This study presents the 51 mixtures of ceramic clays characterized by using XRF, XRD, granulometry, and dilatometry analyses. After firing in a 1000–1250°C range, water absorption (WA) according to EN standards by boiling in water, under vacuum, and by 24h soaking is determined. The results indicated that there was a high and statistically significant correlation between the standard methods, but the testing under vacuum gave the highest saturation of the samples fired at 1200°C and 1250°C. It is determined that these illitic-kaolinitic clays can be used to produce floor ceramic tiles belonging to the BIIa group (water absorption between 3% and 6%).
The study also aimed to reveal which method of WA determination is suitable to read the sintering interval from the gresification diagrams, which is compared to the beginning of sintering as read from dilatometry curves.
Este estudio muestra la caracterización de 51 mezclas de arcillas cerámicas, mediante análisis XRF, XRD, granulometría y dilatometría. Después de calentar las muestras en el intervalo de 1.000-1.250°C, se ha determinado la absorción de agua (WA), de acuerdo con las normas EN mediante ebullición en agua, al vacío y en remojo durante 24 horas. Los resultados confirman que existe una correlación estadística alta y significativa entre los diferentes métodos estándar, aunque los ensayos a vacío dieron la mayor saturación de las muestras calentadas a 1.200 y 1.250°C. Se ha determinado que estas arcillas ilítico-caoliníticas pueden ser utilizadas para la fabricación de baldosas cerámicas de suelo pertenecientes al grupo BIIa (absorción de agua entre 3 y 6%).
El estudio también pretendía demostrar qué método de determinación de WA es adecuado para establecer el intervalo de sinterización, que se compara con el comienzo de la sinterización observado según las curvas de dilatometría.
Ceramic tiles are one of the most important products in the construction sector. Their quality is tested by the standard methodologies, consisting of the obliged and additional methods of the test according to EN standards. The important differentiation between ceramic tiles is based on water absorption (WA), which also defines the applicability of ceramic tiles. There are the tiles belonging to different groups based on WA determined by boiling: ≤0.5%, then 0.5–3%, 3–6%, 6–10%, and ≥10% [1,2]. According to SRPS EN ISO 10545-3, there are the two relevant test methods for determination of WA: absorption by boiling and under vacuum [3,4]. The newer EN standard [4] no longer allows to choose between the two, but only keeps the method in a vacuum. In the future, the standard is expected to be divided into two parts, according to the planned revision which is under voting by the European Cometee for Standardization (CEN), one containing the boiling method and the other the vacuum method.
The method by boiling is persistent in the ASTM standard in the case of extruded tiles [5]. Since the same EN standard proposed two methodologies [3], it was expected that both tests give similar results. However, there are a few studies that concluded the opposite. One study claims that in all available standard methods, both ASTM C373 and EN ISO 10545-3 [3,5,6], the open pores in porcelain tiles are not completely saturated. Porcelain tiles are considered to be those with WA ≤0.5%. The authors propose that the most reliable method is the EN test under vacuum, but the samples should soak longer than prescribed and that is up to 5 days. Another study compared the 3 methods in ceramic tiles with the range of WA values (up to about 14.5%), which covers all types of ceramic tiles. The authors revealed that all the methods give similar results, except in the WA range of 0.25–3.5%, where the ASTM standard method gives higher saturation. Generally, the best correlation is noticed among ASTM boiling and EN vacuum methods. It seems that the time of soaking in water after the initial testing can also significantly influence the results since ASTM standard demands it to be 24h, but according to EN the time is the one needed for the samples to cool after boiling [6]. The authors of this study believe this should be further investigated.
In this study, the naturally occurring illitic-kaolinitic clays were used to produce the laboratory samples: the discs were dry-shaped and the tiles were wet-pressed. This study aimed to compare the methods for water absorption determination in the samples according to SRPS ISO EN 10545: by boiling and under vacuum [3,4]. Additionally, water absorption is determined by immersion in cold water for 24h according to the method used in clay bricks [7]. Although the active EN standard [4] proposes only the WA determination under vacuum, there are not many available studies on comparisons of the two methodologies, especially in different firing temperatures. This study aimed to reveal the usefulness of the studied illitic-kaolinitic ceramic clays in terms of water absorption and firing temperatures and propose their future use in the industry of ceramic products.
An additional concern was the specific usage of the gresification diagram in the Balkans geological rule books on the classification and categorization of mineral raw materials [8–10]. The gresification diagram, which simultaneously presents the linear shrinkage and water absorption, depending on the firing temperature, is presented in many studies [1,11–18]. The temperature at which the maximum shrinkage is reached is considered a moment of maximum densification [19]. In the previous studies, the shrinkage increases up to 1250°C [18,20,21], reaches the plateau between 1150°C and 1250°C [17], or suddenly drops at about 1180°C [19]. In some of the studies, the plateau in shrinkage is reached, after which the shrinkage drops at about 1125°C up until 1275°C [12,14,22]. The maximum shrinkage occurred while increasing closed porosity by the liquid phase at the expense of a decrease in the open porosity. The quantity of the liquid phase depends on the quantity of low-temperature-melting feldspars [23].
In the study that tested the ceramic paste suitable for wall tiles production, firing shrinkage increases after the initial plateau, when open porosity decreases since liquid phase content increased, while more dense compounds occur from metakaolin (gehlenite and anorthite). In this case, maximum densification could be reached at very high temperatures [11]. These changes in firing shrinkage show the shifting of mechanisms from solid-state to the liquid phase sintering [24].
It is believed that the temperature read from the gresification diagram at which 6% of WA occurs is a so-called ceramics’ clinkering point and that in 2% or 3% WA is the sintering point, which determines the sintering temperature range [21,25,26]. The clinkering temperature is considered as the temperature at which the ceramic body reaches the water absorption of the clinker brick (≤6%) [27–29]. The Balkans rule books do not specify which method for determination of WA is suitable [8–10], nor the literature explains why these concrete values of WA are chosen to read these temperatures. This study reports and explores some of the details of this methodology.
Materials and methodsThe mixtures tested were formed of 51 illitic-kaolinitic ceramic clays from the tertiary basin near Šabac, Serbia, which is not yet in the exploitation phase but is an object of interest. The labels from C-1 to C-51 were used to distinguish composite samples. The raw samples were dried in an oven at 105°C to constant mass and subsequently dry-ground in a planetary mill. Afterward, the samples were sieved and the fraction below 0.5mm is used to ensure homogeneity for further testing. The entire amount of the samples was ground, and there were no rejections. The fraction was somewhat finer than the one previously used [14].
The characterization of the clays was done on multi-levels to determine the relevant features as described below.
The chemical compositions were measured using the energy dispersive X-ray fluorescence (XRF) technique (Spectro Xepos instrument with 50W/60kV X-ray tube). The method for clay chemical testing was calibrated using a fundamental parameter approach. It is based on the theoretical relation between the measured X-rays’ intensity and the concentration of elements in the sample. This calibration is made by the manufacturer but allows the possibility of adjusting the calibration curves based on the testing of certified reference materials. The reference materials were: NCS DC 60102 and set of the ten reference material (from JRRM 302 to JRRM 310).
The certified reference materials were used for validation of the method (NCS DC 60104, NCS DC 60105, and NCS DC 60106).
X-ray diffraction analysis (XRD) was carried out to identify the minerals present in the mass using the Philips 1050 X-ray powder diffractometer with Ni-filtered CuKα radiation (λ=1.5418nm) and Bragg–Brentano focusing geometry. The patterns were taken in the 6°–90° 2θ range with the step of 0.05°, while the exposure time was 6s per step. The identification of the minerals was based on the Inorganic Crystal Structure Database. Semi-quantitative analysis was obtained by the built-in program (DIFRAC EVA V.9.0) which uses the stoichiometric calculations presented in the literature [30]. The results obtained by the semi-quantitative analyses were within the measurement errors of the chemical composition.
Particle size distribution is determined on the raw materials (before grinding) by the combined method of hydrometry (fractions below 0.063mm) and wet sieve analysis according to the procedure described in the standard [31]. The samples pre-dried at 105°C were sieved through the standard sieves, and the remains on each sieve were measured with an accuracy of 0.1wt.%. Fractions below 0.063mm were done by the hydrometric method, while the solution of sodium hexametaphosphate was used as a dispersing agent (anticoagulant) of the fine-grained particles. The particle size ranges for the indicated particle sizes classes were as follows: clay (<0.002mm), alevrolite (0.002–0.06mm), and sand (0.06–2mm).
Dilatometry tests are done using the Setaram instrument for thermo-mechanical analysis, in the air atmosphere with a flow of 20ml/min. The calibration curve was recorded on the same equipment and conditions without a sample. The heating was done by the heating speed of 20°C/min until 1000°C, and the soaking time was 1h.
The samples were dry-pressed to discs (50mm in diameter) and wet-pressed to tiles (25×120mm2) using a laboratory hydraulic machine and pressure of 400kg/cm2. The molds were designed to mark the place where the measuring of dimensions is to be conducted by the furrows. The tiles (25×120mm2) of 51 composite samples mostly dissipated and cracked around the blades formed by the furrows in molds after dry-pressing. After the trial-error procedure, the addition of 4wt.% of water was sufficient to gain smooth-surface and stable dry samples. The reasons lie in the fact that the moist materials can be more homogeneously mixed and that better packing of the grains is achieved since the water gives a lubricating effect during pressing [32,33]. The quantity of added water was lower than in other studies [11,14,18,19,32–38] thus minimizing the energy required to dry the products before firing.
After drying, the firing was conducted in an oxidizing environment electric oven at 1000°C, 1100°C, 1200°C, and 1250°C, with 1h soaking at the final temperature and natural cooling in a closed furnace (firing regime was as follows: 70°C/h until 200°C, then 92°C/h up to 520°C, afterward 60°C/h up until 610°C, and finally 140°C/h until the final temperature). The soaking time of 1h was chosen to avoid overfiring [39]. The reported firing regimes used during the firing of ceramic tiles range from 15°C/min to 36°C/min, and soaking times range from 5min to 5h [18,40–43]. The number of samples of all shapes and firing temperatures was 5, as requested by the standard [3,4].
Bulk density (BD) and water absorption (WA) were determined in the usual ways [3,4,41,44]. All of the measurements of weight and dimensions were conducted on a scale with 0.01g and a caliper with 0.01mm precisions. Water absorption under vacuum is performed using the apparatus Isovacum A 2012, produced by Gabtech, Italy. The modulus of rupture was determined by using Crometro CR4/E1 Gabrielli machine (resolution 1N, with a range up to 7kN, and a force increase of 1N/s).
The tests used for WA methods evaluation were the sum of squares and the coefficient of determination. The possible outliers were tested using Mahalanobis distance [45].
Results and discussionProperties of the raw materials and fired samplesTables 1 and 2 show the main characteristics of the studied ceramic clays. The samples were of the illitic-kaolinitic type, with relatively high contents of quartz, and in most of the cases satisfactory content of iron for gray (light) firing color. The stoichiometric formulas of the minerals present are illite – (K,H3O)Al2Si3AlO10(OH)2, kaolinite – Al2Si2O5(OH)4, muscovite – KAl3Si3O10(OH)2, and microcline – KAlSi3O8. However, the structural phase analysis is not sensitive to the relatively small presence of different ions in the mineral composition. For example, sulfur can only be seen if it is in its elemental state and if its presence is greater than 1%. Therefore, the minerals in which Ca, Mg, and Na are present were not determined.
Macro-oxides wt.% contents in the mixtures.
| LOI | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | P2O5 | MnO | TiO2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 4.18 | 62.23 | 19.11 | 1.03 | 0.08 | 0.94 | 0.20 | 2.68 | 0.01 | 0.03 | 0.00 | 0.57 |
| Max | 6.79 | 68.96 | 22.91 | 2.67 | 0.30 | 1.42 | 0.46 | 4.24 | 0.94 | 0.11 | 0.02 | 0.82 |
| Average | 5.49 | 66.42 | 20.65 | 1.58 | 0.19 | 1.21 | 0.28 | 3.16 | 0.03 | 0.04 | 0.01 | 0.71 |
| StDev | 0.58 | 1.48 | 0.85 | 0.32 | 0.05 | 0.09 | 0.05 | 0.28 | 0.13 | 0.01 | 0.00 | 0.05 |
Semi-quantitative phase analysis and particle size distributions.
| Quartz (wt.%) | Illite (wt.%) | Kaolinite (wt.%) | Muscovite (wt.%) | Microcline (wt.%) | Clay fraction (wt.%) | Allevrolite fraction (wt.%) | Sand fraction (wt.%) | |
|---|---|---|---|---|---|---|---|---|
| Min | 39.10 | 16.00 | 5.30 | 6.40 | 2.20 | 6.00 | 44.00 | 14.00 |
| Max | 62.20 | 37.90 | 14.10 | 14.10 | 8.60 | 31.00 | 69.00 | 38.00 |
| Average | 50.55 | 25.46 | 8.94 | 8.87 | 4.93 | 20.20 | 53.75 | 26.06 |
| StDev | 5.91 | 5.05 | 2.11 | 2.14 | 1.25 | 5.33 | 5.65 | 5.08 |
The chemical content of the clays was somewhat similar to illitic clays from the previous study [1], but the content of quartz was higher, and that of clay minerals was lower. LOI values were a bit higher than ideally, containing both organic matter and low amounts of carbonates. Black cores formed due to the presence of organic matter and a partially reducing atmosphere during firing weakened the products [46]. Mineralogically, the clays consisted of quartz, illite, kaolinite, muscovite, and feldspar (microcline). Granulometry analyzes showed that the clays contained mostly of the alevrolite-sized fraction (Table 2.). The highest differences in the samples were in the quantity of quartz and illite and that the clay fraction quantity significantly varied. The sample C-7 contained the highest quantity of clay minerals, and on the contrary, in the C-50 the highest content of quartz among all the samples is observed.
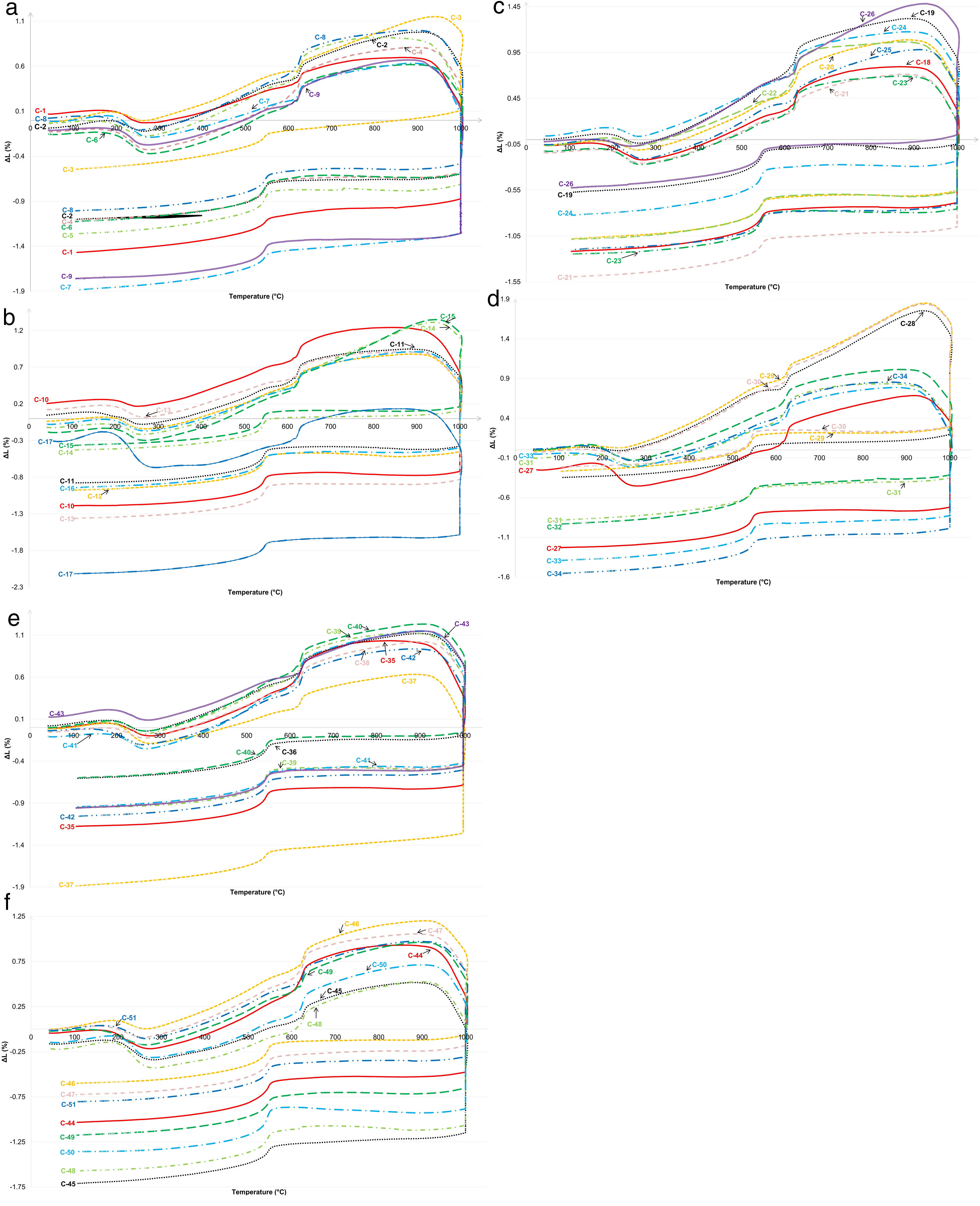
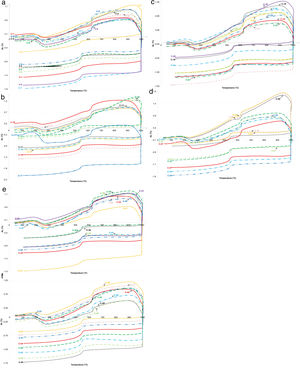
The dilatometry analyzes are shown in Fig. 1. Most of the samples underwent similar patterns during firing typical to illitic clays [47]. The samples suffered a slight spread up to about 180°C, due to the loss of adsorbed water [1]. Thereafter, a sudden shrinkage to a temperature of 270°C occurred. The highest shrinkage in this period was detected in the samples C-17 and C-27 (Fig. 1b and d). Following was the continual expansion up until 620°C, which could indicate the conversion of kaolinite to metakaolinite and loss of chemically bound water [1]. After a short intensive shrinkage until about 630–640°C due to dehydroxylation of clay minerals, mostly a slight shrinkage took place in the samples until a period without dimensional changes is reached up to 880°C at the latest, which could be attributed to the completion of dehydroxilation process and solid-state sintering [47]. The exceptions are noticed in the case of C-3, C-14, C-15, C-26, C-28, C-29, and C-30 samples, in which the period without dimensional changes is reached by rapid expansion by 915–935°C, when the vitrification started [1]. Significant quantities of illite (about 30%) caused the shrinkage to start mostly above 900°C, when sintering, the formation of vitreous phase, decarbonization, and recrystallization of the new phases occurred [48]. The mentioned samples were those which showed the lowest total shrinkage at the end of testing.
Dilatometric curves of ilitic-kaolinitic ceramic clays: (a) Composites C-1 to C-9, (b) Composites C-10 to C17. Dilatometric curves of ilitic-kaolinitic ceramic clays: (c) Composites C-18 to C-26, (d) Composites C-27 to C-34, (e) Composites C-35 to C-43, and (f) Composites C-44 to C-51. Dilatometric curves of ilitic-kaolinitic ceramic clays: (e) Composites C-35 to C-43, and (f) Composites C-44 to C-51.
During the cooling period, all samples collected, while a peak resulting from quartz conversion is noticeable around 560°C. The total shrinkage at the end of the study was between 0.21% and 2.12% (average 1.05%). The lowest overall shrinkage exhibited the sample C-17 that contained the highest quantity of quartz (Fig. 2). An increased shrinkage is expected in the samples containing higher amounts of fluxes [18], which was not the here presented case since the increased fluxes content was observed in the sample C-29 which shrank very low (0.22%). The somewhat higher amount of quartz and illite in these samples indicated the lower shrinkage [1]. Low shrinkage in the production of ceramic tiles is very important since it affects the reduced occurrence of contractions and therefore the stress during the firing phase [49].
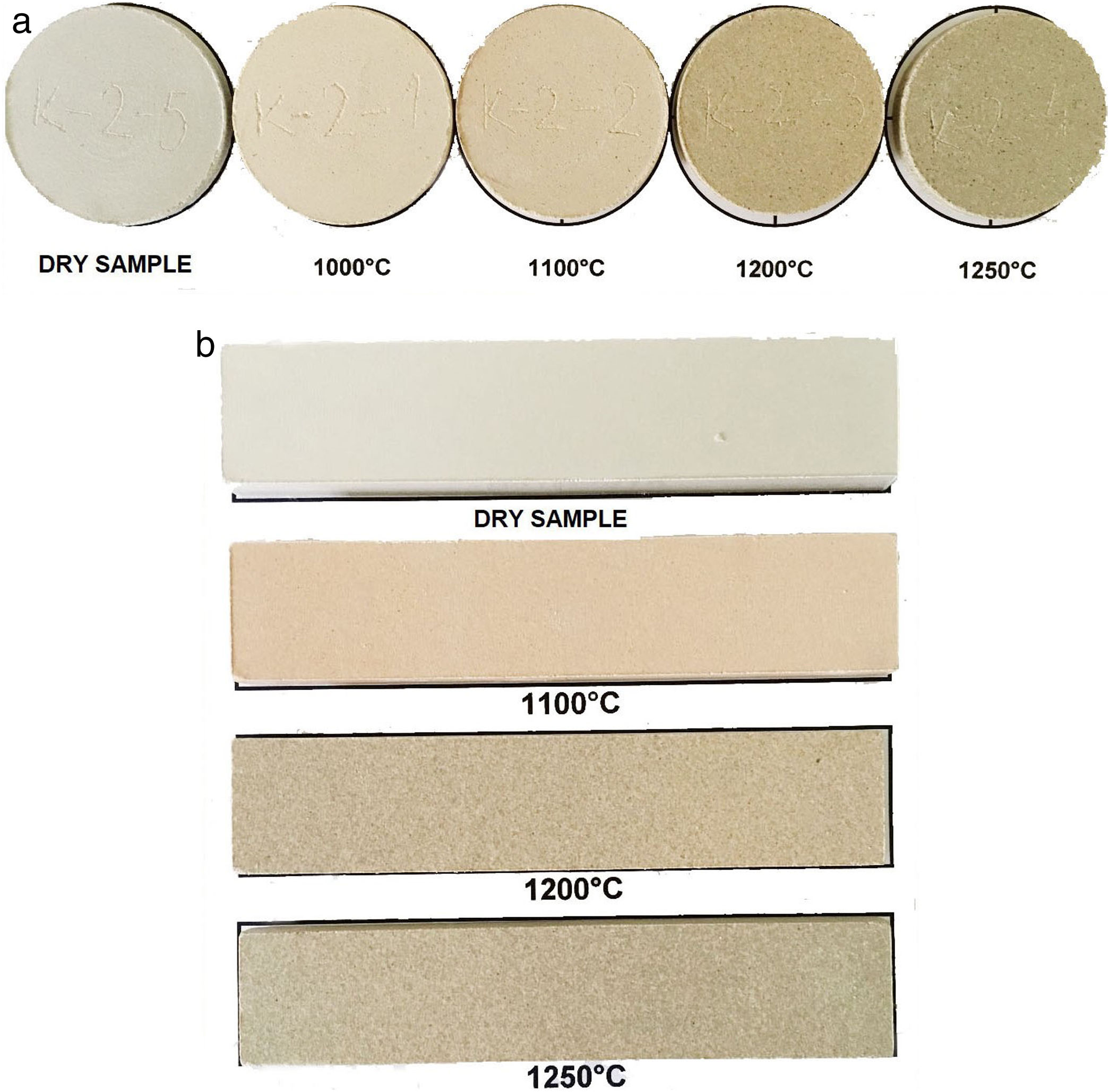
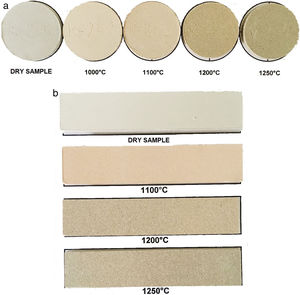
All of the samples belonged to the white-firing clays as stated by Piltz [50]. According to C. E. C. card, the colors ranged from A1 to D6 (pale red to gray by increasing the firing temperatures). At the firing temperature of 1250°C, the colors were A7/A8 in most of the cases [51]. The red color diminishes after firing at 1200°C and 1250°C due to the formation of mullite from kaolinite, which hosted Fe3+ in the structure [35,46]. Fig. 2 presents the appereance of the samples in the shape of discs (C-2) and tiles (C-17). In principle, a Fe2O3 content of 3wt.% is considered as the boundary separating light-fired and dark-fired clays [52], but in practice, it is often required that the raw material for ceramic tiles contains less than 2wt.% of this oxide.
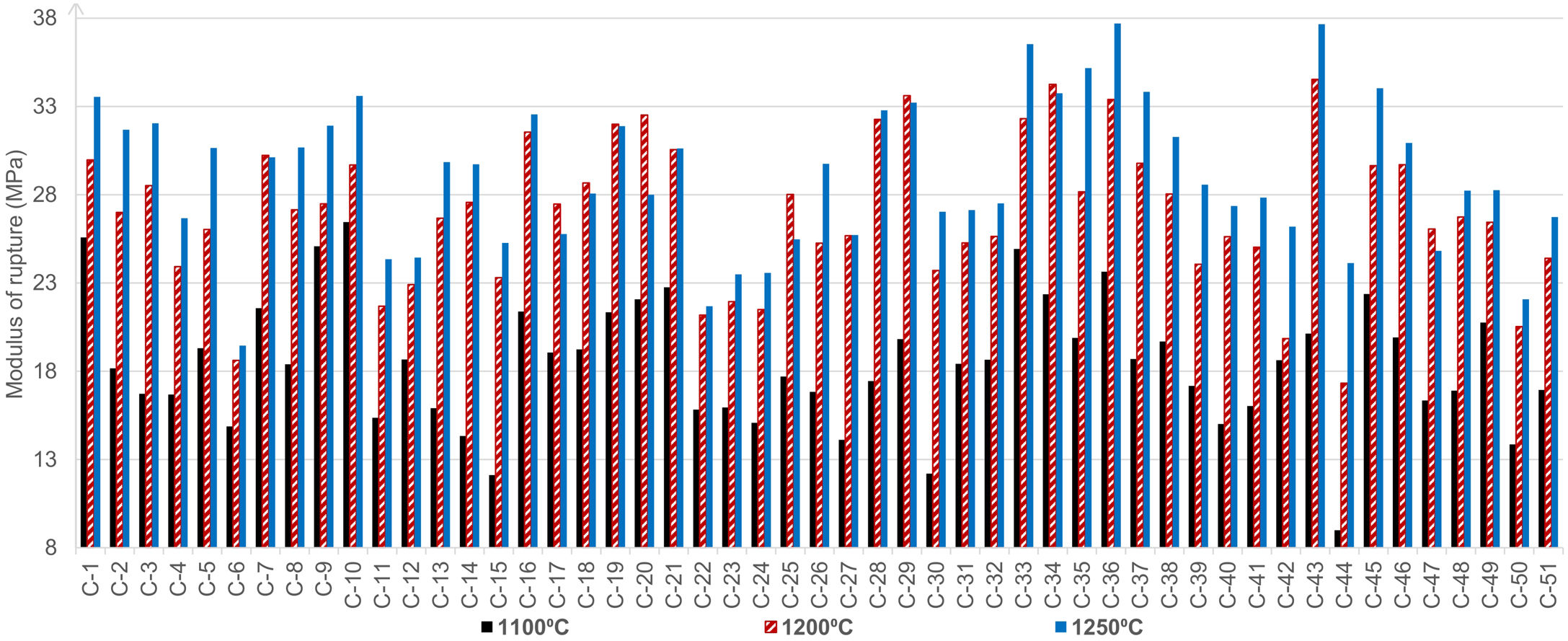
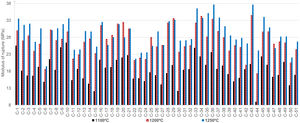
Bulk densities of the obtained products showed the lowest differences while firing the discs and tiles at the peak temperature of 1000°C (Table 3). The highest obtained densities were observed in the case of wet-pressed tiles fired at 1250°C, as expected. The sample C-50, containing the lowest quantity of clay minerals and the highest content of quartz, showed the lowest BDt while firing at 1250°C. Fig. 3 presents the obtained modulus of rupture of all the tested composite samples. The modulus of rupture mainly increased with temperature, and the values are comparable to the literature data for ceramic tiles [53].
Bulk density at different firing temperatures for discs and tiles.
| BDd (g/cm3) | BDt (g/cm3) | |
|---|---|---|
| 1000°C | ||
| Min | 1.86 | 1.84 |
| Max | 2.11 | 2.15 |
| Average | 1.97 | 1.99 |
| StDev | 0.04 | 0.06 |
| 1100°C | ||
| Min | 1.95 | 2.05 |
| Max | 2.22 | 2.29 |
| Average | 2.07 | 2.19 |
| StDev | 0.04 | 0.05 |
| 1200°C | ||
| Min | 2.04 | 2.23 |
| Max | 2.39 | 2.45 |
| Average | 2.22 | 2.35 |
| StDev | 0.07 | 0.05 |
| 1250°C | ||
| Min | 2.08 | 2.27 |
| Max | 2.39 | 2.49 |
| Average | 2.26 | 2.38 |
| StDev | 0.06 | 0.06 |
BDd – bulk desity of the dry-pressed discs.
BDt – bulk desity of the wet-pressed tiles.
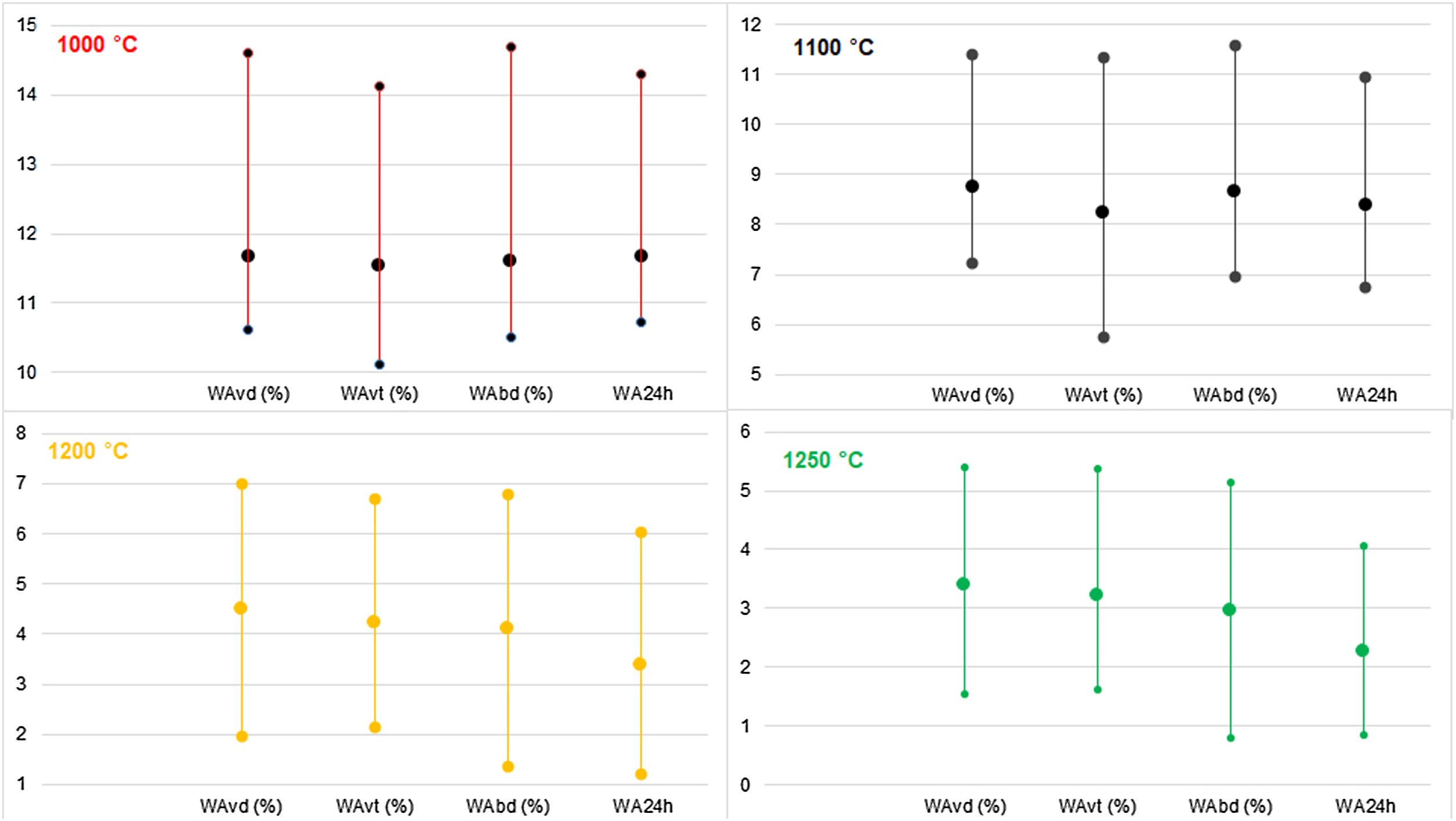
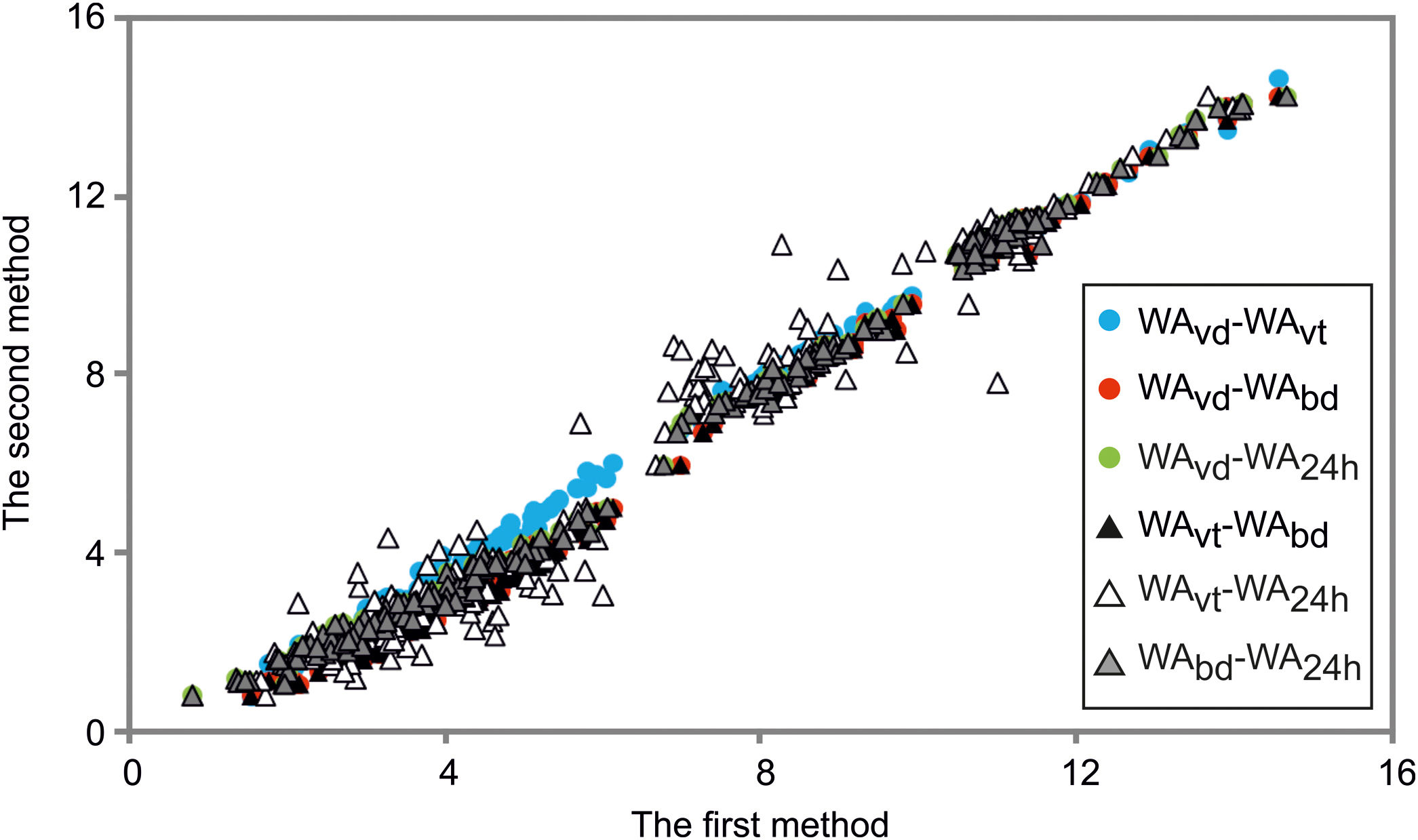
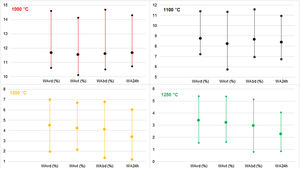
The WA and BD results gained are tested using the Mahalanobis distance [45]. The critical Mahalanobis’ chi-square was 233, and the highest Mahalanobis’ distance was 20, which showed that there were no outliers in the results. The results are presented in Fig. 4.
Comparison of water absorption determined by boiling, vacuum and soaking methods in the case of dry-pressed discs and wet-pressed tiles fired at the temperatures from 1000°C to 1250°C.
WAvd – water absorption under vacuum for dry-pressed discs, WAvt – water absorption under vacuum for wet-pressed tiles, WAbd – water absorption in boiling water for dry-pressed discs, WA24h – water absorption after soaking for 24h for dry-pressed discs.
Water absorption determined by all the used methods in both discs and tiles decreased with increased peak firing temperature. At all the temperatures, the average WAvd showed the highest values since these samples contained a higher quantity of small pores than tiles, and the vacuum method enabled the highest saturation of the samples. The observed values in the case of WAvt were averagely the lowest at the firing temperatures of 1000°C and 1100°C, while at higher temperatures these were amongst the highest since significant densification happened at the higher temperatures. The lowest water absorptions at 1200°C and 1250°C are observed during soaking discs in distilled water.
By comparing the average results gained using the three methods of WA determination in dry-pressed discs, the values were similar or the same at 1000°C, but some differences were noticed in the higher firing temperatures. The latter might mean that the complete open porosity in the material is easily accessible, and thus any method of WA determination is equally good. If these types of clays were used for clay brick production, since the firing is conducted below 1000°C, the 24h cold-water immersion test is completely satisfying. In all the firing temperatures, the lowest values on average were found after 24h soaking tests [7], as expected. As for the testing of discs, with increasing temperature, there are increasing differences in a vacuum and by boiling methods. The decrease of average values by boiling in relation to vacuum increases in the following order: 0.60% (1000°C), 0.80% (1100°C), 8.89% (1200°C), and 13.24% (1250°C). The differences in WAs were about 0.4% on average at 1200°C and 1250°C, which can be a high value when deciding on the group to which ceramic tiles belong to, especially if the results are near the limits defined by the standard. The reason is that in the higher temperatures smaller open pores are gained, which are more difficult to get accessed by water [6,54].
The differences between WAs of disks and tiles by testing under vacuum dropped by 1.11% at 1000°C, 5.83% at 1100°C, 5.78% at 1200°C, and by 5.29% at 1250°C. At high temperatures, these samples showed a tendency to reduce the differences between these methods. The only factor that differed here was the bulk density of the products since the materials’ chemical and mineralogical contents were the same, as well as granulometry, firing temperature, and pressure used for shaping [55]. The addition of water in these ceramic materials influenced the reduction of porosity and the average pore size [32], which was less prominent in higher firing temperatures.
Generally, it is expected that WAs are higher under vacuum than that by the boiling water, with the larger difference in the case of higher quantities of smaller pores [6,54]. In this study, the vacuum method was also proved to be more appropriate in all the firing temperatures in the range of 1000°C – 1250°C, since the samples were better saturated with water.
Since the firing temperatures in the ceramic industry vary from about 1000°C up until 1200°C [18,32,56–58], depending on the final usage of the products, in the case of the studied ceramic clays it would be the best to fire at 1200°C and gain the tiles belonging to the group BIIa with the water absorption between 3% and 6% [2]. In the previous study [1], the clays that had higher contents of clay minerals and a lower quantity of quartz then here presented showed water absorptions between 3.3% and 3.8% while firing at 1200°C at the industrial scale.
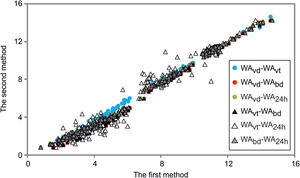
The statistical comparison of the methodsThe correlation coefficients between the results of four types of WA values obtained by statistical analysis are shown in Fig. 5.
Comparison of different methods for water absorption determination in ceramic tiles (WAvd – water absorption under vacuum for dry-pressed discs, WAvt – water absorption under vacuum for wet-pressed tiles, WAbd – water absorption in boiling water for dry-pressed discs, WA24h – water absorption after soaking for 24h for dry-pressed discs).
The evaluation of the four cases was done by using robust statistical comparison [59]. The intercept was found to be close to 0, statistically significant at p<0.01 level, while the slope was close to 1, for all 6 comparisons. The correlations for all comparisons were extremely good – close to 0.99, statistically significant at p<0.01 level. Higher correlation coefficients imply a good match of the results between the two methods. The correlation between the two methods is related to the detection limits, which means that a cross-calibration between the two observed techniques is possible. The results of the harder-to-obtain method can be predicted by the calculation of the results from the simpler-to-gain method, if necessary.
The gresification diagram and the sintering range determinationAt the beginning of the densification of a ceramic material, the clay particles start to fuse, which refers to the solid-state sintering which happens around 900°C in illitic-kaolinitic clays. At this stage, there is still a considerable porosity in the product, and changes in shrinkage are low. The so-called ceramics’ clinkering temperature is the point where a melt occurs within a clay material and is a starting point of the liquid-phase sintering. Open porosity decrease while the number of smaller pores starts to be progressively reduced. It comes to considerable shrinkage, with the maximum at the sintering temperature, which is theoretically the point of maximum densification when also a great reduction in water absorption occurs [8–10,14,19,24]. The range between the clinkering and sintering temperatures is considered a sintering range. The sintering range up to 50°C is considered low for ceramic clays but is satisfying if it is over 100°C [25].
Since the dry-pressed discs in this study were more saturated by water, those samples were chosen for further analysis. Table 4 presents the temperatures of clinkering and sintering of the 51 samples as obtained from the figures WA – firing temperature, for WAs determined under vacuum and by boiling in water. The values were close to one another, which is proved by the statistically significant correlations shown in Table 5. It can be concluded that both methods can be used to obtain the approximate sintering range from the gresification diagrams. Although, since the WAs determined by testing under vacuum are higher, this is the method chosen as the more reliable one. In most of the studied clays, the determined sintering range was above 100°C difference.
Characteristic temperatures obtained from the gresification diagrams.
| Min | Max | Average | StDev | |
|---|---|---|---|---|
| Tcv (°C) | 1124.0 | 1223.0 | 1169.7 | 24.5 |
| Tsv (°C) | 1221.0 | 1377.0 | 1288.0 | 33.8 |
| ΔTv (°C) | 85.0 | 168.0 | 118.3 | 17.0 |
| Tcb (°C) | 1117.0 | 1215.0 | 1159.5 | 24.2 |
| Tsb (°C) | 1207.0 | 1370.0 | 1272.8 | 33.8 |
| ΔTb (°C) | 83.0 | 164.0 | 113.3 | 16.3 |
| Tbs (°C) | 845.0 | 987.0 | 914.3 | 25.3 |
Index c refers to ceramics’ clinkering temperature, and index s to the temperature of sintering.
v – testing under vacuum, b – testing by boiling water.
Tbs– the temperature of the beginning of sintering read from dilatometric curves.
The correlations between the specific temperatures obtained from diagrams of 51 ceramic clays samples.
| Tsv | ΔTv | Tcb | Tsb | ΔTb | Tbs (dilat.) | |
|---|---|---|---|---|---|---|
| Tcv | 0.877+ | 0.302* | 0.936+ | 0.873+ | 0.424+ | 0.454+ |
| Tsv | 0.722+ | 0.827+ | 0.949+ | 0.743+ | 0.241 | |
| ΔTv | 0.295* | 0.627+ | 0.865+ | −0.176 | ||
| Tcb | 0.895+ | 0.374+ | 0.457+ | |||
| Tsb | 0.748+ | 0.245** | ||||
| ΔTb | −0.171 |
Index c refers to the ceramics’ clinkering temperature, and index s to the temperature of sintering.
v – testing under vacuum, b – testing by boiling water.
Tbs (dilat.) – the temperature of the beginning of sintering read from dilatometric curves.
The temperature determined as a beginning of sintering according to dilatometric curves varied widely (Table 4). It is seen in this study that this temperature is statistically significantly correlated to the temperature of clinkering at p<0.01 level (Table 5), and so can be considered as an approximate and fast determination method of the beginning of more intensive shrinkage during firing and the liquid phase occurrence. In the studied clays, the temperature of sintering is mostly predicted in WA – firing temperature figures, since it was above 1250°C.
The discs showed firing shrinkages between 3.06% and 5.66% (4.16% on average) while firing at 1200°C, and in the range of 3.10–5.78% (4.57% on average) at 1250°C. The similar results on FS are determined in a previous study considering illitic ceramic clays fired at 1200°C [46]. In most of the samples, after the initial almost constant growth in FS in the 1000–1200°C range, the shrinkage started to slow down, and the maximum densification is expected to happen above 1250°C.
ConclusionAccording to the tested parameters, it was obtained that the newly opened deposits of illitic-kaolinitic clays are suitable to be mixed and used in the production of BIIa ceramic tiles while firing at 1200°C.
This study showed that the most reliable EN standard method for determining water absorption of ceramic clays is testing under vacuum for the samples fired at 1200°C and 1250°C. At lower temperatures, the higher saturation of the samples was obtained by boiling in water. In a view of not yet finished debate about whether the EN standard describing the water absorption method by boiling will be still kept, it is important to do more studies on various raw clay materials and compare these methods’ accuracy and differences according to the peak firing temperature.
The specific use of the gresification diagrams proposed by the geological rule books in the Balkans is proved in this study to roughly represent the sintering range of these clays. The results of the so-called clinkering temperatures of ceramics are closely related to the temperature of the beginning of sintering obtained by reading the dilatometric curves. The sintering temperature is the predicted moment of maximal densification.
The authors are grateful for the support by the Ministry of Education, Science and Technological Development of the Republic of Serbia for this work (Contract No. 451-03-68/2020-14/200012).