |
| Total cholesterol (mg/dl) | 200 ± 45 | 210 ± 43 | 207 ± 41 | NS |
| LDL cholesterol (mg/dl) | 125 ± 38 | 123 ± 35 | 127 ± 42 | NS |
| HDL cholesterol (mg/dl) | 45 ± 12 | 44 ± 11 | 40 ± 10 | NS |
| Triglycerides (mg/dl) | 155 ± 73 | 150 ± 65 | 157 ± 67 | NS |
| Body mass index (kg/m2) | 24.2 ± 3.1 | 24.6 ± 3.0 | 25 ± 3.2 | NS |
| Diabetes Mellitus % | 14.5 | 15.3 | 22.1 | 0.023 |
| Hypertension % | 39.1 | 35 | 41 | NS |
| Current smoking % | 41.2 | 42 | 49 | 0.033 |
| CAC score | 38 ± 25 | 305 ± 129 | 640 ± 301 | < 0.01 |
CAC: Coronary artery calcium.
EAT and PCFT measurements according to group.
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| EAT (cm) thickness | 3.2 ± 1.1 | 4.7 ± 1.9 | 7.1 ± 2.7 |
| PCFT (mm) | 10.9 ± 1.7 | 14.0 ± 1.3 | 16.3 ± 2.1 |
EAT: Epicardial adipose tissue
PCFT: Pericoronary fat tissue
Group 1: No atherosclerosis
Group 2: Nonobstructive atherosclerosis (luminal narrowing <50% in diameter)
Group 3: Obstructive atherosclerosis (luminal narrowing ≥50%) in a single vessel or obstructive atherosclerosis in the left main coronary artery and/or multiple vessels.
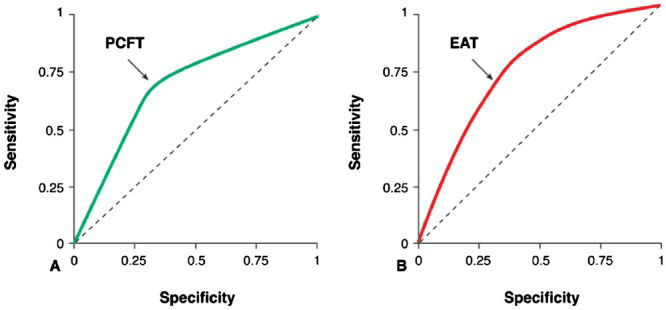
A ROC curve for OCAD was assessed to verify the optimum cut-off point for PCFT, which was 13.8 mm. The area under the curve (AUC) for PCFT was 71.4% (95% confidence interval [CI], 0.665-0.764), with a sensitivity of 72.2% and a specificity of 68.1%. A ROC curve for OCAD was assessed to verify the optimum cut-off point for EAT, which was 6.8 cm. The AUC for PCFT was 71.5% (95%CI, 0.750-0.777), with a sensitivity of 73.5% and a specificity of 69.3% (Figure3).
A) ROC curve for PCFT for the development of obstructive coronary artery disease (OCAD). A ROC curve for OCAD was plotted to verify the optimum cut-off point for PCFT, which was 13.8 mm. The AUC for PCFT was 71.4% (95%CI, 0.665-0.764), with a sensitivity of 72.2% and a specificity of 68.1%. B) ROC curve for EAT for the development of OCAD. A ROC curve for OCAD was plotted to verify the optimum cut-off point for EAT, which was 6.8 cm. The AUC for PCFT was 71.5% (95%CI, 0.750-0.777), with a sensitivity of 73.5% and a specificity of 69.3%.
Thus, the results demonstrate that the EAT and PCFT scores were higher in patients with obstructive coronary artery diseases.
DISCUSSIONThe major finding of this population-based study was that EAT directly surrounding the coronary arteries is related to obstructive atherosclerosis in patients. Our study showed that EAT and PCFT were particularly high in the high-risk group (diabetics and patients with high CAC scores). Previous studies on EAT thickness were mainly based on echocardiography and measured EAT thickness in the right ventricle only or in the combined epicardial and pericardial adipose tissue surrounding the heart (1,9-12). Differentiation between epi- and pericardial fat may be difficult with echocardiographic examination (13). On CT, the pericardium is readily identified, resulting in easy differentiation between epi- and pericardial fat. In contrast to previous studies, we focused on the epicardial fat surrounding the arteries because of the notion that it is the local fat that may drive the development of atherosclerosis. Multislice CT provides an accurate and reproducible quantification of EAT due to its high temporal and spatial resolution. Ahn et al. (14) studied the association between the thickness of EAT on the free wall of the RV and CAD in patients who underwent conventional coronary angiography due to chest pain. A positive association was found between EAT thickness and the presence of significant coronary stenosis (luminal narrowing -50%) and with the number of coronary arteries with significant stenosis. In contrast, a study by Chaowalit et al. (15) in 139 patients who were referred for conventional coronary angiography did not show a significant correlation between EAT thickness and number of atherosclerotic coronary segments.
In our study, we found a positive correlation between not only EAT but also PCFT and the number of diseased vessels and calcified lesions. Previous studies evaluating the relationship between EAT and angiographic CAD have yielded conflicting results, most likely due to differences in the measurement techniques and study populations (16-18). A positive correlation was shown between EAT thickness and CAD severity. In our present study, we showed that EAT and PCFT could be predictors of coronary atherosclerosis. MSCT can be used to provide an estimate of the severity of EAT and PCFT. In line with previous findings (19), the amount of EAT and pericoronary fat was strongly associated with obesity in patients with suspected CAD. Adipose tissue is a major driver of insulin resistance, an essential pathophysiological feature associated with the development of metabolic disorders, including hyperglycemia, hypertension, and low HDL cholesterol (2,20). In our study, LDL cholesterol levels did not differ between the two groups. This result demonstrates the importance of EAT and PCFT, as they could be risk factors independent of the level of LDL cholesterol.
Several limitations of our study should be considered. The present study was performed in a heterogeneous study population. The present analysis was restricted to an evaluation of the association between coronary atherosclerosis and EAT thickness. The proatherogenic process, which relates EAT and predisposition to atherosclerosis, was not investigated.
In conclusion, EAT and PCFT are related to severe coronary atherosclerosis. MDCT may be an invaluable diagnostic tool with which to determine EAT and PCF thickness.
AUTHOR CONTRIBUTIONSDemircelik MB and Yilmaz OC conducted the research, designed and conceived the study. Demircelik MB, Yilmaz OC and Selcoki Y planned the methods to achieve the results. Demircelik MB, Bozkurt A, Gurel OM and Eryonucu B were responsible for the project supervision. Eryonucu B, Akin K, Atar IA, Bozkurt A and Demircelik MB were vital to the project staff and provided space, financial resources, tools and equipments. Yilmaz OC, Demircelik MB and Bozkurt A performed the patient tracking and data reporting. Demırcelık MB, Bozkurt A and Atar IA provided the logical explanations and presentation of the findings. Demircelik MB, Yilmaz OC and Selcoki Y conducted the review of the literature. Eryonucu B, Bozkurt A, Akin K, Gurel O and Atar IA provided final approval and grammar checks.
No potential conflict of interest was reported.