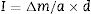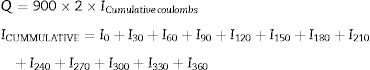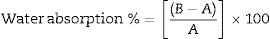This paper presents an experimental investigation on the durability properties of metakaolin (MK) and bottom ash (BA) blended geopolymer under different environmental exposure. The blended geopolymer concrete (GPC) was prepared with sodium based alkaline activators under ambient curing temperature. The concentration of sodium hydroxide used was 8M. The ratio of sodium silicate to sodium hydroxide was kept as 2.0. The performance of blended geopolymer concrete was compared with conventional concrete (CC). The test results reveal that blended geopolymer concrete develops a better performance against sulphate and acid resistance. Also, MK-BA GPC shows enhanced performance over the conventional concrete in terms of sorptivity, rapid chloride and water absorption.
Este artículo presenta una investigación experimental sobre las propiedades de durabilidad del geopolímero mezclado con metacaolín (MK) y cenizas de fondo (BA) bajo diferentes exposiciones ambientales. El hormigón de geopolímero mezclado (GPC) se preparó con activadores alcalinos a base de sodio a temperatura ambiente de curado. La concentración de hidróxido de sodio utilizada fue 8M. La relación de silicato de sodio a hidróxido de sodio se mantuvo en 2,0. Se comparó el desempeño del concreto geopolímero mezclado con el concreto convencional (CC). Los resultados de las pruebas revelan que el concreto geopolimérico mezclado desarrolla un mejor desempeño frente a la resistencia a los sulfatos y ácidos. Además, MK-BA GPC muestra un rendimiento mejorado sobre el hormigón convencional en términos de sortividad y de rápida absorción de cloruro y agua.
Ordinary Portland cement is the most popular binder material for producing concrete. The usage of cement is ever increasing due to its ability to gain early strength as well as prolonged strength gain. However, it is energy intensive and also consumes large amount of natural materials for its production. Further, production of every one tonne of cement releases about 1 tonne of CO2 in to the atmosphere promoting global warming and deterioration of ecosystem [1–3]. It is being noted from the last decade and extensive research works have been carried out in the field of advance growth in inorganic geopolymers because of its extensive scope of prospective applications of these supplementary materials. Different studies are being found in the literature on the performance, synthesis, behaviour and function of geopolymer materials [4,5].
Geopolymer is an inorganic alumino-silicate compound synthesized from geological material or industrial by-products such as fly ash, slag, rice husk ash etc. Further, geopolymers are inorganic in nature and it can be synthesized by means of external activators under alkaline medium and also the proper selection of source materials which are rich in Si and Al content. The chemical mechanism between SiAl minerals and alkaline liquids consist of destruction of Si and Al atoms from source materials followed by coagulation and condensation of precursor into monomers and finally monomers into polymeric crystalline structures [6–8]. Eventually the polymerization process develops fast accelerated chemical reaction under alkaline environment on Si:Al minerals that forms three dimensional polymeric chain and ring structure consisting SiAlO bonds [9].
In general, when the bottom ash is grined finer it will be more reactive and thereby directly imparts on the high compressive strength in geopolymer specimens [10,11]. The effect of curing mode on the properties of geopolymer mortar made using combustion coal bottom ash shows that dry curing decreases in compressive strength than ambient curing. Also, dry curing exhibits porous microstructure whereas ambient cured specimen exhibit homogenous and compact matrix [12]. The geopolymeric reaction depends on the fineness and pore size of the material used. It has been observed that fine bottom ash improves the performance of geopolymer in terms of sulphate resistance as well as sorptivity [13]. Subsequently, the effect of molar ratio of Si to NaOH and Na2SiO3 to NaOH on compressive strength of geopolymer mortar. It is noted that mortar with low Si to NaOH attained maximum compressive strength. Further, proper selection of molar ratio can cause high geopolymer reaction at ambient temperature [14]. Further, the durability performance of lignite bottom ash was studied and the test results indicated that finer bottom ash imparted high compressive strength and also exhibit better performance in terms of durability than cement mortar [10,15].
Metakaolin is generally used as pozzolanic micro filler material in terms of high strength and as well as high performance concrete. The characteristics of metakaolin based geopolymer in terms of strength parameters as well as durable properties shows tremendous performance when compare with OPC [16]. Also, when compared with individual score materials performance, blended nature exhibits better results [17,18]. The calcination temperature and period of calcination plays an important role in developing the strength of the matrix [19]. Consequently, the kinetics of geopolymeric reaction of metakaolin and activators shows that silica and alumina content will have direct impact on early strength. Also, increase in molar ratio of Si/Al imparts strength gain at later ages [20]. The increase in fineness of metakaolin enhances the strength of binder material [21]. Also, metakaolin synthesized with sodium hydroxide and sodium silicate imparts the dense texture and high resistance to surface scratching geopolymer [22].
The performance of geopolymer materials depends on the various factors such as choice of selection of source materials, fineness of the materials used and mode of curing [19,23]. Mode of curing has been one of the major issue factors for geopolymer technology. But by taking into account of proper selection of source materials as well as the suitable activators consideration one can be able to cast the geopolymer concrete in cast – in situ under ambient temperature itself [22,24,25]. Further, it has been noted that proper selection of molar ratio can cause greater geopolymeric reaction at ambient temperature [14].
In the present experimental investigation, durability properties such as sulphate attack, acid attack and their weight loss and its strength retention were tested at different period of time. Also, sorptivity, rapid chloride penetration test and water absorption tests were also carried out to explore the performance of metakaolin – bottom ash geopolymer concrete activated by sodium based alkaline activators under ambient temperature.
A profuse research work was done earlier in the field of geopolymer concrete by using different alumina–silicate based materials as source materials. Moreover, the performance of geopolymer concrete when they are blended with two or more source materials together under different curing conditions are being investigated by different researchers. But, the synthesis effects on metakaolin and bottom ash geopolymer source materials have not been explored so far. In this background, a venture has been taken up to study the durability performance of MK-BA geopolymer concrete when it is exposed to different period of time under various environment circumstances.
MaterialsCementOrdinary Portland cement (OPC) of 53 grade, satisfying the requirements of BIS 12269-2013 [26] has been used to produce control mix. The physical properties of the cement were measured as per BIS 4031 – 1988 & 1999 [27,28] and the results are summarized in Table 1. Also, the chemical properties of cement were analyzed using the procedure prescribed by BIS: 4032-1985 [29] and the result are presented in Table 2.
Physical and mechanical properties of cement.
| S. no. | Characteristics | Result | Specifications as per BIS 12269-2013 |
|---|---|---|---|
| 1 | Specific gravity | 3.14 | 3.15 |
| 2 | Fineness by Blaine's air permeability | 305 m2/kg | Not less than 225m2/kg |
| 3 | Normal consistency | 30% | – |
| 4 | Initial setting time | 48min | Not less than 30min |
| 5 | Final setting time | 246min | Not more than 600min |
| 6 | Soundness Le-Chatelier method | 1.2mm | Not more than 10mm |
| 7 | Compressive strength | ||
| 3 days | 28.6MPa | Not less than 27MPa | |
| 7 days | 37.3MPa | Not less than 37MPa | |
| 28 days | 55.4MPa | Not less than 53MPa | |
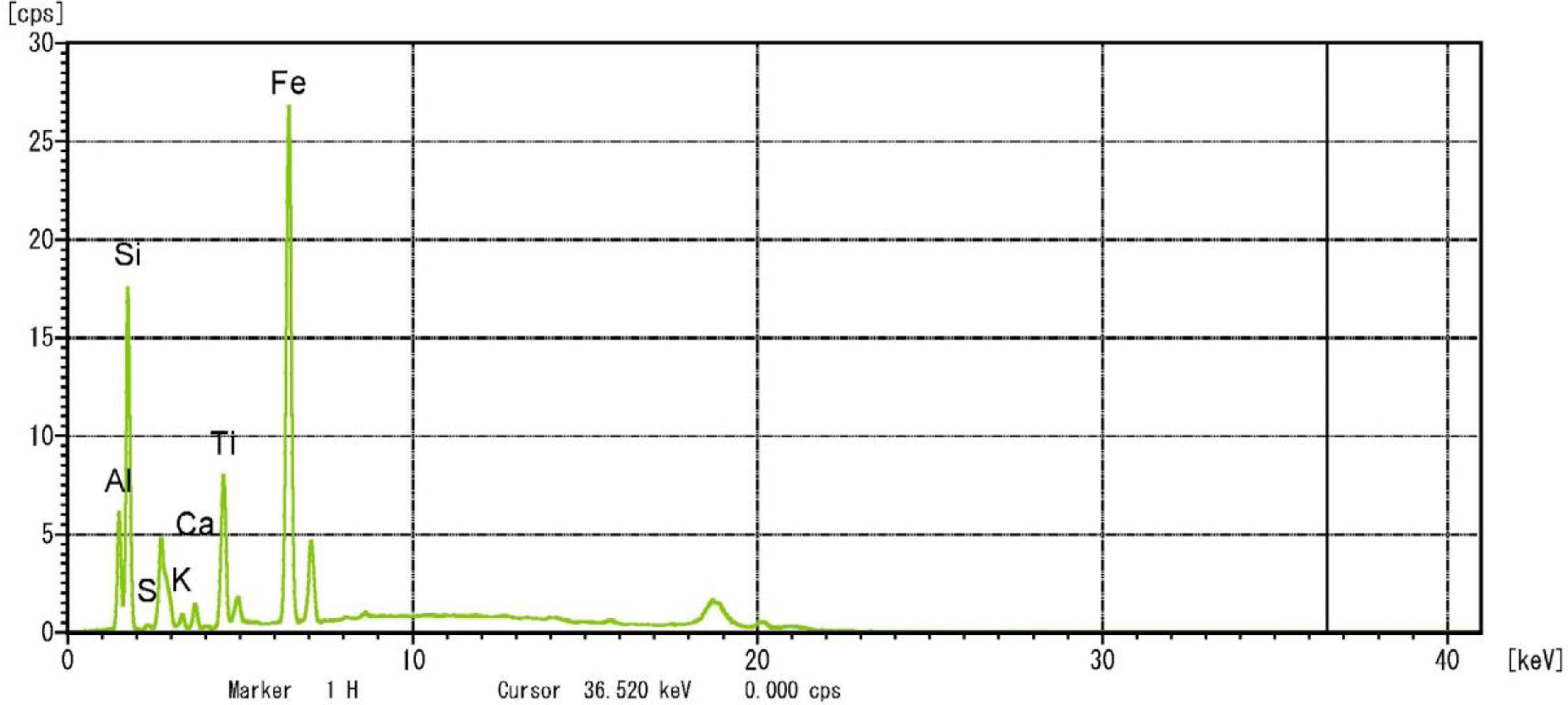
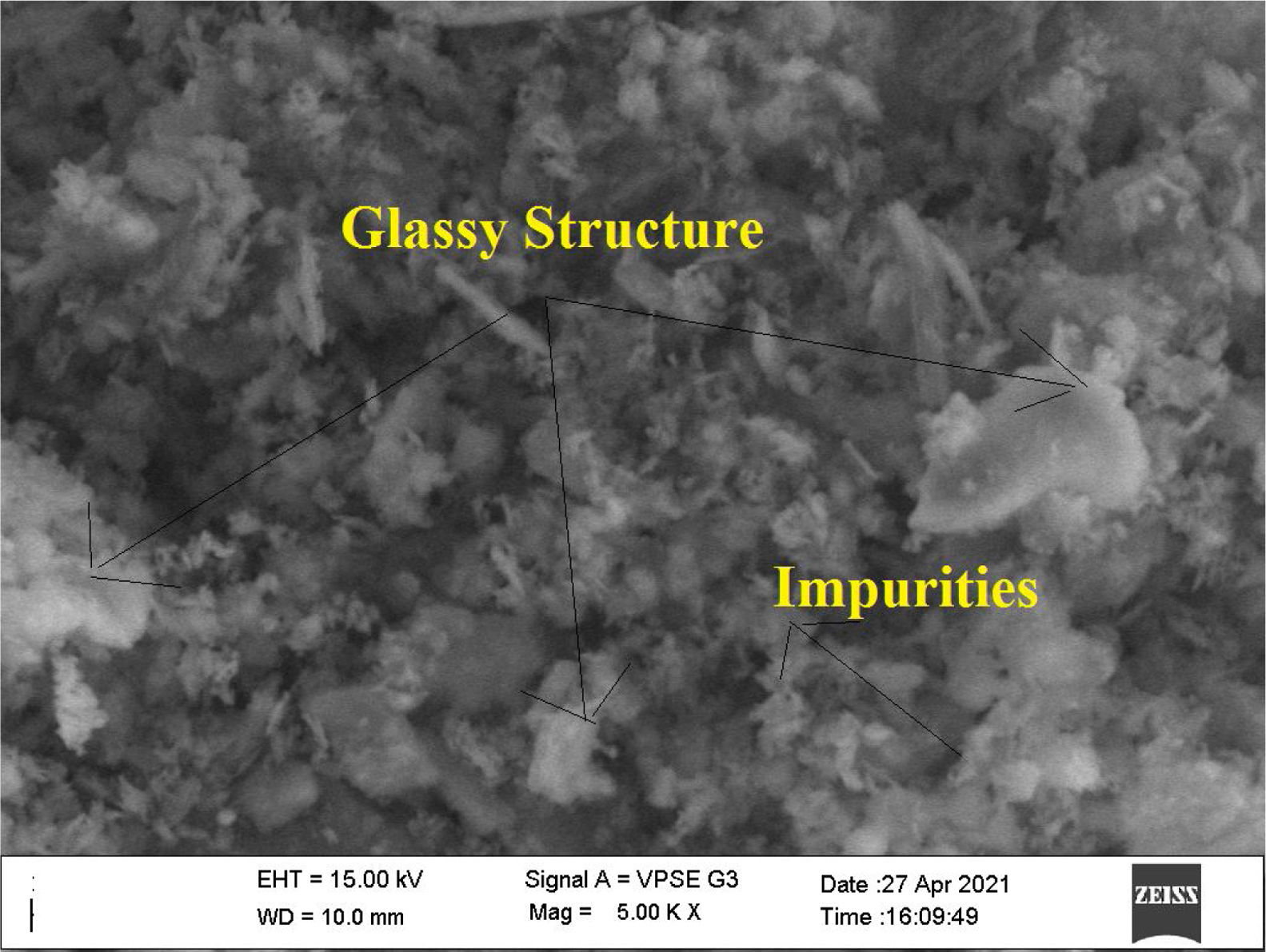
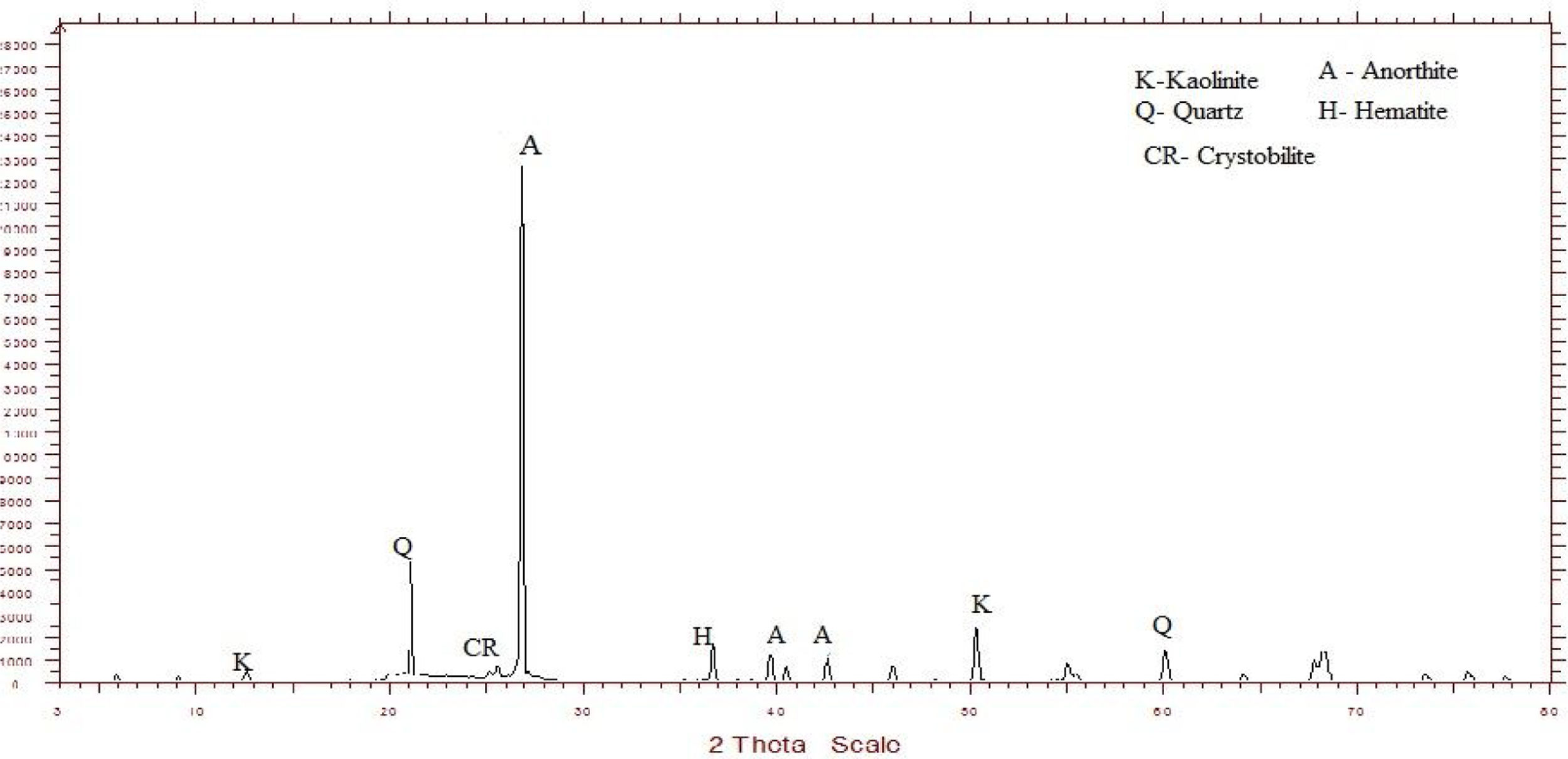
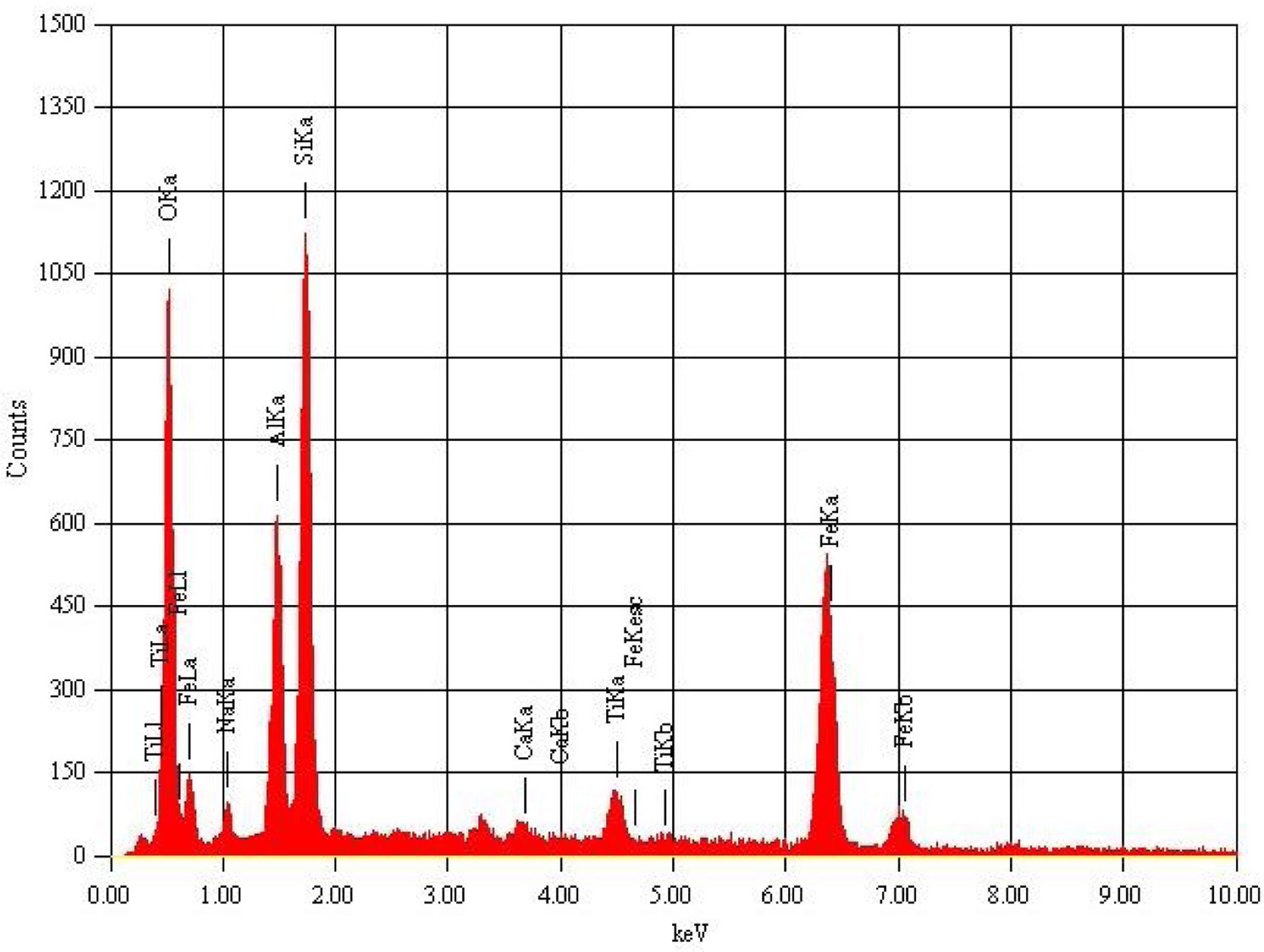
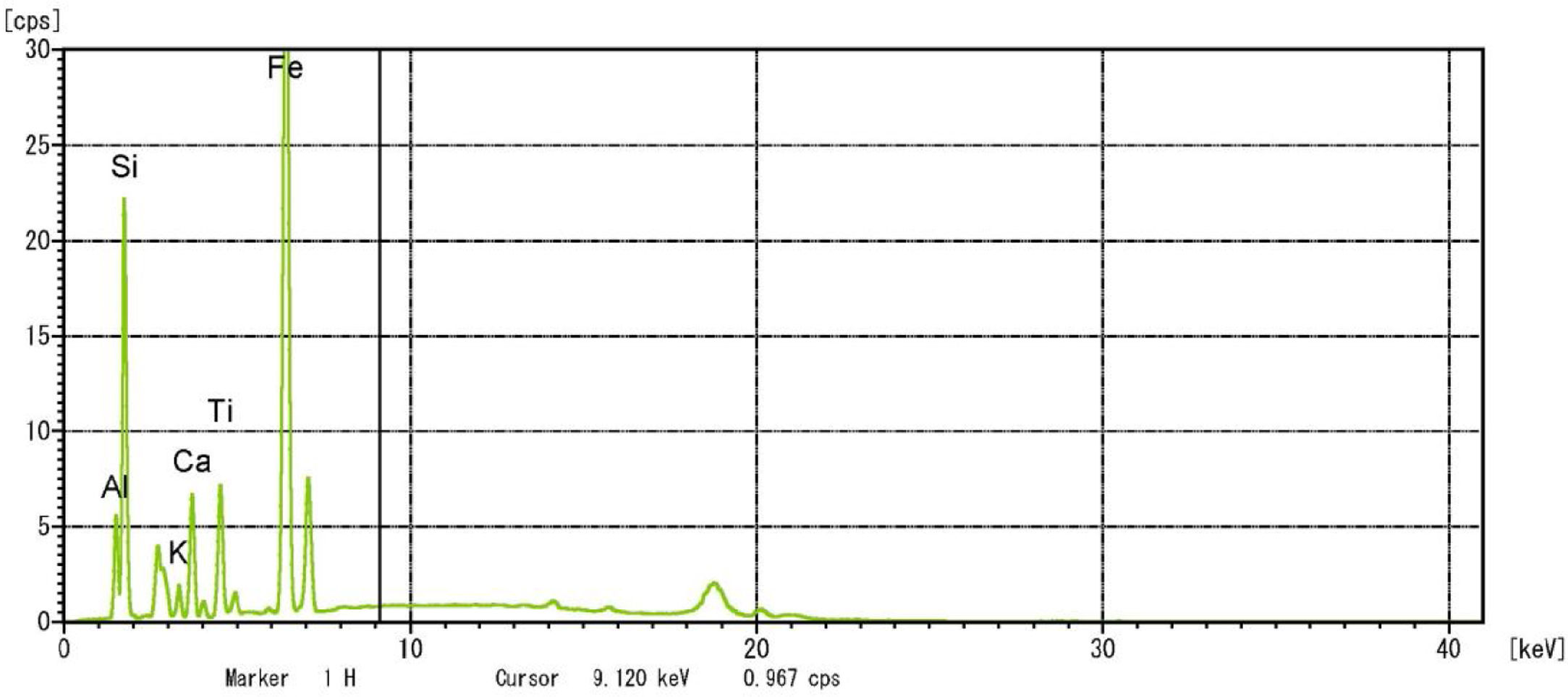
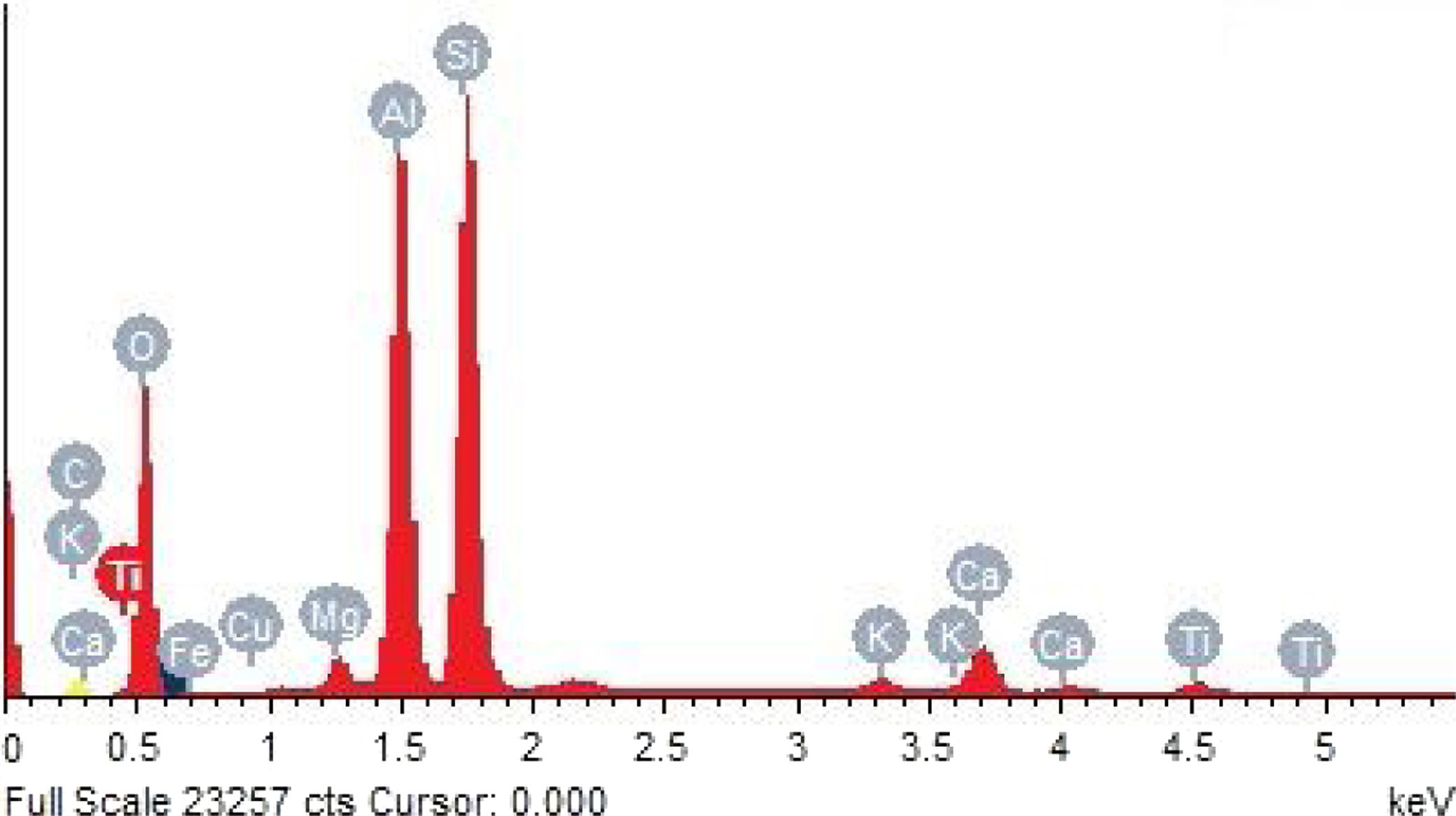
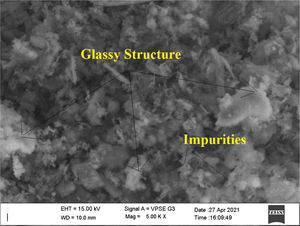
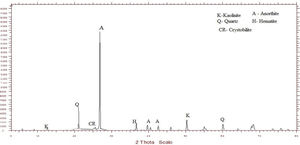
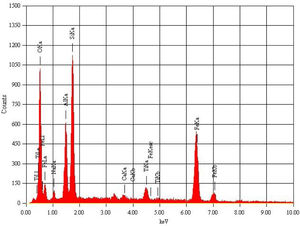
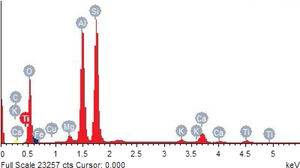
Metakaolin (MK) is the product which is obtained by the calcinations of kaolin under 650–750°C. Metakaolin used in this study was procured from Jeetmul industries, Chennai. The metakaolin having average particle size 5μm and specific surface area of 20,000m2/g. The specific gravity of metakaolin was found to be 2.5. The chemical composition and mineral compositions are determined by X-ray fluorescence (XRF) as shown in Fig. 1 and Table 3. The microstructure of the metakaolin material were carried out by Scanning Electron Microscope (SEM) analysis and from the image it has been inferred that particles are irregular in shape, glassy structure and as well as some impurities are also found and it has been shown in Fig. 2. Also, XRD and EDAX were carried out to study the mineralogical composition, percentage of minerals existed in metakaolin. The XRD of metakolin endorses the presence of anorthite, quartz, cristobalite, kaolinite and hematite as the major mineralogical phases along with minor percentage of impurities. The details of the percentage of elements present in the material are shown in Table 4 and Fig. 3. Further, EDAX confirms the presence of silica, alumina and iron as major elements in metakaolin and are shown in Table 5 and Fig. 4.
Quantitative analysis of metakaolin and bottom ash.
| Element | Metakaolin (%) | Bottom ash (%) | ||
|---|---|---|---|---|
| Mass | Atom | Element | Atom | |
| O | 23.38 | 42.09 | 55.53 | 64.25 |
| Na | 1.39 | 1.74 | 8.50 | 13.10 |
| Al | 11.22 | 11.97 | 13.09 | 8.98 |
| Si | 21 | 21.54 | 16.40 | 10.81 |
| Ca | 0.52 | 0.37 | 1.96 | 0.91 |
| Ti | 4.38 | 2.64 | 0.52 | 0.20 |
| Fe | 38.12 | 19.66 | 2.47 | 0.82 |
| Total | 100 | 100 | 0.44 | 0.21 |
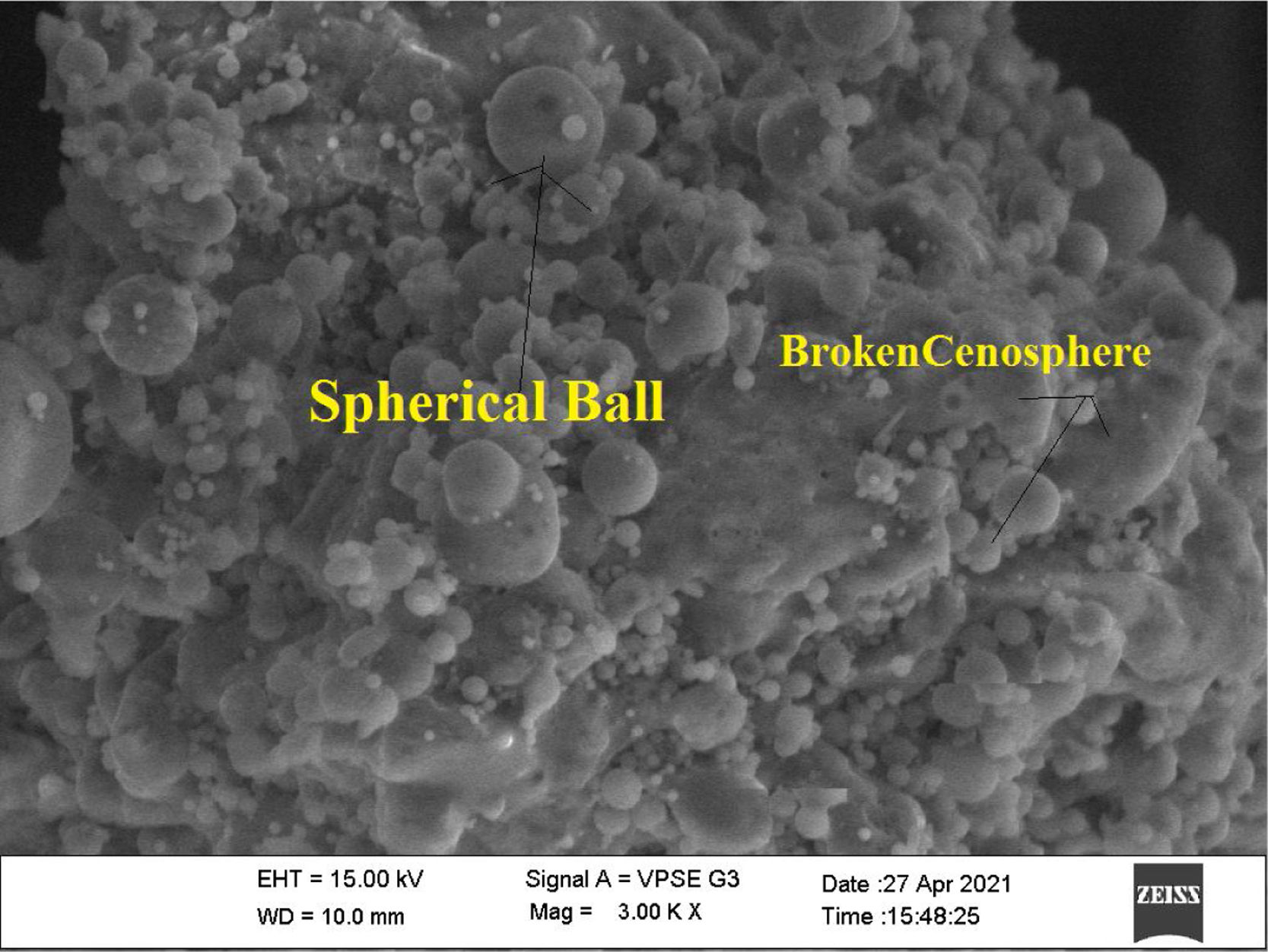
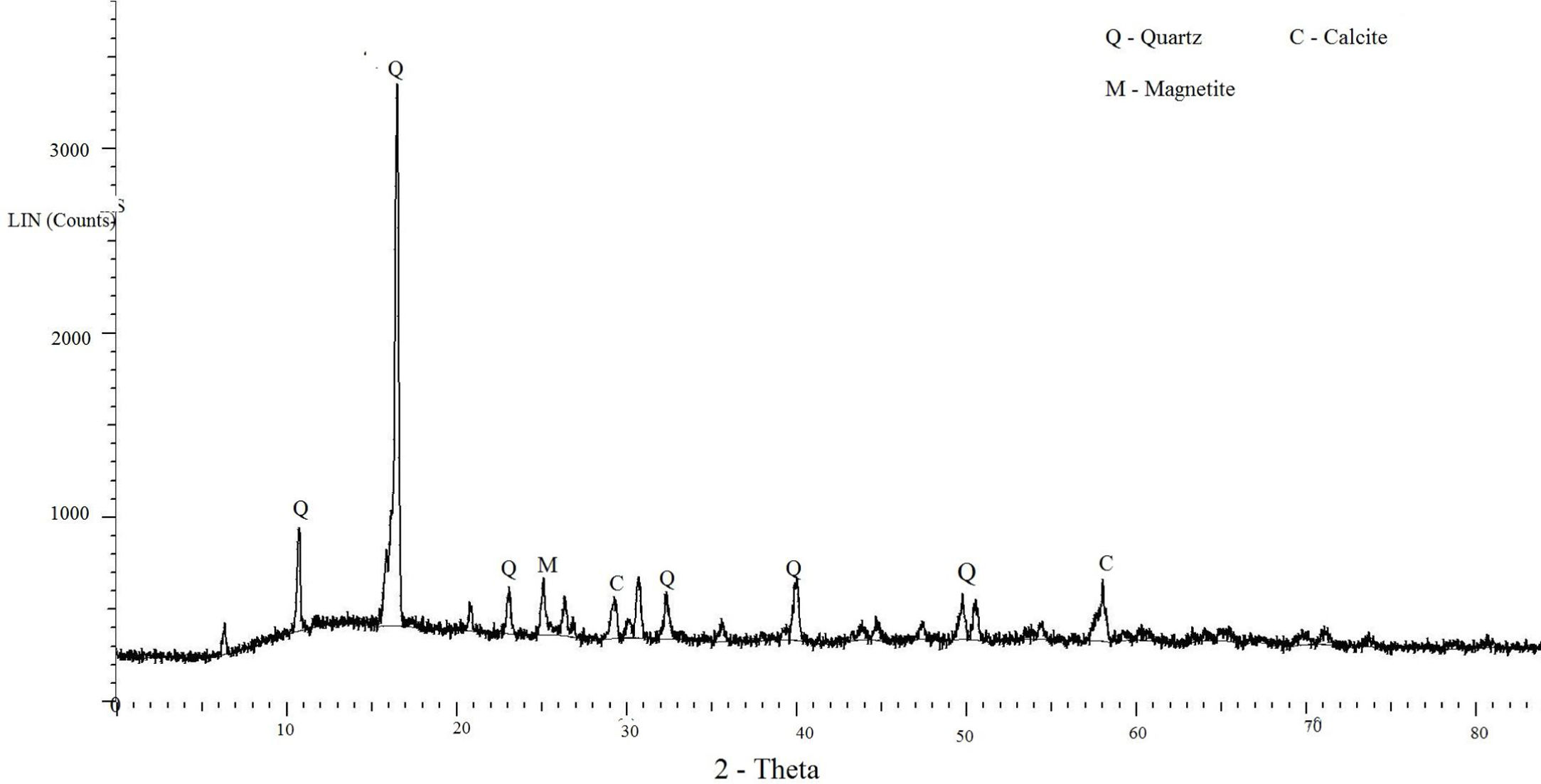
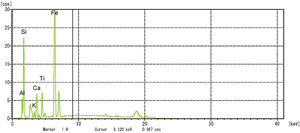
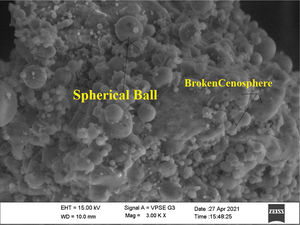
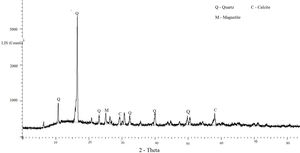
In this study, bottom ash (BA) which was collected from the Mettur Thermal power plant station. Bottom ash is in a moist state condition, it was dried well and grained as much as possible to attain low particle size. The specific surface area of bottom ash is 3460cm2/gm and the specific gravity was found to be 2.17. The chemical composition and mineral compositions are determined by X-ray fluorescence (XRF) as shown in Fig. 5 and Table 3. Also, SEM images reveal that the particles are almost spherical in shape with some minor broken cenosphere revealing the existence of silica and alumina in high proportions and it has shown in Fig. 6. Further, XRD study has been carried out to know the mineralogical composition and other characteristics of bottom ash and they are shown in Table 4 and Fig. 7. The XRD analysis of bottom ash indicates the presence of quartz, calcite and magnetite as the major mineralogical phases. EDAX spectrogram image confirms the presence of silica and alumina in prevailing proportion and they are shown in Table 5 and Fig. 8.
Alkali activatorThe chemical solution which is responsible for the process of polymerization is known as alkaline activator solutions. To stimulate the source materials, a mixture of sodium hydroxide solution and sodium silicate solution were used in this work. The sodium silicate and sodium hydroxide solution were purchased from the local supplier. The ratio of silica modulus in sodium silicate solution is 1.0.
A commercial grade sodium hydroxide flakes with 95–97% purity was purchased in bulk from local supplier. The sodium hydroxide solution was prepared by dissolving flakes in water. The mass of NaOH solids in a solution varies depending on the concentration of the solution expressed in terms of molarity (M).
Further, the chemical composition of sodium silicate solution is 29.4% of SiO2, 13.7% of Na2O and water of 55.9% by weight. Also, the alkaline activator solution was prepared a day before the time of casting which is a key responsible for inducing the polymerization process.
AggregatesThe fine aggregate used in this study was locally available river sand confirming to zone III. The specific gravity of fine aggregate is as 2.63. Also, locally available coarse aggregate having specific gravity of about 2.26 was used. The characteristics of fine and coarse aggregates are in conformity with the requirements prescribed BIS 383-1970 [30]. Further, the physical properties of coarse and fine aggregates are tested as BIS 2386-1963 [31] and the test results are presented in Table 6.
Experimental programmeBased on the earlier studies made by the authors [32] on geopolymer mortar of metakaolin and bottom ash varied with liquid to binder ratio from 0.48 to 0.52 with 8M for different mix proportions under ambient curing. It has been found that the source material such as metakaolin and bottom ash of equal proportions with liquid to binder ratio of 0.5 identified as promising mix to make geopolymer concrete. For casting the geopolymer concrete (GPC), the density was taken as 2400kg/m3 similar to conventional concrete. For geopolymer concrete casting, the mix design was carried out as per the guidelines prescribed [33,34]. To compare the test results, the conventional concrete (CC) was casted as per the guidelines [35]. From the test results, it has been observed that geopolymer concrete Exhibits 49% of higher compressive strength than that of CC. Also, the similar trend was exhibited in other mechanical properties also [36]. The same mix proportion will be adopted to cast and test the durability properties of geopolymer concrete specimens. The mix proportion of MK-BA GPC is presented in Table 5. In addition, control mix using cement as binder was designed as per prescribed [35] and the material quantity are also shown in Table 7.
Mix proportions of CC and GPC.
| Materials (kg/m3) | CC | MK-BA GPC |
|---|---|---|
| Cement | 381 | – |
| Metakaolin | – | 200 |
| Bottom ash | – | 200 |
| Sodium silicate | – | 133.38 |
| Sodium hydroxide | – | 66.7 |
| L/S ratio | – | 0.5 |
| Sodium hydroxide molarity (M) | – | 8M |
| Fine aggregate | 833 | 540 |
| Coarse aggregate | 1084 | 1260 |
| Coarse aggregate size (mm) | 20 | 20 |
| Water | 162 | – |
To study the behaviour of geopolymer samples under different environment such as sulphate, and acidic environment exposure, the geopolymer concrete samples were exposed to 5% of magnesium sulphate solution and 2% of sulphuric acid solution respectively. All the geopolymer concrete specimens are exposed to different environment only after 28 days of curing at ambient temperature. It is to be noted that, the duration of exposure on different solutions was 1, 3, 6 and 12 months and all the specimens were kept fully immersed in their corresponding solutions having total volume as four times the volume of specimens immersed. The effects of magnesium sulphate and sulphuric acid on the geopolymer concrete and control concrete specimen were regularly observed through visual inspection and measurement of pH values. At the time of testing, the surface of specimen was wiped out and they were tested for weight and compressive strength at surface dry conditions. A total of 24 specimens were casted to determine the sulphate resistance of GPC and CC as prescribed [37], whereas 24 specimens were casted and tested to determine the acid resistance of GPC and CC in accordance with the guidelines [38].
To study the rate of sorptivity of the GPC and CC specimens, cylindrical disc specimens having 100mm diameter and 50mm thickness were cast for determining the rate of sorptivity of GPC as well as CC. The sorptivity test was conducted in accordance with the standard [39]. The test was carried out on 28 day cured specimens. The specimens were placed in a tray such that their bottom surface up to a height of 5mm was in contact with water. This is to allow free movement of water through the bottom surface of specimen by capillarity. The sides of the specimens were covered with adhesive glue as show in Fig. 9. The top surface which was not exposed to water was covered with plastic sheet. The mass of the specimen was recorded at the intervals as per mentioned in the code. The amount of water absorbed was calculated using the following equation.
where I is an absorption in mm, Δm is the change in mass of the specimen in gm at the time t, a is the exposed area of the specimen in mm2, d is the density of water in g/mm.To evaluate the electrical conductance of concrete, a rapid indication of resistance to the dispersion of chloride ions has been determined by Rapid Chloride Penetration Test (RCPT) as per the guidelines prescribed [40]. A concrete disc having 50mm thick and 100mm diameter has been determined for the period of 6h. A potential difference of 60V direct current was maintained across the specimen immersed in a 3% sodium chloride solution and the other in a 0.3M sodium hydroxide solution.
The passage of current was monitored at every 30min till completion of 6h along with the temperature of the solution in each cell. The charge passed can be computed by using trapezoidal rules and it is expressed as coulombs [1 coulombs is 1 ampere seconds] and it has been calculated by the formula expressed in Eq. (3.3). A total of 18 specimens were cast to carry out rapid chloride penetration test on GPC and control concrete. The test was carried out on GPC and CC at the age of 28 days, 56 days and 90 days of curing at room temperature and the specimens casted are shown in Fig. 10.
where Q=Charge passed (coulombs), I0=Current (amperes) immediately after voltage is applied, It=Current (amperes) at ‘t’ minute after voltage is applied.The water absorption characteristics of MK-BA GPC and CC were determined at the age of 7 and 28 days using the procedure prescribed [41]. The test specimens of size 100×100×100mm were used and they are shown in Fig. 11. The 7 and 28 days cured specimens of GPC and CC were dried in an oven at a temperature of about 100°C for 24h. Then the cured specimens were endorsed to cool at room temperature for 3min. The initial mass of the specimens was noted as (A). Then the final mass of the specimens was recorded only after the immersion of specimens in water for 24h and it is noted as (B). Water absorption was calculated from equation 2.2 given below.
Results and discussionResistance towards magnesium sulphate attackVisual appearanceThe physical appearance of both geopolymer specimens and control concrete specimens exposed to magnesium sulphate solution over a period of 12 months shows that the shape and dimensions of the specimens are quiet intact and they are shown in Figs. 12 and 13. However, precipitation of white salt has been noticed on the surface of CC but GPC specimens are free from precipitate. The reason for precipitate over CC is due to magnesium taking part in the reaction replacing calcium in solid phases as a result of which brucite and magnesium silicates hydrates are formed. Existence of brucite on the control concrete surface lowers the pH of pore solution and then decomposes the calcium silicate hydrates as a result of which calcium is precipitated as gypsum in form of white precipitation [42]. As the GPC is free from calcium hydroxide and calcium silicate hydrates, the white precipitate has not been formed on its surface [43–45].
pH concentration of solutionThe variation in the pH concentration of the magnesium sulphate solution containing for the geopolymer specimens and control concrete are studied. The initial value of pH for 5% of MgSO4 prior to immersion of specimens was 8.32. After 1 month of exposure, GPC specimens increased to about 8.48 whereas for the control concrete it was 8.87. After 3 months of exposure GPC Exhibits 9.41 of pH whereas CC shows 9.69 of pH. Thereafter, the rate of increase was steady and it doesn’t show any increase in pH value. In control concrete, its surface by means of physical appearance displays some white patches in it but it does not formed as much as thick which tends to leaching. In GPC, no such while patches are formed and leaching does not occur on the surface. This may be due to the rate of migration of alkali being high up to certain period and thereafter no migration has happened because of constant pH value [44,46].
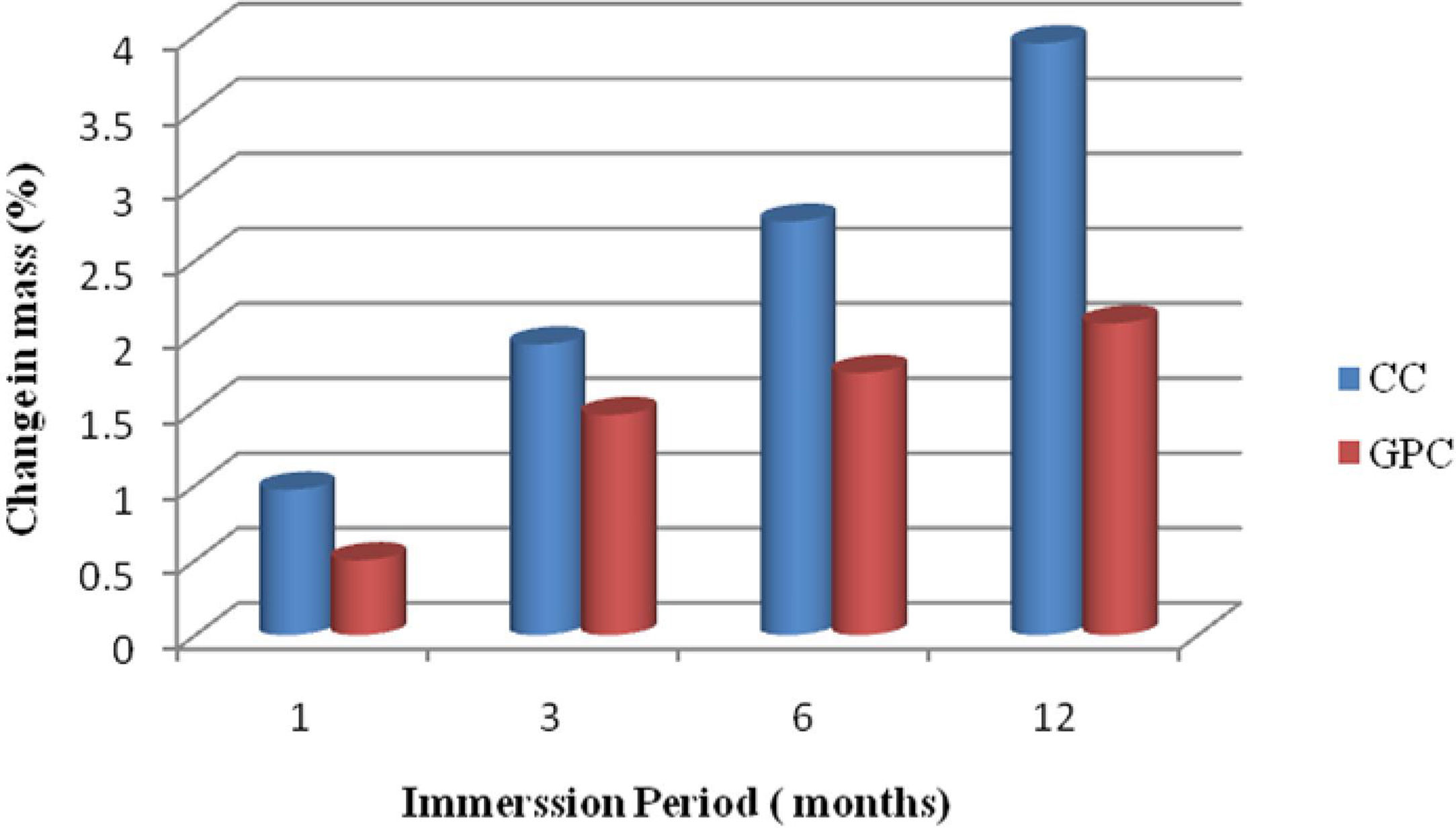
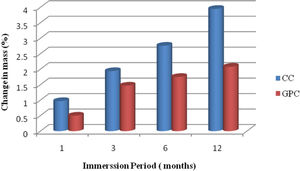
Change in massThe results of the mass gain in control concrete and MK-BA GPC are shown in Table 8 and they are also depicted in the Fig. 14. It is obvious from the test results that mass of both concrete specimens is increased in mass marginally. However, when compared with the control concrete, GPC has demonstrated relatively less gain of mass. After 12 months of exposure, GPC shows 2.08% of mass gain, whereas CC explicit 3.95% of mass gain. Generally, the concrete having higher porosity, permeability and sorptivity allow more sulphate solution to occupy pore spaces. The reason for high mass gain in CC is because of precipitation. The GPC has exhibited relatively less gain in mass than control mix as GPC has relatively less permeability and sorptivity than CC.
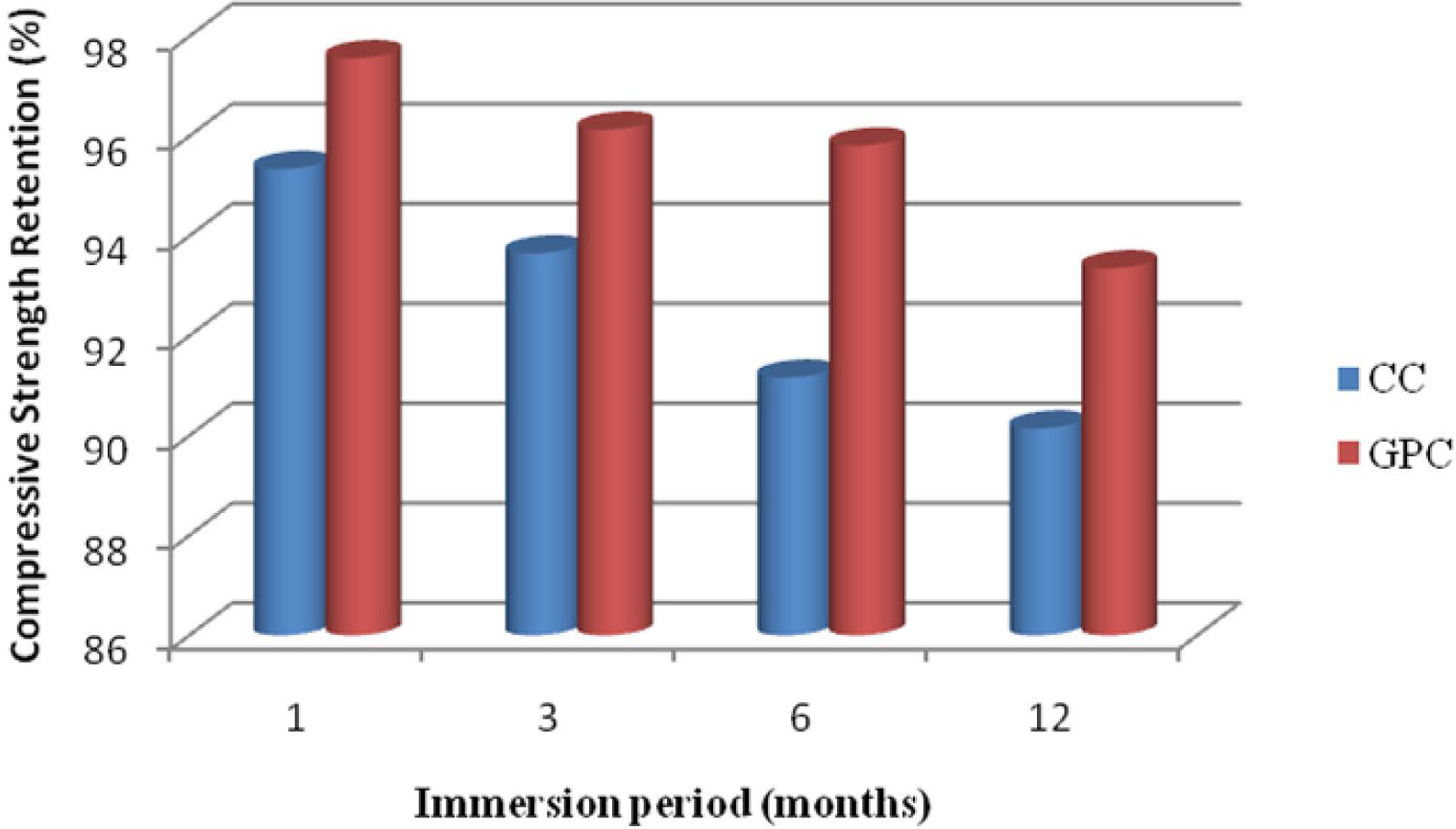
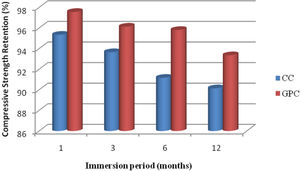
Change in mass and compressive strength retention of CC and GPC due to sulphate atack.
| S. no. | Immersion period (months) | Change in weight (%) | Residual compressive strength (%) | ||
|---|---|---|---|---|---|
| CC | GPC | CC | GPC | ||
| 1 | 1 | 0.97 | 0.50 | 95.34 | 97.56 |
| 2 | 3 | 1.94 | 1.47 | 93.65 | 96.13 |
| 3 | 6 | 2.76 | 1.75 | 91.16 | 95.81 |
| 4 | 12 | 3.95 | 2.08 | 90.14 | 93.35 |
From the test results of both GPC and CC have lost their compressive strength marginally on exposure towards sulphate solution. But when compared with CC the range is comparatively higher. Further, GPC has exhibited 2.7%, 3.34%, 1.97% and 1.91% of higher residual compressive strength than control concrete at the exposure period of 1, 3, 6 and 12 months respectively. Geopolymer specimens have demonstrated better performance in resisting sulphate attack in cement concrete due to the formation of ettringite and gypsum presence of sulphate [47,48]. Also, the formation of brucite lowers pH and then decomposes the CSH leading to deterioration of concrete [42]. The test results are displayed in the Table 8 and they are depicted in Fig. 15.
The microstructure SEM image of both GPC and CC for 12 months of exposure on sulphate solution is shown in Figs. 16 and 17. From the microstructure matrices deterioration of cement based concrete are mainly because of sulphate with Ca (OH)2 and calcium monosulfo aluminate which leads to form gypsum and ettringite resulting in loss of strength retention, precipitation [49,50]. But in case of geopolymer specimens, it exhibits a different mechanism. The geopolymer products were not alike as the normal control concrete because there is no hydration products existing and they are not as much vulnerable towards the sulphate attack [44]. More number of needle like structures were visible in CC SEM image where as in GPC it was found very less and also the exposure to the magnesium sulfate solution resulted in a dense micro structure in the surface region.
Resistance towards sulphuric acid attackVisual appearanceThe physical appearance of CC and GPC are depicted in Figs. 18 and 19. The GPC specimen indicates that the shapes of the specimens are quiet intact and also free from cracks. In contrary, some of the researchers reported in their research that GPC had suffered severe erosion on the surface when exposed to acidic medium. It must be due to 5% of sulphuric acid used to determine the acid attack [51]. Further, in case of CC it seems that some white precipitation exists along with some minor cracks and surface getting damaged. But the shape of the specimens is intact and dimensionally unchanged.
pH concentration of solutionThe change in the pH concentration of the sulphuric acid solution containing the geopolymer specimens and control concrete are studied. The initial value of pH for 2% of H2SO4 prior to immersion of specimens was 5.2. After 1 month of exposure, towards the acidic medium, GPC specimens shows the pH value increased to about 5.72 where as for the control concrete consideration its value is about 5.99. After 3 and 6 months of exposure in acidic medium in GPC specimens they exhibit the value of about 6.13 and 6.34 respectively. Moreover, for control concrete at the period of 3 and 6 months exposure its value was about 6.27 and 6.38 respectively. Beyond that there was no much deviation in pH value that was observed in both GPC and CC. Generally, the concrete having high porosity, permeability and sorptivity allows more acid solution to penetrate into the pore spaces. The acid solution leach away calcium compounds present in concrete and leaching was not observed in GPC specimens [51].
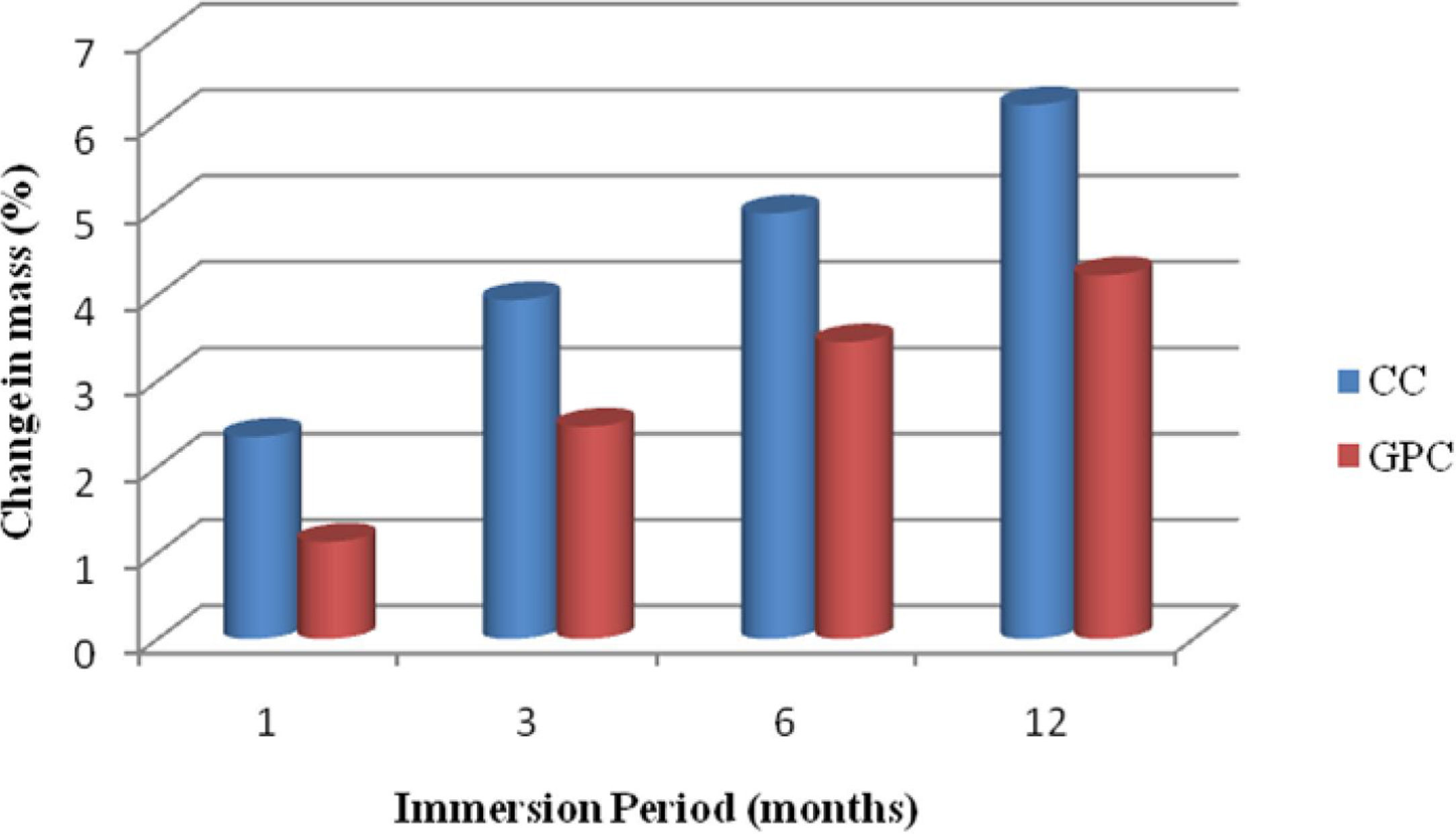
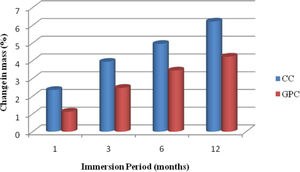
Change in massFrom Table 9 and Fig. 20 it shows that loss of weight for control concrete and geopolymer concrete specimens when they are exposed to acidic medium for different immersion period. As for as cement based concrete specimens are concerned, the loss of weight has taken place due to the reaction between calcium hydroxide in the concrete specimens and acid, which in turn induces the tensile stresses resulting in formation of minor cracks and scrabbling of surfaces [10]. In GPC, it exhibits better performance than CC. The reason for the excellent performance of GPC is because of fineness of the source material used for dense packing and also high amount of Si and Al content and also GPC is relatively free from calcium compounds. [52]. Also, the geopolymer specimens had less weight loss under acidic environment mainly due to the low sorptivity, water absorption and also calcium content is less. This observation is in conformity with the other researchers also [24,53].
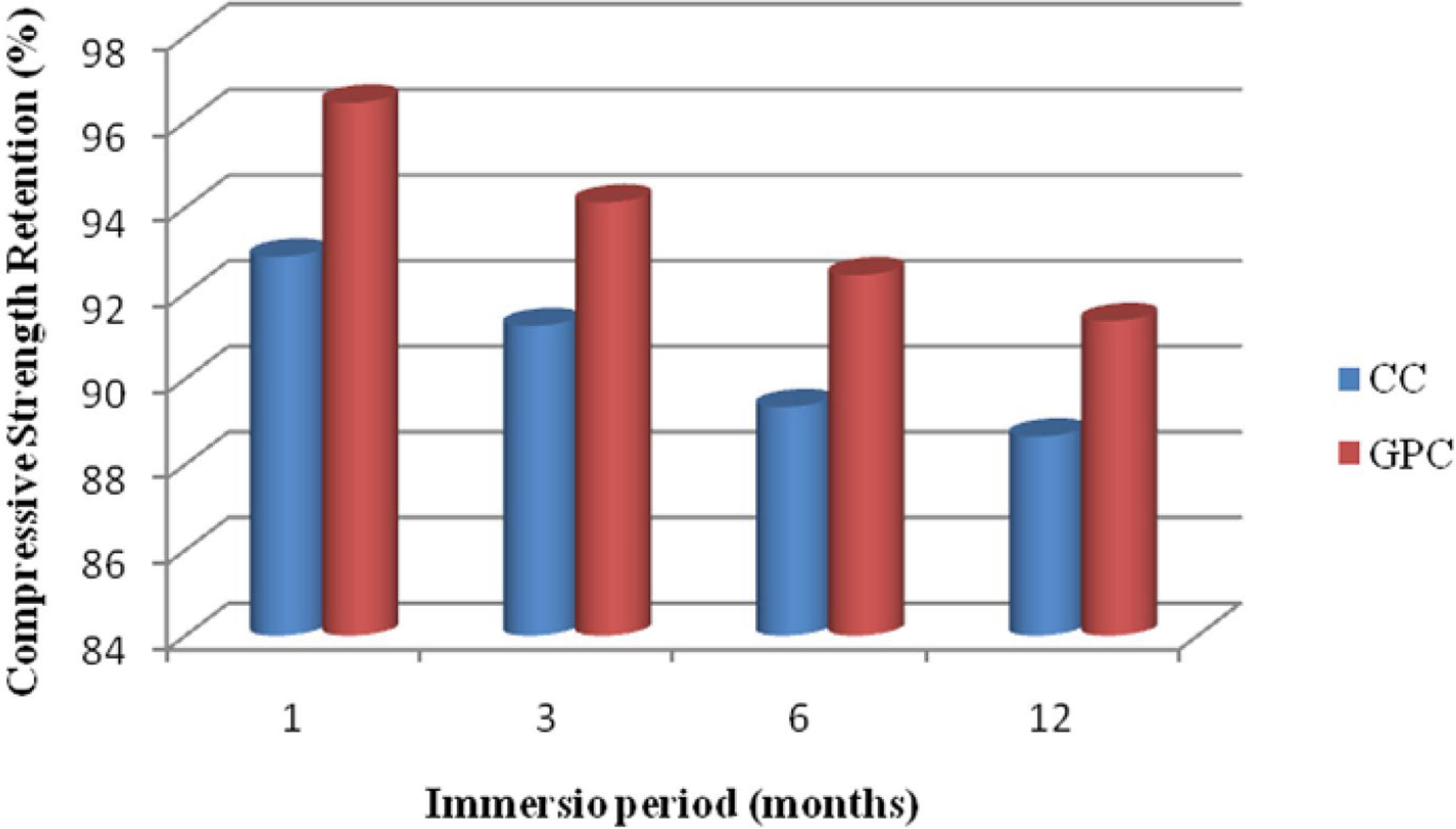
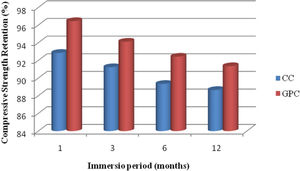
Change in mass and compressive strength retention of CC and GPC due to acid attack.
| S. no. | Immersion period (months) | Change in weight (%) | Residual compressive strength (%) | ||
|---|---|---|---|---|---|
| CC | GPC | CC | GPC | ||
| 1 | 1 month | 2.35 | 1.13 | 92.85 | 96.45 |
| 2 | 3month | 3.94 | 2.47 | 91.23 | 94.12 |
| 3 | 6 month | 4.95 | 3.45 | 89.34 | 92.42 |
| 4 | 12 month | 6.21 | 4.23 | 88.65 | 91.35 |
The strength retention of geopolymer concrete and control concrete are portrayed in Fig. 21. From the test results, it is observed that both GPC and CC have lost their compressive strength retention marginally when they are exposed to acidic medium. The strength retention shows that GPC has demonstrated better performance than CC in resisting acid attack. Further, GPC has exhibited 3.6%, 2.9%, 3.1% and 2.7% higher strength attention than control mix at different exposure period. The strength retention values are depicted in Table 9. In addition, Bakharev investigated the resistance of fly ash base geopolymer concrete against sulphuric acid and acetic acid over a period of 5 months. Test results shows, geopolymer material had excellent performance than cement concrete and the same observation had been replicated in this findings also.
The SEM image of both GPC and CC specimens after 365 days of immersion in sulphuric acid solution are shown in Figs. 22 and 23. From the morphological image of control concrete, it is noticed that formation of elongated crystalline structures of gypsum as it indicates the existence of high amount of gypsum. This may be the reason for high weight loss and for the specimen surface become porous. In case of geopolymer specimens it may be noted that the particles are densely packed because of the fineness of the materials used so that the GPC specimens are more resistant towards the acid attack when compared to CC [54].
SorptivityDamage of the concrete structures occurred due to the movement of destructive substances from the contiguous areas into the concrete. Actually, various construction materials are porous in nature and they are mainly affected by means of moisture access. It is obvious that the higher porosity will adversely affect the performance of the concrete [53,55,56]. The various factors by which the sorptivity of concrete will get affected are mix composition, compaction, aggregate orientation; distribution etc. The existence of moisture content in the structure becomes the primary source of several durability issues. It is to be noted that low absorption properties of concrete will adhere good quality and also the quality of concrete will improve with curing age and the source and type of materials used [57].
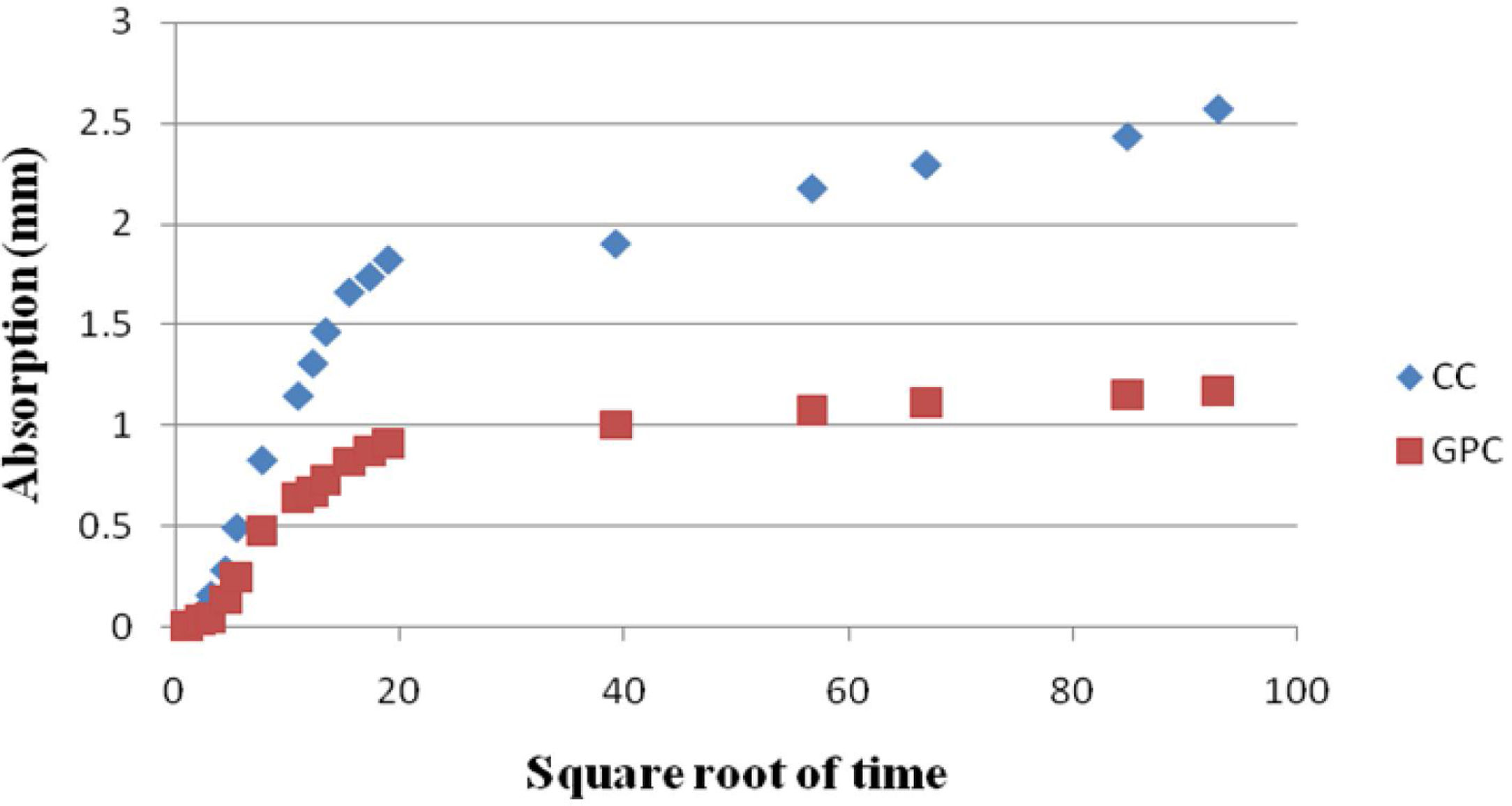
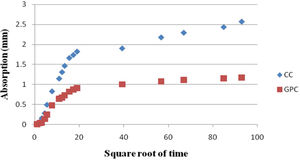
The sorptivity of MK-BA GPC and Control Concrete were assessed up to 6 days (8640min) as per the guidelines prescribed. The test results of geopolymer concrete and control concrete are portrayed in Table 10. From the table it can be noted that, the initial rate of absorption of control concrete as well as geopolymer concrete specimens are found to be 0.096 and 0.048mm/t1/2 respectively for the first 6h of duration. The final rate of absorption is 0.029 and 0.013. From the values obtained at different time period, it is clear that both CC and GPC had exhibited reduction in sorptivity with increase in time. It is mainly because of pore refinement of matrix occurred with the age of concrete. The sorptivity value of GPC is low which means the quality of concrete endorses to be good. Further, higher compressive strength of GPC [36,56] impart a low absorption of water. The low sorptivity value indicates MK-BA GPC was much denser, homogeneous matrix and less permeable when compared to CC. This has been achieved by means of filler effect of source materials micro particles which are finer and also geopolymerization reaction between source materials and alkaline activators.
Sorptivity of CC and GPC.
| Time ‘t’ (min) | Square root of time (t1/2) | Mass (g) | Absorption I | Sorptivity mm/t1/2 | |||
|---|---|---|---|---|---|---|---|
| CC | MK-BA GPC | CC | MK-BA GPC | CC | MK-BA GPC | ||
| 1 | 1.00 | 0.148 | 0.069 | 0.019 | 0.01 | 0.019 | 0.010 |
| 5 | 2.24 | 0.557 | 0.278 | 0.071 | 0.04 | 0.032 | 0.016 |
| 10 | 3.16 | 1.273 | 0.424 | 0.163 | 0.05 | 0.052 | 0.017 |
| 20 | 4.47 | 2.240 | 1.087 | 0.287 | 0.14 | 0.064 | 0.031 |
| 30 | 5.48 | 3.875 | 1.935 | 0.497 | 0.25 | 0.091 | 0.045 |
| 60 | 7.75 | 6.488 | 3.705 | 0.832 | 0.48 | 0.107 | 0.061 |
| 120 | 10.95 | 8.973 | 4.985 | 1.150 | 0.64 | 0.105 | 0.058 |
| 150 | 12.24 | 10.228 | 5.224 | 1.311 | 0.67 | 0.107 | 0.055 |
| 180 | 13.42 | 11.445 | 5.722 | 1.467 | 0.73 | 0.109 | 0.055 |
| 240 | 15.49 | 12.977 | 6.388 | 1.664 | 0.82 | 0.107 | 0.053 |
| 300 | 17.32 | 13.566 | 6.783 | 1.739 | 0.87 | 0.100 | 0.050 |
| 360 | 18.97 | 14.228 | 7.079 | 1.824 | 0.91 | 0.096 | 0.048 |
| 1540 | 39.24 | 14.850 | 7.788 | 1.904 | 1.00 | 0.049 | 0.025 |
| 3220 | 56.75 | 16.985 | 8.365 | 2.178 | 1.07 | 0.038 | 0.019 |
| 4474 | 66.89 | 17.894 | 8.620 | 2.294 | 1.11 | 0.034 | 0.017 |
| 7200 | 84.85 | 18.990 | 8.952 | 2.435 | 1.15 | 0.029 | 0.014 |
| 8640 | 92.95 | 20.050 | 9.130 | 2.571 | 1.17 | 0.028 | 0.013 |
A graph has been plotted between the absorbed water and square root of time and it is depicted in Fig. 24. From the figure it has been observed that the absorption curves are precipitous at the initial period and at the later periods a gentle smooth curve has been noted for both CC and GPC. Further, it has been noted that at the early age the steeper curve indicates the high rate of water absorption whereas the time prolonged the uptake of water due to capillary force was reduced extremely. Moreover, the rate of water absorption with respect to time was relatively less for GPC, while it is increased for CC.
Rapid chloride penetration testIn general, one of the major forms of environmental attack on concrete is that ingression of chloride leads to the corrosion of rebars and subsequent reduction in strength, serviceability and aesthetics of the structures. Rapid chloride penetration test has been carried out on geopolymer concrete and control concrete specimens at the age of 28 days and 90 respectively. The test results are displayed in Table 11 and it is evident from the test results that both GPC and CC exhibited low chloride permeability into the specimens with respective to the age of curing. However, GPC has demonstrated significantly better resistance to chloride ion ingression than control concrete at all ages. The results are in conformity with the results reported by other researchers also [58,59]. RCPT value of cement concrete presents higher value due to the presence of higher concentration of OH ions in pore solution. However, the OH ions in geopolymer concrete are completely consumed during geopolymeric reactions. Moreover, the micro filler effect of the fine particles of MK and BA inhibit the pores and develop more dense and compact micro structure, and thereby reduce the chloride ingression [60].
Water absorptionIt is one of the important properties which influences the strength and durability of the concrete. The concrete with higher water absorption indicates the existence of porous microstructure. The higher porosity in concrete will reduce the strength and durability. From Table 12, it is noticed that water absorption for CC and GPC were found as respectively for 7 and 28 days cured specimens. From the test results, as expected GPC shows a lower percentage of water absorbed than CC. A low percentage of water absorption is a reliable reason for higher compressive strength of geopolymer concrete. From the test results it is observed that, as the day prolongs the percentage of water absorption are comparatively less because of the fineness and also the density of the geopolymer matrix. Also, it is very well known that in the study, water absorption was much lower, which indicates a high degree of geopolymerization [61]. Also, other researcher found that water absorption for GPC is less than 5% and the test results are in conformity with those results [62,63].
DiscussionsThe test results of the study provide a clear understanding about the geopolymer specimens behaviour when they are exposed to different environmental mediums. In fact, the results of the experiments conducted shows that geopolymer materials had good durability when compared with control concrete.
Concrete may be susceptible to sulphate attack in the presence of moisture. Deterioration of concrete occurs when it is exposed to sulphate environment resulting in sulphate transport through the pore system, chemical interaction with the hydration products, stresses due to the formation of the expansive products and also precipitation of salt in pore spaces [45,64]. These reactions cause reduction in strength parameters and also spoil the concrete structure [47]. The GPC has demonstrated relatively higher resistance to sulphate due to absence of Ca (OH)2. Therefore, there is no possibility of gypsum and ettringite to form in geopolymer when it is exposed to sulphte medium [45,65]. Eventually, geopolymer displays relatively higher resistance to sulphate attack.
Usually, acid attacks leach away the calcium compounds of cement paste. MK-BA GPC accomplished good resistance against acid similar to geopolymer concrete made with geopolymer binders such as fly ash, metakaolin, bottom ash, GGBS and blended geopolymer binders [66]. It is due to less content of calcium hydroxide present in GPC. In case of geopolymer, acid attack causes predominantly depletion of sodium consequently and the ejection of tetrahedral aluminium from the alumino-silicate framework reduces the strength marginally but it is on par when compared with CC [67]. In case of control concrete, sulphuric acid reacts with Ca(OH)2 leading to the formation of expansive gypsum. Subsequently, the gypsum reacts with C3A and forms an ettringite However, the formation of expansive gypsum, ettringite, decalcification of CSH due to the attack of sulfuric acid reduce the strength and durability of control concrete significantly [15].
It is clear that both the GPC and control concrete exhibited reduction in sorptivity with increase in age of concrete. It is obvious that the pore refinement of the matrix takes place with the age of concrete resulting in reduction in sorptivity of concrete. However, the sorptivity of GPC is relatively less than control at all ages. The sorptivity of GPC at various ages is in perfect agreement with compressive strength. The compressive strength of MK-BA geopolymer concrete has continuously increased with decrease in sorptivity [56]. Further, the reduction in pore size of geopolymer concrete thereby avoiding the access of hostile liquid into the pore system [13]. Also, the higher sorptivity value of control concrete may be due to the existence of CaOH2. Further, the formation of calcium hydroxide during the hydration of cement results in poor microstructure and thereby higher sorptivity value [61,68].
One of the major forms of environmental attack on concrete is chloride ingression leading to corrosion of rebars and subsequently reduction in strength serviceability and aesthetics of the structure. Moreover, the poor quality of concrete and also shallow reinforcement quickly brings chlorides to some depth in the concrete and also reduces diffusion distance to reach rebar [69,70]. The rate of ingression of chloride ions depends on pore structure of concrete and in turn depends on type of binder, chemical admixture, mineral admixture, W/B ratio, gradation of aggregates, mix proportion, etc. [71–73]. In the present study, GPC had exhibited low chloride ion ingression as per the codal provision and also it confirms with the other researcher values too. Further, the same trend has been followed in the water absorption.
ConclusionsIn the present experimental study, the effect of metakaolin and bottom ash blended geopolymer concrete which are cured under ambient temperature was studied and their durable properties were investigated. Also, the performance of geopolymer concrete was compared with control concrete in terms of strength retention, mass gain, chloride penetration and sorptivity. From the test results, the following conclusions were drawn:
- 1.
The geopolymer concrete specimens had very good durability characteristics when they are exposed to sulphate medium. The solidity of the tested geopolymeric specimens depends on the type of activators used for the GPC preparation, concentration and type of cations existed in the sulphate medium. In addition to the exodus of alkalis from the geopolymer specimens into the sulphate solution, there is also diffusion of Mg and Ca in the specimens which has impact on strength improvement. But in case of control concrete, sulphate reacts with CaOH2, C-A-S and CSH leading to the formation of ettringite and also gypsum at later age which results in strength decrement.
- 2.
The deterioration of geopolymer materials in acidic medium is connected with depolymerization of aluminosilicate polymers and also condensation of siliceous polymers and zeolites formation which in turn direct to the loss of strength retention. In acidic environment, the deterioration of geopolymer materials is mainly because of formation of fissures in geopolymer matrix which leads to fragile grainy structures. However, the formation of expansive gypsum, ettringite and decalcification of CSH due to the attack of sulphuric acid leads to reduction in strength of the control concrete.
- 3.
Geopolymer specimens show considerably less sorptivity than the control concrete. The low sorptivity indicates that the GPC is comparatively more impervious than control concrete and also it can be control chloride ion ingression, also in water absorption GPC shows better performance than the control concrete.