The initiative of the Spanish Ministry of Health “Commitment to quality of scientific societies”, aims to reduce unnecessary interventions of healthcare professionals.
MethodsThe Spanish Association of Surgeons has selected 22 experts from the different sections that have participated in the identification of 26 proposals “do not do” to be ordered by the expected impact its implementation would have according to the GRADE methodology. From these proposals, the Delphi technique was used to select 5 recommendations presented in more detail in this article.
ResultsThe 5 selected recommendations are: Do not perform cholecystectomy in patients with asymptomatic cholelithiasis; do not keep bladder catheterization more than 48h; do not extend antibiotic prophylaxis treatments more than 24h after a surgical procedure; do not perform routine antibiotic prophylaxis for uncomplicated clean and no prosthetic surgery; and do not use antibiotics postoperatively after uncomplicated appendicitis.
ConclusionThe Spanish Association of Surgeons's participation in this campaign has allowed a reflection on those activities that do not add value in the field of surgery and it is expected that the spread of this process serves to reduce its performance.
La iniciativa del Ministerio de Sanidad «Compromiso por la calidad de las sociedades científicas» tiene como objetivo disminuir las intervenciones innecesarias de los profesionales sanitarios.
MétodosLa Asociación Española de Cirujanos ha seleccionado a 22 expertos de las diferentes secciones que han participado en la identificación de 26 propuestas de «no hacer» que se ordenaron por el impacto esperado que tendría su puesta en marcha según la metodología GRADE. A partir de estas propuestas, se ha utilizado una técnica de Delphi para seleccionar las 5 recomendaciones más importantes en relación con el impacto potencial que tendría su aplicación.
ResultadosLas 5 recomendaciones seleccionadas son: no realizar colecistectomía en pacientes con colelitiasis asintomática; no mantener sondaje vesical más de 48h; no prolongar más de 24 h, tras un procedimiento quirúrgico, los tratamientos de profilaxis antibiótica; no realizar profilaxis antibiótica de rutina para la cirugía no protésica limpia y no complicada, y no emplear tratamiento antibiótico postoperatorio tras apendicitis no complicada.
ConclusiónLa participación de la Asociación Española de Cirujanos en esta campaña ha permitido una reflexión sobre aquellas actuaciones que no aportan valor en el ámbito de nuestra especialidad y es esperable que la difusión de este proceso sirva para reducir su realización.
Some years ago, the National Physicians Alliance in the US initiated a project called “Choosing wisely”.1 The purpose of the project was for scientific societies to create a list of 5 recommendations aimed at promoting a more efficient use of healthcare resources and the indication of diagnostic tests or treatments. Currently, 60 American scientific societies have contributed more than 200 key clinical recommendations to help promote practical improvement and avoid unnecessary medical interventions and those with potential risks.
Simultaneously, in 2007 the National Institute for Health and Care Excellence (NICE), while involved in the process of defining their guidelines, identified certain clinical practices that they recommend not to do,2 either because they provide no benefits, because the risk/benefit ratio is unclear, or because there is not sufficient evidence to recommend their systematic use. As of March 2014, the “Do not do” database contains 972 recommendations.2
In this context, in April 2013, the Spanish Ministry for Healthcare, Social Services and Equality started the project known as “Compromiso por la calidad de las sociedades científicas”, or “Commitment to quality of scientific societies”.3 This project is included within the activities of the Spanish Network of Agencies for the Evaluation of Healthcare Technologies, and its aim is to reduce the number of unnecessary interventions, defined as those that have not demonstrated efficacy, have little or uncertain effectiveness, or are not cost-effective. A total of 12 scientific societies proposed “Do not do” recommendations, and currently 39 medical societies have become associated.
The purpose of our study is to present the process and recommendations of what “not to do” that were finally selected by the Ministry of Health and the Spanish Association of Surgeons (AEC).
MethodsIn April 2014, a panel of 25 expert surgeons were selected, 22 of which finally participated. For the selection of the panelists, the AEC mainly based is criteria on clinical experience, in addition to also considering that their age and sex were representative of the sociodemographic profile of the society. Thus, as for age, 50% of the participants were between 36 and 50 years old, and 41% were between 51 and 65. In terms of years of experience, 82% had more than 15 years of experience and belonged to different sections of the AEC. The selected experts then created and assessed a preliminary list which was agreed upon by several members of each section. All the experts had previously signed a declaration of interests.
The first phase of the project was coordinated, according to a calendar agreed upon by Guía Salud (Spanish public healthcare entity) and the Quality Management section of the AEC, which collected and communicated to the different panelists the documentation that was generated over the course of this project.
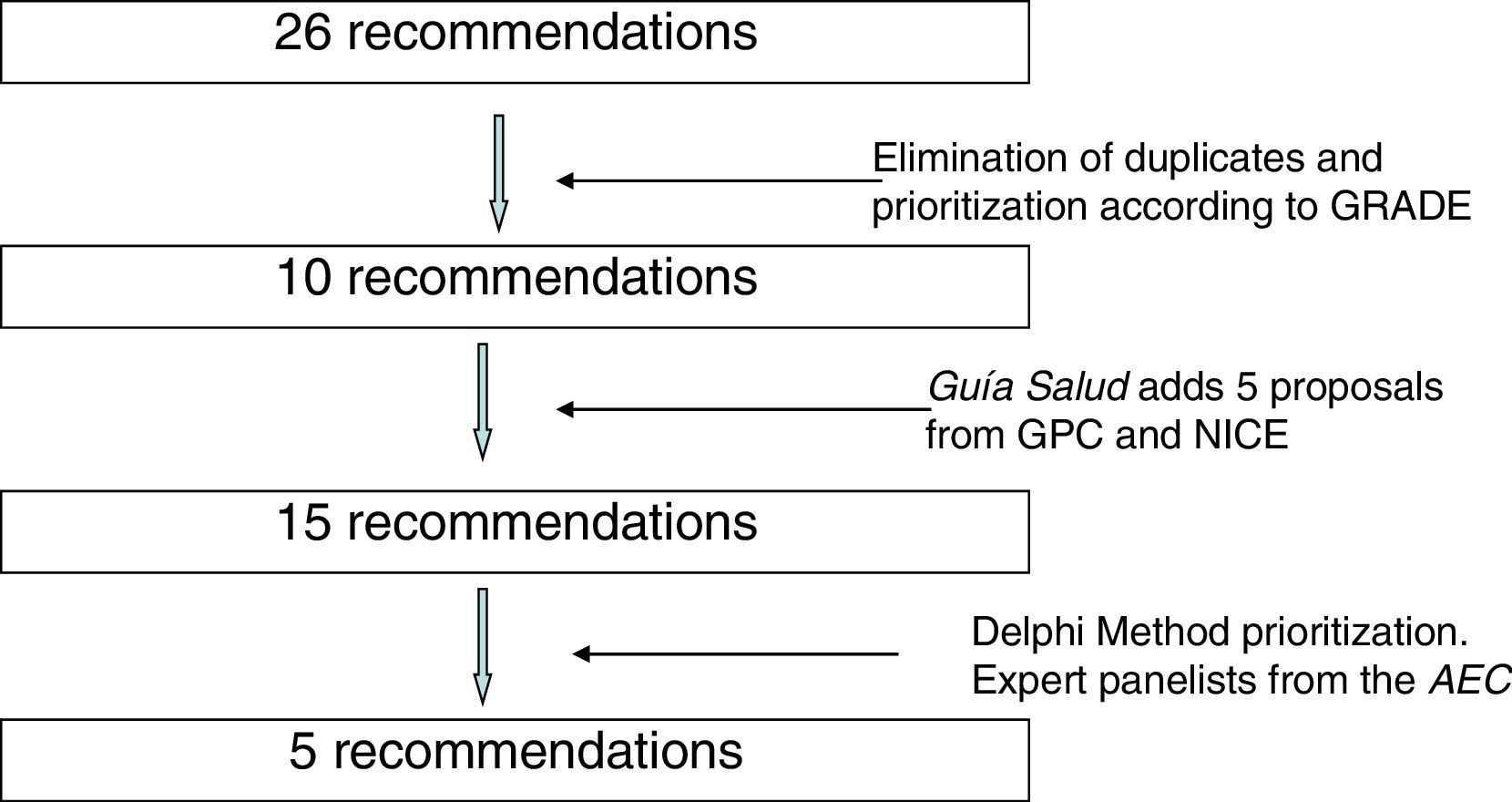
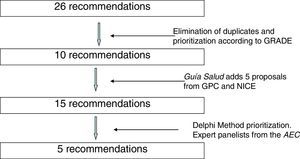
During the months of July and August 2014, 26 recommendations were compiled. An effort was made to avoid duplications, and the recommendations were prioritized by their expected impact according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.4 To this end, the literature was reviewed related with the benefits in health, safety and risks, validity, sensitivity and cost/effectiveness of the interventions. In this manner, 10 recommendations were selected that were sent to Guía Salud. Guía Salud surveyed the different panelists, who assessed 15 recommendations (10 from the AEC and another 5 that they themselves included) selected from the clinical practice guidelines of the Program of Guidelines of the National Healthcare System,5 the NICE “Do not do” database2 and other clinical practice guidelines.
The main statistics that were used in the Delphi technique6 are measures of central tendency and dispersion: median, interquartile range and mean. The median (central value after ordering the assessments of all the panelists) and the mean indicated the central tendency of the distribution or groups of answers by the experts. The interquartile range (IR) showed us the degree of dispersion in the responses. The following criteria assessment criteria were used: median (M): sufficiently high score to be selected or go on to the second round of circulation, between 7 and 9; insufficient score to be selected or go on to the second round of circulation, less than 7. Interquartile range (IR): low dispersion: IR<2; high dispersion: IR>2. If in the first survey round consensus had not been reached, a second circulation would have been done. In this round, the recommendation would be sent to each panelist, together with its score from the previous round, the median and the IR of the aggregate scores of the rest of the panelists in the first round. In the second round, only those recommendations with an average score >7 and IR<2 would be assessed. Consensus was reached when 5 recommendations were obtained with the highest median and the lowest interquartile range. For the analysis of the results, SPSS version 22 statistical software was used.
ResultsThe AEC panel took place between November 21 (launch date) and December 6, 2014 (end date). Two online reminders were sent to the panel on November 25th and 27th.
Twenty-two experts participated (from a total of 25), 17 men and 5 women. As for age, 50% were between 36 and 50 years of age, and 41% were between 51 and 65. In terms of years of professional experience, 82% had more than 15 years of experience.
Only one round was conducted since, out of the 15 recommendations scored, 5 recommendations were obtained with a median of 9 and an interquartile range of 1. Therefore, a second round was not necessary. The recommendation in position 6 presented an interquartile range higher than the first 5 (1.5). Table 1 shows a complete list of recommendations organized by median values, interquartile range and mean.
Recommendations, in Order by Median, Interquartile Range and Mean.
| Order | Recommendation | Median | IR | Mean |
|---|---|---|---|---|
| 1st | Do not perform cholecystectomy in patients with asymptomatic cholelithiasis. | 9 | 1 | 8.45 |
| 2nd | Do not use urinary catheters for more than 48h after gastrointestinal surgery. | 9 | 1 | 8.32 |
| 3rd | Do not prolong antibiotic prophylaxis for more than 24h after a surgical procedure. | 9 | 1 | 8.19 |
| 4th | Do not use routine antibiotic prophylaxis for clean, uncomplicated, non-prosthetic surgery. | 9 | 1 | 8.18 |
| 5th | Do not use postoperative antibiotic therapy after uncomplicated appendicitis. | 9 | 1 | 8.14 |
| 6th | Do not perform axillary lymphadenectomies for clinical stages I and II breast carcinomas with negative lymph nodes without having used the sentinel lymph node technique. | 9 | 1.5 | 8.05 |
| 7th | Do not use a femoral central catheter in patients that require prolonged intravenous therapy if it is possible to use the jugular or subclavian access. | 9 | 2 | 8.09 |
| 8th | Do not order routine preoperative studies, especially in healthy, asymptomatic patients. | 9 | 2 | 7.77 |
| 9th | Do not use antibiotic prophylaxis during elective cholecystectomy due to uncomplicated symptomatic cholelithiasis. | 8.5 | 2 | 7.95 |
| 10th | Do not create a stoma without preoperative site marking, preferably done by an expert in stomatherapy. | 8.5 | 3 | 7.50 |
| 11th | Do not routinely perform mechanical bowel preparation to prevent surgical wound infection. | 8 | 2 | 7.43 |
| 12th | Do not use plastic adhesives to protect and stabilize the surgical field. | 8 | 3 | 7.55 |
| 13th | Do not perform elective surgery on all asymptomatic inguinal hernias to prevent clinical situations of complicated hernias (incarceration or strangulation). | 8 | 3.25 | 7.05 |
| 14th | Do not routinely place dressings over surgical wounds with primary closure as a measure for preventing infection of the surgical site. | 7.5 | 3 | 7.36 |
| 15th | Do not routinely remove body hair to reduce the risk of infection of the surgical site. | 7 | 2 | 7.18 |
Median: 7 to 9=sufficiently high score to be selected or go on to the second round; less than 7=insufficient score to be selected or to go on to the second round. Interquartile range (IR): low dispersion, IR<2; high dispersion, IR>2.
Fig. 1 shows the process of incorporation and prioritization of the recommendations.
The 5 selected recommendations used in the previously described process are:
- 1.
Do not perform cholecystectomy in patients with asymptomatic cholelithiasis.
- 2.
Do not use urinary catheters for more than 48h.
- 3.
Do not prolong prophylactic antibiotic treatments for more than 24h after surgery.
- 4.
Do not administer antibiotic prophylaxis on a routine basis for clean, non-prosthetic and uncomplicated surgery.
- 5.
Do not use postoperative antibiotic treatment after uncomplicated appendicitis.
The main objective of the collaboration project between the scientific societies and the Ministry of Healthcare is to reduce the use of unnecessary medical interventions, meaning those that have no demonstrated efficacy, have limited or uncertain effectiveness, are not cost-effective or are not a priority. Secondary objectives include reducing variability in clinical practice, diffusion among physicians and patients to guide the decision-making process, proper use of healthcare resources and, last of all, promotion of clinical safety and reduction of iatrogenesis. The following is an explanation of the 5 recommendations selected.
Do Not Perform Cholecystectomy in Patients With Asymptomatic CholelithiasisAsymptomatic cholelithiasis is the presence of gallstones that may be detected incidentally in patients that do not present any abdominal symptoms, or by palpation of the gallbladder during surgery for another reason.
There are no clinical trials that have evaluated the benefit of cholecystectomy in asymptomatic patients, nor have any prospective studies analyzing the clinical evolution of asymptomatic cholelithiasis demonstrated the efficacy of cholecystectomy. Approximately 0.7 and 2.5% of patients with asymptomatic cholelithiasis develop cholelithiasis-related symptoms each year, and the annual incidence of complications like cholecystitis, acute pancreatitis, obstructive jaundice or cholangitis is 0.1%–0.3%.7 Cholecystectomy in patients with asymptomatic cholelithiasis does not increase life expectancy because the risk of surgery in terms of mortality and morbidity is higher than the complications of cholelithiasis itself.8 In addition, the costs are lower if patients with gallstones are not treated until they present symptoms or some type of complication.9 Likewise, diabetic patients would also not benefit from prophylactic cholecystectomy.10
In Western countries, where the prevalence of gallbladder carcinoma is very low,11 cholecystectomy is not justified in general.12 It is estimated that, depending on additional risk factors, between 67 and 769 cholecystectomies would have to be done to avoid one case of gallbladder cancer.13 Some cases, however, deserve special consideration. In asymptomatic patients with porcelain gallbladder, cholecystectomy could be indicated due to the possibility of developing gallbladder cancer, although in the most recent studies the correlation between porcelain gallbladder and cancer has not been clearly established.14 As for gallbladder polyps, according to the recently published guidelines of the European Association for the Study of the Liver,15 cholecystectomy is recommended in patients with polyps ≥1cm with or without lithiasis, regardless of patient symptoms. Cholecystectomy should also be considered in patients with asymptomatic cholelithiasis and polyps between 6 and 10mm and in cases of polyp growth. Cholecystectomy is also recommended in asymptomatic patients with sclerosing cholangitis and gallbladder polyps, regardless of size. Nonetheless, cholecystectomy is not indicated in patients with asymptomatic cholelithiasis and polyps ≤5mm.
In the same manner, cholecystectomy is not recommended in patients with asymptomatic cholelithiasis treated with another surgical intervention, including bariatric surgery.15 Cholecystectomy should be considered in patients with spherocytosis or sickle-cell anemia if splenectomy is going to be performed.
In short, although there are no prospective studies comparing surgical intervention with conservative treatment for patients with asymptomatic cholelithiasis, routine cholecystectomy should not be recommended.
Do Not Use Urinary Catheters for More Than 48hThe increased number of surgical procedures and the need to monitor diuresis have made urinary catheterization a routine practice in a large number of patients. Occasionally, however, the duration of the catheterization is prolonged with no justification. Most urinary tract infections (UTI) occur in the presence of instrumentation, especially after catheterization.16 If urinary catheter use is prolonged for more than 4 days, the prevalence of bacteriuria is virtually 100%, with up to 20% UTI.17 Furthermore, prolonged catheterization also indirectly interferes in the recovery of surgical patients. The current tendency is to avoid catheterization or to minimize its use.18 The literature about the withdrawal of urinary catheters in the postoperative period is limited, but it has not been demonstrated that systematically maintaining catheterization provides any benefit. The existence of scheduled alerts and the implementation of protocols that program and justify the need for catheterization are associated with reduced UTI rates.19,20 Not many studies have focused on the early withdrawal of urinary catheters, but one prospective cohort study in the US analyzed the advantage of justifying and optimizing the use of urinary catheters in colorectal surgery, observing a very significant reduction in the incidence of UTI when these aspects were monitored.21 In conclusion, the lack of scientific evidence to recommend maintaining urinary catheterization for more than 48h in an ordinary postoperative situation make it an avoidable measure.
Do Not Prolong the Administration of Antibiotic Prophylaxis for More Than 24h After a Surgical ProcedureOne of the principles of antibiotic prophylaxis (AP) in surgery is not extending the infusion of antibiotics beyond the first 24h.22 In spite of this, audits about the compliance with prophylaxis protocols have shown that the most frequent error made is precisely excessive duration.23 Clinical studies have been unable to demonstrate any benefits of the prolonged administration of antibiotics beyond the preoperative dose. In a meta-analysis of 25 general surgery studies,24 surgical site infection (SSI) rates did not decrease with the prolongation of AP after surgery.24 The same results were observed in several meta-analyses focused on bowel surgery.25 Systematic reviews that focus on other types of surgery have confirmed identical results. In a cohort study, the administration of AP>48h was not associated with a lower SSI (OR 1.2; CI: 0.8–1.6), but there was an associated higher risk for bacterial resistance (OR 1.6; CI: 1.1–2.6).26 The epidemic increase of infections by Clostridium difficile has been related with the increased use of antibiotic therapies and, specifically, prolonged AP.27 In conclusion, the prolongation of prophylaxis beyond 24h cannot be justified. Current clinical guidelines insist on limiting AP to a single preoperative dose or, at the very most, treatment within the first 24h post-op.28,29 Extending AP increases pharmacological toxicity, costs, risk for bacterial resistance and postoperative infection by C. difficile.
Do Not Use Routine Antibiotic Prophylaxis for Clean, Uncomplicated, Non-prosthetic SurgeryIn general, AP is indicated when the probabilities of infection are high or when the consequences of a postoperative infection are potentially serious. The indication of AP in this surgery depends on the type of operation, patient comorbidities and the existence of prosthesis. In clean, non-prosthetic surgery lasting less than 2h without a lot of tissue attrition, the use of prophylaxis is not necessary. Table 2 summarizes the situations in which AP is not necessary in surgery.30 Contrarily, AP would be indicated when the effects of infection are very severe or irreversible (infection of a mesh or vascular access device) or when there are relevant risk factors, such as obesity or immunosuppression. In hernia surgery, meta-analyses found a protective effect of AP in hernioplasty (OR 0.61; 95% CI: 0.40–0.92),31 so it is therefore recommended in inguinal hernia repair and, by inference, abdominal hernioplasties or abdominoplasties.32 In breast surgery, AP is indicated when implants are inserted for esthetic reasons, in reconstruction due to neoplasm and breast reduction.33 AP is also effective in oncologic surgery without reconstruction, with an evident reduction of the incidence of SSI (RR 0.67; 95% CI: 0.53–0.85).34 AP does not significantly reduce SSI in clean surgery of the head and neck (2.4 with AP versus 3.7% in control groups; OR 0.49; 95% CI: 0.19–1.23; P=.13), so it is not routinely recommended.35 In conclusion, AP should not be administered in short (<2h) clean surgeries with no implantation of prosthesis, in patients with good immune response or in those in whom the consequences of SSI would not be catastrophic.
Do Not Use Postoperative Antibiotic Treatment After Uncomplicated AppendicitisThe definition and use of the concept “uncomplicated” refers to the initial appearance in clean-contaminated surgery in which the focus is eliminated and, therefore, only requires a single dose of antibiotic prophylaxis.36 Uncomplicated appendicitis has no abscess, no perforation and the patient presents no peritonitis.37 Currently, there is no evidence demonstrating that prolonged antibiotic treatment after acute uncomplicated appendicitis provides any sort of benefit, not even for the prevention of surgical site infections.38,39 Thus, the recommendation to not use antibiotics in the postoperative period of an uncomplicated appendicitis is mainly based on the absence of evidence recommending its use. Rational use that respects this recommendation would generate savings in medication costs, and probably also for hospitalization, by avoiding prolonged hospital stay for the administration of the antibiotic itself, the generation of resistances and the prevention of complications.40,41 There are few reports in the literature, although they provide very interesting data in favor of no antibiotic use and no data in favor of their systematic use. In this context, there are very extensive observational cohort studies comparing the incidence of any type of infection depending on the use or not of postoperative antibiotics, with no observed differences.42 There are 2 randomized clinical trials that have studied the subject. One found no differences in the appearance of infectious complications after comparing 7 days of antibiotic treatment versus placebo in the postoperative period of 756 patients with appendicitis of all types.43 Another compared one dose versus 3 and versus 5 doses in 269 cases of uncomplicated appendicitis, and, once again, there were no differences in the appearance of infectious complications or in hospital stay; however, the number of diarrheas was significantly greater when 5 doses were administered.44 Therefore, there is currently no scientific evidence to recommend the use of postoperative antibiotics for uncomplicated appendicitis.
We can conclude that the “what not to do” program, developed by the Ministry of Health, informs healthcare professionals which medical actions do not add value to the healthcare process. This program is based on the selection of the best evidence available to avoid exposing patients to potential risks and to improve the adaptation of healthcare resources. The participation of AEC in this campaign has provided reflection on which interventions contribute no added value in our specialty, and it is expected that the communication of this identification and selection process will lead to improved patient care.
FundingThis article was completed without the support of any company, either public or private.
Authorship/collaboratorsThe 4 authors have contributed to the study design and data collection. Likewise, the 4 authors have contributed to the composition of the article, critical review and approval of the final version.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to acknowledge the Board of the Spanish Association of Surgeons (AEC) for their support and the members of the panel of experts and collaborators for their participation in the Delphi study.
Dr. Raquel Sanchez Santos
Dr. Elena Fernández Martín
Dr. Lourdes Sanz Alvarez
Dr. Victor Soria Aledo
Dr. Carlos Emparan Garcia De Salazar
Dr. Elias Rodríguez-Cuellar
Dr. Manuel Romero Simo
Dr. Roger Cabezali Sanchez
Dr. Pere Rebasa Cladera
Dr. Julio Cesar Jordan Balanzá
Dr. Josep M Badia Pérez
Dr. José Ma Balibrea del Castillo
Dr. Susana Ros López
Dr. Guzman Franch Arcas
Dr. Juan Manuel Martos Martínez
Dr. Arturo Soriano Benitez de Lugo
Dr. Juan Manuel Bellón Caneiro
Dr. Rosa Carmen Fernández Lobato
Dr. José Vicente Roig Vila
Dr. Francisco Sánchez Bueno
Dr. Eduardo Ma Targarona Soler
Dr. José Ma Jover Navalón
Professor José Luis Balibrea Cantero
Dr. Luis Grande Posada
Dr. Mario Javier de Miguel Velasco
Please cite this article as: Soria-Aledo V, Romero Simó M, Balibrea JM, Badia JM. Recomendaciones de «no hacer»: propuestas de la Asociación Española de Cirujanos al proyecto de «Compromiso por la calidad de las sociedades científicas». Cir Esp. 2016;94:453–459.
Part of the content of this article was presented at the National Meeting for Surgery on October 22, 2015 in Granada (Spain) under the title: “Commitment to Quality Project”