Epstein-Barr virus exposure appears to be an environmental trigger for rheumatoid arthritis that interacts with other risk factors. Relationships among anti-cyclic citrullinated peptide antibodies, the shared epitope, and smoking status have been observed in patients with rheumatoid arthritis from different populations.
OBJECTIVE:To perform an association analysis of anti-Epstein-Barr nuclear antigen-1 antibodies, anti-cyclic citrullinated peptide antibodies, the shared epitope, and smoking status in Brazilian patients with rheumatoid arthritis.
METHODS:In a case-control study, 140 rheumatoid arthritis patients and 143 healthy volunteers who were matched for age, sex, and ethnicity were recruited. Anti-Epstein-Barr nuclear antigen-1 antibodies and anti-cyclic citrullinated peptide antibodies were examined using an enzyme-linked immunosorbent assay, and shared epitope alleles were identified by genotyping. Smoking information was collected from all subjects. A comparative analysis of anti-Epstein-Barr nuclear antigen-1 antibodies, anti-cyclic citrullinated peptide antibodies, the shared epitope, and smoking status was performed in the patient group. Logistic regression analysis models were used to analyze the risk of rheumatoid arthritis.
RESULTS:Anti-Epstein-Barr nuclear antigen-1 antibodies were not associated with anti-cyclic citrullinated peptide antibodies, shared epitope alleles, or smoking status. Anti-cyclic citrullinated peptide antibody positivity was significantly higher in smoking patients with shared epitope alleles (OR = 3.82). In a multivariate logistic regression analysis using stepwise selection, only anti-cyclic citrullinated peptide antibodies were found to be independently associated with rheumatoid arthritis (OR = 247.9).
CONCLUSION:Anti-Epstein-Barr nuclear antigen-1 antibodies did not increase the risk of rheumatoid arthritis and were not associated with the rheumatoid arthritis risk factors studied. Smoking and shared epitope alleles were correlated with anti-cyclic citrullinated peptide-antibody-positive rheumatoid arthritis. Of the risk factors, only anti-cyclic citrullinated peptides antibodies were independently associated with rheumatoid arthritis susceptibility.
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease with a pathogenesis that is not fully understood. The pathophysiology of RA is complex and multifactorial and involves both genetic and environmental factors. Many studies have demonstrated the importance of certain human leukocyte antigens (HLA) molecules that contain the shared epitope (SE) as the major genetic risk factor for RA. Nevertheless, genetic factors fail to completely explain the occurrence of RA.1,2
In addition, antibodies to cyclic citrullinated peptides (anti-CCP) are highly specific predictors of RA. Anti-CCP have been observed many years prior to disease onset, indicating an important role for them in the development of RA. An association between the positivity of anti-CCP and the presence of the SE in RA has been reported by numerous researchers. The presence of SE alleles appears to be a risk factor related to anti-CCP production.1,3 Interactions between tobacco exposure and SE status in anti-CCP-positive patients have been demonstrated by several studies, whereas neither smoking nor SE status confers an increased risk of developing anti-CCP negative RA.4–10
Among other environmental factors, infectious agents, most notably viruses, have long been suspected of promoting the development of RA and other autoimmune diseases.11–13 Links between Epstein-Barr virus (EBV) and RA have been a focus of research for the last three decades. Studies have demonstrated that EBV is a potent B-cell stimulator, antibodies in RA cross-react with EBV nuclear antigens, and EBV glycoproteins share sequence similarities with the HLA-DRB1 SE. These viral properties support the theory that EBV is a strong risk factor for RA.12–15
It has been observed that the levels of some anti-EBV antibodies (anti-EBNA, anti-VCA, and anti-EA) are higher in RA patients than in controls.16,17 In particular, Epstein-Barr nuclear antigen-1 (EBNA-1) contains glycine/alanine sequences that constitute a dominant EBNA-1 epitope against which specific reactivity has been demonstrated.18,19 An antibody against this EBNA-1 sequence also reacted with a protein expressed by synovial lining cells in RA patients.20 Identical glycine/alanine repeat sequences of EBNA-1 are also present in collagen, cytokeratin, and actin.21
Many studies have suggested that some RA risk factors are highly associated with EBV in the complex etiopathogeny of RA. Among the antibodies against EBV, anti-EBNA-1 appears to be the most prominent in the interactions with other risk factors. In the present study, we evaluated associations between anti-EBNA-1, anti-CCP, shared epitope alleles, and smoking status in Brazilian RA patients.
METHODS AND MATERIALSPatients and controlsA total of 140 RA Brazilian patients (including 122 females) aged 25-82 years (mean = 54.24 years), comprising 74 Caucasians, 20 Blacks, and 46 Mulattoes were evaluated at the University of Campinas Teaching Hospital in Brazil. All the patients fulfilled the 1987 RA revised criteria of the American College of Rheumatology. For the control group, we screened 143 healthy individuals who were matched for age, sex, and ethnicity and had no personal or family history of autoimmune disease. Smoking exposure was measured in pack-years, and all subjects were classified as never smoker, past smoker or current smoker. Clinical information was collected using a questionnaire at the time of enrollment. This study was approved by the ethics committee of the University of Campinas, Brazil, and informed consent was obtained from all patients and controls.
Serology and genotypingAnti-EBNA1 antibodies were analyzed using an enzyme-linked immunosorbent assay (ELISA) kit containing synthetic peptides (ETI-EBNA-G, DiaSorin, Saluggia, Italy). Anti-CCP antibodies were determined by ELISA (QUANTA Lite CCP3 IgG, Inova Diagnostics Inc., USA). Rheumatoid factor was evaluated by a nephelometric immunoassay (BN ProSPec System, Siemens, Marburg, Germany). All assays were performed according to the manufacturers' instructions.
HLA-DRB1 typing was undertaken by polymerase chain reaction (PCR), using specific primers and hybridization with sequence-specific oligonucleotides, as previously described.22 HLA-DRB1∗01, HLA-DRB1∗04, and HLA-DRB1∗10 were the SE alleles studied.5 Patients carrying zero, one, or two alleles of SE were classified as SE−/−, SE+/−, or SE +/+, respectively.
Statistical analysisThe chi-squared and Fisher's exact tests were used to compare categorical variables between the groups. The differences between the mean or median values of two groups were analyzed using the Mann-Whitney test. The Kruskal-Wallis test was used when the subjects were separated into three or more groups. To evaluate RA risk, we calculated odds ratios (OR) with 95% confidence intervals (CI). A logistic regression analysis with univariate and multiple models was used with stepwise variable selection. The significance level for statistical tests was set at 5% (p<0.05). All statistical analyses were performed using SAS (Statistical Analysis System) software for Windows, version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
RESULTSPatient and control characteristicsThe demographic, clinical, and laboratory characteristics of the RA cases and matched controls are listed in Table 1.
Demographic, clinical, and laboratory characteristics.
| Variables | RA patients (n = 140) | Controls (n = 143) |
|---|---|---|
| Women, n (%) | 122 (87.1) | 123 (86.0) |
| Ethnicity, n (%) Caucasian | 74 (52.9) | 83 (58.0) |
| Mulattoes | 46 (32.9) | 39 (27.3) |
| Black | 20 (14.3) | 21 (14.7) |
| Age (years) | 54.2±11.3 | 55.8±13.4 |
| Duration of disease (years) | 10.2±6.9 | NA |
| RF-positive, n (%) | 111 (79.3) | ND |
Data are reported as numbers of subjects with percentages or Mean ± Standard Deviation (SD) in parentheses. RA = Rheumatoid Arthritis; RF = Rheumatoid Factor; NA = not applicable; ND = not determined.
The rates of anti-EBNA-1 and anti-CCP positivity, as well as the mean antibody levels detected using these assays, along with the percentages related to SE status and smoking status in the two groups, are listed in Table 2. No significant differences were observed between the patient group and the control group in terms of the positivity and the mean levels of anti-EBNA-1 antibodies. The positivity and mean levels of anti-CCP antibodies were significantly higher in the patients than in the controls (p<0.001). The presence of the SE alleles (SE+/− and SE+/+) and a smoking history of at least 15 pack-years were significantly higher in the patients than in the controls (p<0.001 and p = 0.003, respectively).
Comparative analysis of anti-EBNA-1, anti-CCP, SE status, and smoking status in RA patients and controls.
| RA patients | Controls | p-value | |
|---|---|---|---|
| Anti-EBNA1-positive, n (%) | 127 (90.7) | 130 (90.9) | 0.955 |
| Mean anti-EBNA1 levels (U/ml) | 246.1 | 277.1 | 0.111 |
| Anti-CCP-positive, n (%) | 109 (77.9) | 2 (1.4) | <0.001 |
| Mean anti-CCP levels (U/ml) | 117.0 | 6.8 | <0.001 |
| Shared epitope status, n (%) SE −/− | 52 (37.1) | 81 (56.6) | <0.001 |
| SE +/− | 67 (47.9) | 55 (38.5) | |
| SE +/+ | 21 (15.0) | 7 (4.9) | |
| Smoking status, n (%)Non-smokers | 82 (58.6) | 107 (74.8) | 0.003 |
| <15 pack-years | 24 (17.1) | 22 (15.4) | |
| ≥15 pack-years | 34 (24.3) | 14 (9.8) |
Data are reported as numbers of subjects with percentages or mean levels in parentheses. RA = Rheumatoid Arthritis; Anti-EBNA-1 = Anti-Epstein-Barr Nuclear Antigen 1 Antibodies; Anti-CCP = Anti-Cyclic Citrullinated Antibodies; SE = Shared Epitope; p = significance based on Pearson's chi-squared or Mann-Whitney test. Differences were considered to be significant at p<0.05.
Anti-EBNA-1 levels were standardized in quartiles (1 = <63.71 U/ml; 2 = 63.71-125.26 U/ml; 3 = 125.27-333.44 U/ml; 4 = ≥333.45 U/ml). No significant differences were found in the distribution of anti-EBNA-1 antibodies according to anti-CCP positivity, SE status, or smoking status (Figure 1).
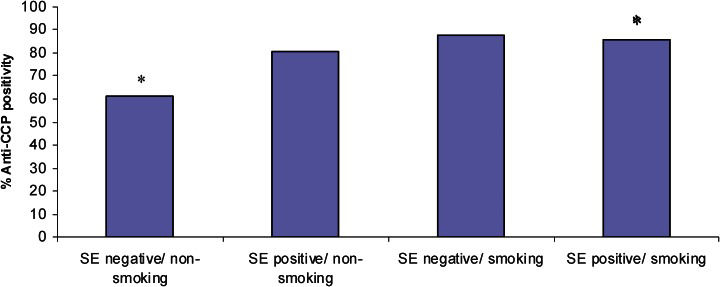
Anti-CCP profile according to SE status and smoking status in patientsAlthough it was not significant, there was a trend for patients with higher levels of anti-CCP to carry SE alleles (p = 0.059). Smokers had significantly higher levels of anti-CCP (p = 0.018). SE carriers and smoking patients had a significantly higher risk of being positive for anti-CCP (Figure 2, OR = 3.82; 95% CI = 1.28−11.40; p = 0.016).
Anti-EBNA1, anti-CCP, SE status, and smoking status in RA riskThe univariate logistic regression analysis showed that anti-CCP positivity carried a highly significant RA risk (p<0.001; OR = 247.49). The presence of one or two SE alleles increased the risk of RA 1.90-fold (p = 0.012) and 4.67-fold (p = 0.001), respectively. A smoking history of 15 or more pack-years led to a 3.17-fold increased risk of RA (p = 0.001). Anti-EBNA-1 levels were not found to be a risk factor for RA in this model.
In the multivariate logistic regression analysis with stepwise selection, anti-CCP was the only selected variable that was independently associated with RA.
DISCUSSIONThere is increasing evidence of the roles of a number of environmental factors, including viruses exposure and smoking, in the pathogenesis of RA. EBV has been studied for many decades; however, it remains unclear how its interactions with other RA risk factors develop. In 1976, Alpsaugh and Tan observed a higher serologic reactivity to the nuclei of EBV-infected B cells in RA patients than in the controls.23 The target B cell antigen was named “rheumatoid arthritis nuclear antigen” (RANA). Reactivity to RANA was not observed in uninfected B cells and was not influenced by the HLA-DR phenotype or the presence of rheumatoid factor. Subsequently, it was established that RANA was, in fact, the same molecule as EBNA-1. The QKRAA sequence present in HLA-DRB1∗0401 shares sequence homology with EBV gp110 protein, a target of the immune response to infection.24 Deblon et al. demonstrated that RA patients who carried the SE had a stronger humoral response to EBNA-1 than did SE-negative RA patients.25 These data are conflicting but suggest that EBV infection is related to the presence of HLA-DR molecules that contain the SE.
The anti-citrulline immune response is strongly involved in RA etiology, and anti-citrulline antibodies can precede RA development by several years. Anti-CCP antibodies are highly specific and are considered a valuable disease marker and a strong risk factor for RA. A gene-environment interaction among anti-CCP antibodies, SE status, and smoking has been observed in several studies. The presence of HLA-DR SE only confers a risk of RA to patients who are positive for anti-CCP.1,10,26,27 Smoking is associated with the increased presence of citrulline-modified proteins in genetically predisposed individuals who carry HLA-DR SE alleles.8–10 In this study, we did not perform the HLA-DRB1 subtyping to identify HLA-DR SE alleles, since Lundström et al. observed that interactions of SE status with anti-CCP antibodies and smoking were independent of HLA-DRB1 subtyping and occurred with ∗01, ∗04, and ∗10 groups, regardless of fine specificity.5
EBV antigens can also undergo citrullination, thus becoming targets of anti-CCP. Antibodies to citrullinated EBV peptides have been identified in sera from RA patients, and the viral-citrullinated-peptide antibody levels of these patients correlated with their anti-CCP levels.28 A relationship between EBV and citrullinated peptides was supported by the observation that anti-citrulline antibodies reacted with deiminated EBNA-1.29 Toussirot and Roudier suggested that EBV should be considered as an environmental factor associated with anti-CCP production, just as smoking is associated with anti-CCP development in RA patients.15
It remains unknown how EBV influences the risk of developing RA. Anti-EBNA-1 antibodies are increased in individuals who are exposed to EBV and remain increased for a lifetime. Multiple sclerosis (MS) is a good example of an autoimmune disease for which anti-EBNA-1 antibodies have been shown to be a strong risk factor. Interestingly, this risk is increased by smoking but is independent of the MS susceptibility HLA-DR15 allele.30,31 In the present study, we showed a high positivity of anti-EBNA-1 in both patients and controls, and there was no association between anti-EBNA-1 levels and anti-CCP levels, SE status, or smoking status in RA patients. The relative risk for RA was not increased in patients with higher levels of anti-EBNA-1. Thus, we have demonstrated that anti-EBNA-1 serology cannot evaluate the interactions between EBV and the most well-known RA risk factors. Further studies could assess the role of EBV in RA pathogenesis. The accurate quantification of EBV by real-time polymerase chain reaction and the evaluation of antibodies to viral citrullinated peptides are promising techniques that could aid in this process.28,32,33
Brazil has a heterogeneous population with a distinct genetic profile that has been influenced by various racial and ethnic groups, predominantly Africans and Europeans. As reported in more homogeneously Caucasian populations5–8, we showed that SE alleles and tobacco exposure are related to anti-CCP antibodies in RA patients. Several previous studies observed these same relationships in Brazilian RA patients.34,35
In this study, we demonstrated the contributions of anti-CCP antibodies, the presence of the SE, and smoking history to RA risk. However, through a multivariate logistic regression analysis, we showed of the risk factors, only anti-CCP antibodies were independently associated with RA. Anti-EBNA-1 antibodies were neither correlated with the other factors nor influenced disease risk, unlike in other autoimmune conditions, such as MS. Despite some supporting evidence, the role of EBV in the development of RA remains unclear and needs to be studied further. We emphasize the importance of establishing the role of EBV in conjunction with other factors involved in RA pathogenesis.