The purpose of this study was to improve the use of 64-channel multidetector computed tomography using lower doses of ionizing radiation during follow-up procedures in a series of patients with endovascular aortic aneurysm repair.
METHODS:Thirty patients receiving 5 to 29 months of follow-up after endovascular aortic aneurysm repair were analyzed using a 64-channel multidetector computed tomography device by an exam that included pre- and post-contrast with both arterial and venous phases. Leak presence and type were classified based on the exam phase.
RESULTS:Endoleaks were identified in 8/30 of cases; the endoleaks in 3/8 of these cases were not visible in the arterial phases of the exams.
CONCLUSION:The authors conclude that multidetector computed tomography with pre-contrast and venous phases should be a part of the ongoing follow-up of patients undergoing endovascular aortic aneurysm repair. The arterial phase can be excluded when the aneurism is stable or regresses. These findings permit a lower radiation dose without jeopardizing the correct diagnosis of an endoleak.
Endovascular aortic aneurysm repair (EVAR) has been shown to be a safe technique.1 It was first described by White et al.,2 and the most common complication is extra-luminal leakage from the prosthesis or from the interior portion of the aneurysmal sack (i.e., an endoleak). Experimental studies have supported the concept of “endotension,” which is the persistent or recurrent pressurization of the aneurysmal sack after EVAR.3 In theory, the pressure maintained in the interior of the sack by a low-flow endoleak outside of the prosthesis lumen is indicative of an increase in aneurism size. These endoleaks are not visible on conventional imaging exams, such as computed tomography (CT) or digital angiography (DA).3
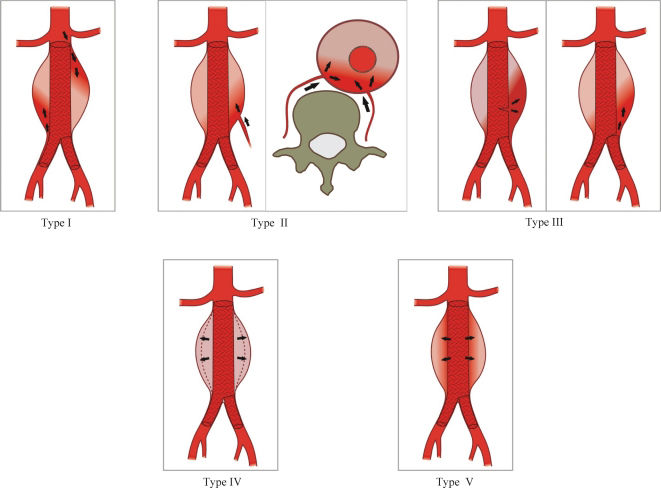
Veith et al.4 proposed a detailed classification system for endoleaks (Figure 1) in an attempt to describe the features and to facilitate the management of patients with this complication. Endotension is considered a type-V endoleak5,6 and is defined as an elevation of pressure within the aneurysmal sack, an undetected endoleak occurring in the late phases of the CT, an ultra-filtration of blood across the sleeve of the prosthesis, or a transmission of pressure by an aneurysmal thrombus.
Illustration of endoleaks using the Veith classification (Veith et al.4).
The incidence of endoleaks after EVAR ranges from 2.4% to 45.5%;2,4,7–9 several factors may contribute to the large disparity among the reported results. It is important to note that a variety of prosthesis types and diagnostic methods were used to evaluate the endoleaks in these studies.10–12
Despite the wide disparity in the reported results, multidetector CT (MDCT) has proven to be the most useful technique for preoperative evaluation. MDCT enables luminal and extra-luminal assessment of an aneurism using high-resolution spatial images that provide a diagnostic sensitivity for endoleaks that is superior to DA.13,14 The images obtained using this technique also allow for three-dimensional reconstruction and multi-planar reformations, which are useful for evaluating the aneurysmal sack volume. However, there is still a need for examination methods with greater diagnostic accuracy and that use a lower dose of ionizing radiation.15–17
The objective of this study was to improve the use of MDCT in the follow-up of a series of patients with EVAR to ensure adequate evaluation of aneurysmal sack parameters and to accurately diagnose endoleaks with the lowest possible dose of ionizing radiation.
MATERIALS AND METHODSPatientsThis study was approved by the research ethics committee of the Santa Casa de Misericórdia de São Paulo, and all patients signed an informed consent form. We included 30 patients who were undergoing EVAR during a period of 5 to 29 months post-surgery (average 14.4 months; median 13.5 months). The post-operative follow-up protocol of our service and the MDCT predefined protocol were used in this study. All subjects were adults (26 men and 4 women) with an age range of 55–83 years (mean 70.9 years; median 72 years). The endoprostheses used were chosen based on anatomic criteria determined by pre-operative CTs without bias from this study. All prostheses were of the aorto-bi-iliac type and included a variety of brands: Talent® (Meditronic Vascular, Sunrise, FL; n = 18); Excluder® Endoprothesis (W.L. Gore & Associates, Sunnyvale, CA; n = 1); Zenith® (Cook Inc., Bloomington, IN; n = 8); and Apolo® (Nano Endoluminal, Florianópolis, Brazil; n = 3). Patients with renal failure or allergic problems or those who did not agree to participate in the protocol were not included.
ExamsA 64-channel MDCT (Brilliance, Philips, Eindhoven, Holland), which covered the region between the diaphragm and the common femoral arteries, was used in three phases. The pre-contrast phase was acquired with a collimation of 2.5 mm, 120 kVp, and 322 mAs. The arterial and venous post-contrast phases were both acquired with 0.625-mm slice collimation, a pitch of 0.703, a tube rotation velocity of 0.75 per second, 120 kVp, and 350 mAs; they were then reconstructed to a 1.0-mm slice thickness. X-ray tube current modulation was used to reduce the total radiation dose. Iodine contrast (concentration 300 mg/ml) at a dose of 1.5 ml/kg (average 100 ml per patient) was injected in the antecubital vein using a 20-gauge catheter and an injection pump with a 5 ml/second velocity followed by 30 ml of physiologic saline. For the post-injection phase, a device was placed on the aorta at the celiac trunk (bolus tracking) to detect when 180 Hounsfield Units (HU) were reached, which marked the arrival of half of the contrast material and the end of the arterial phase. The venous phase occurred 60 seconds after the first phase. The apneic period varied from 12 to 18 seconds for all phases of the exam.
Image AnalysisAll images were analyzed independently on a workstation (GE Medical Systems, Milwaukee, WI) by two radiologists (RMB and RB) who had 11 and 15 years of experience, respectively, with vascular CT imaging, and the final results were reached by consensus.
An endoleak was deemed absent or present based on the classification criteria proposed by Veith et al.4 according to the characteristics of the leak and the pertinent information regarding the aneurism, the prosthesis and the prosthesis integrity. When present, the endoleak was measured at the largest transverse diameter. At the endovenous pre-contrast phase, calcifications or areas of greater attenuation of the aneurysmal sack due to recent bleeding that could hamper the post-contrast evaluation were assessed. Aneurism measurements were also assessed on pre-operative exams.
The full set of arterial phase images was used to create the following three-dimensional angiographic image displays: “volume rendered” (VR) and “maximum intensity projections” (MIP).
Statistical AnalysisThe McNemar test was used to evaluate the difference in the detection of endoleaks between the arterial and venous phases. Wilcoxon's Test was used to determine the differences in the dimensions of the endoleak sites between the arterial and venous phases and to compare the diameter of the neck and the aneurism between the preoperative phase and the time of our analysis. Fisher's exact test was used to determine the presence of an endoleak based on the different types of endoprostheses. An ANOVA test for repeated measurement with transformation of ranks was used to compare the diameter of the neck of the aneurism preoperatively and postoperatively and in the presence or absence of an endoleak.
RESULTSThe protocol utilized in this study yielded an average of 1079 image slices per exam (minimum 741; maximum 1263), with the average pre-contrast phase having 16.8% (181/1079), the arterial phase having 41.6% (449/1079), and the venous phase having 41.6% (449/1079) of the total slices obtained. The effective radiation dose in the pre-contrast phase was 732 mGy.cm (22%); for both arterial and venous phases, it was 1302 mGy.cm (39%).
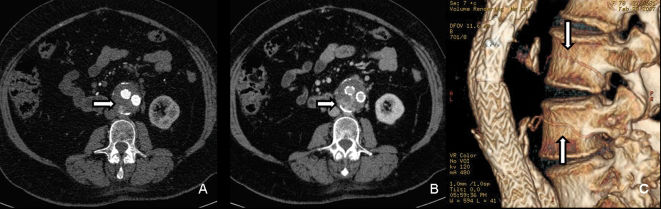
The venous phase analysis resulted in the diagnosis of type-II endoleaks for 8/30 cases (26.7%), while the arterial phase revealed only 5/8 leaks (Table 1). All diagnosed endoleaks were type II, three of which were fed by the inferior mesenteric artery, one of which was supplied by a lumbar artery (type IIA); the other four leaks were fed by two or more lumbar arteries (type IIB, Figure 2). The endoleak sites corresponded to the nutrient artery exits; the type-II endoleaks originating from the lumbar arteries (n = 5) were posterior, and the type-II leaks stemming from the inferior mesenteric arteries (n = 3) were anterior. The maximum transverse diameter of the endoleak sites was 4.8 cm in the arterial phase and 7.0 cm in the venous phase, with a trend toward increased size in the venous phase of the exam.
Type-IIB from the lumbar arteries (arrows) in the arterial (A) and venous (B) phases of the MDCT reveal an increase at the site of contrast during the venous phase. Using the volume-rendering algorithm (C), it is clear that two distinct levels of lumbar arteries are filling the aneurysmal sack (arrows).
One case was initially interpreted as a type-I leak on MDCT, but on consensus evaluation, it was decided that this represented a focal bulging of the aortic wall in the proximal segment free of tissue (i.e., free-flow).
DA was not used for the majority of the subjects in this study due to the stability or reduction in the aneurysmal dimensions measured with MDCT. Only two patients had DA performed because of the expansion of the aneurysm and to determine the appropriate course of therapy. The first case was treated with a translumbar puncture and injection of glue, and the other case underwent conservative treatment with regression of the aneurysm.
Comparative analysis of the preoperative tomographic study showed a reduction in the maximal transverse diameter of the aneurysm (p = 0.0003) in the majority of patients. Preoperative diameters ranged from 4.9 to 8.7 cm (mean 6.2 cm; median 6.05 cm); in the postoperative phase, the diameters ranged from 3.0 to 9.9 cm (mean 5.5 cm; median 5.65 cm). Only one patient had an increase in aneurysm diameter. This increase was associated with a type-II leak measuring 3.7 cm, which was only observed in the venous phase. No statistically significant relationship was identified between the maximum diameters of the aneurysms and the presence or absence of endoleaks.
Measurement of the maximum transverse diameter of the neck proximal to the aneurysm revealed a statistically significant increase (p = 0.0084), ranging from 2.0 to 3.3 cm (mean 2.5 cm; median 2.4 cm) in the preoperative period and from 2.1 to 3.7 cm (mean 2.7 cm; median 2.6 cm) in the postoperative phase. There was no statistically significant relationship between the maximum transverse diameters of the necks of the aneurysms and the presence or absence of endoleaks.
All of the prostheses used remained intact with no evidence of fracture. There was no statistically significant difference between the presence of endoleaks and the various brands of prostheses used (p = 0.0529). Excluder Endoprothesis® was used in only one patient, and it was not considered in the statistical analysis.
DISCUSSIONThe technical advances in endovascular surgery have yielded a safe alternative for the treatment of aortic aneurysms. However, although the perioperative complication rates of EVAR are lower than the rates of conventional surgical treatment,18 we believe that the short follow-up time of these patients prohibits the evaluation of late complications.
Follow-up periods at 1, 6, and 12 months after surgery and then every year thereafter for the life of the patient have been suggested for cases with no complications.16,18,19 Endoleaks were the most frequent complication identified, and they require shorter follow-up intervals depending on the type of leak and the change in aneurysmal sack dimensions.18 The number of exams that these patients will undergo during their lifetimes, together with the continuing increases in radiation dose and the constant technological improvements of the imaging machines used, has led to a great deal of discussion regarding the best protocol for MDCT examinations.
Some CT protocols have been proposed for better characterization of the aneurysm, the prosthesis and the complications of EVAR.7,20,21 Golzarian et al.21 proposed the use of a biphasic protocol with arterial and venous phases after the administration of an intravenous contrast agent. Rozenblit et al.7 demonstrated the advantages of using fine image slice sampling for diagnosing leaks, attributing the higher sensitivity in the diagnosis to the use of a biphasic protocol and advocating the use of the pre-contrast phases to resolve difficult cases. These authors stated that the increase in ionizing radiation associated with the use of a biphasic protocol is justified because it affords a more precise diagnosis of an endoleak.
In 2006, Iezzi et al.22 used 4-channel MDCT to study 50 patients with follow-up times of 1, 6, and 12 months (150 exams) after the placement of a prosthesis. The results suggested that during follow-up visits within the first month, a protocol using pre-contrast and arterial phases was most appropriate. However, they reported that for the later follow-ups at 6 and 12 months, a protocol with only the arterial phase was sufficient. The authors acknowledged that any increase in the aneurysm size may be related to an undiagnosed type-II endoleak, possibly due to the lack of a venous phase exam, and may be interpreted as an endotension. In these cases, Iezzi et al.22 suggested the use of a complete protocol with three phases or ultrasound with contrast, as proposed by Napoli et al.23
Also in 2006, Macari et al.17 analyzed 110 triphasic CT exams with a 4- and 16-channel MDCT, calculated the effective radiation dose and found that of the 28 patients with type-II endoleaks diagnosed in the venous phase, only 25 were also diagnosed in the arterial phase. They concluded that the arterial phase did not diagnose additional endoleaks but did increase the radiation dose by 36.5%. However, Macari et al.17 had two reservations about eliminating the arterial phase in postoperative patients: there may be diagnostic difficulty in differentiating between type-II and type-III endoleaks, as modular prostheses are being used more frequently, and the elimination of the arterial phase may limit the diagnosis of arteriovenous fistulas or pseudo-aneurisms of the common femoral artery.
To our knowledge, according to a search of the Medline database for reports in English, no previous reports in the literature have evaluated the incidence of endoleaks using 64-channel tomography. In this study, the equipment detected endoleaks in 26.7% (8/30) of the patients in our series. This detection rate agrees with the recent literature on multidetector equipment,17,22 but it is higher than that reported in exams using helicoid equipment with a single tier of detectors (9.2 to 18.5%).24,25
In agreement with the findings of Macari et al.,17 our protocol in the arterial phase, while exposing the patient to a larger dose of radiation (39%), was unable to reveal a number of the endoleaks that were visualized only in the venous phases (3/8). The venous phase had a greater capacity to diagnose endoleaks in the late follow-up of EVAR. We think that this finding could be better evaluated in a larger patient series or a longitudinal series.
In our view, the lack of an endoleak diagnosis using a protocol with only an arterial phase could be erroneously interpreted as endotension (i.e., a type-V endoleak) if the diagnosis is principally associated with an increase in the diameter of the aneurysm. Consequently, a patient may be deprived of treatment for a type-II leak, which increases the risk of an aortic rupture and death. This possibility has been previously described by Iezzi et al.22, although they defended a protocol with only the arterial phase in the later patient follow-up visits.
Tolia et al.19 demonstrated that although the rate of spontaneous resolution of type-II endoleaks was high (62.5%), an evaluation of the persistence and dimensions of a type-II endoleak is fundamental for prognosis. Similarly, in an evaluation of 348 EVAR patients followed for 10 years, Timaran et al.24 concluded that the maximum diameter of an endoleak is an important predictive factor for the increase in the size of an aneurysm in a patient with a type-II endoleak. They also established that endoleak sites with a diameter of 15 mm or more had a 10-fold greater chance of an increase in aneurysm size, which would justify a more aggressive therapeutic intervention. Because we encountered a tendency for an increase in the measurements during the venous phase compared to the arterial phase, our results reinforce the evidence that the venous phase is important for the precise evaluation of the dimensions of the endoleak site. In our patient series, the one case with an increase in the diameter of the aneurysm was associated with a type-II leak demonstrated only in the venous phase; it was later treated by a translumbar puncture.
This study is limited by the small number of cases and the lack of correlation with DA, which was not performed on the majority of patients due to the stability or reduction in the size of their aneurysms. Additionally, this study was based on the retrospective evaluation of measurements of aneurysm diameters and their necks that were obtained from preoperative film examinations and provided by different services using varying protocols. We believe that Duplex Scan could also be an option in clinical practice for EVAR follow-up; it is noninvasive and could help to reduce ionizing radiation exposure. However, further studies are needed to define its most suitable application for the purpose of delineating endoleaks.
In conclusion, the MDCT protocol in the late follow-up of patients undergoing EVAR must include the venous post-contrast phase, which depicted a significantly greater number of leaks compared to the arterial phase. The venous phase also more accurately demonstrated the niche dimensions compared to the arterial phase. The arterial phase should be avoided in patients with stable or decreasing aneurysm size, thereby reducing the radiation dose.
No potential conflict of interest was reported.
Bastos RM was responsible for the study design, collection, analysis and interpretation of data, statistical analysis, article writing, final approval, and overall procedures related to the study. Razuk Filho A was responsible for the study design, statistical analysis, and article revision. Blasbalg R was responsible for the collection, analysis and interpretation of data. Caffaro RA was responsible for the study design and final approval. Karakhanian WK was responsible for the data collection. Rocha AJ was responsible for the study design, statistical analysis, article revision, and final approval.