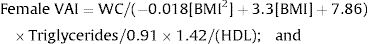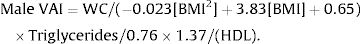Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in children and it is more prevalent in Hispanic males. The gender differences can be explained by body fat distribution, lifestyle, or sex hormone metabolism. We evaluated anthropometric and metabolic differences by gender in children with and without NAFLD.
MethodsWe included 194 participants (eutrophic, overweight, and individuals with obesity). The presence of NAFLD was determined using ultrasonography, and we evaluated the association between this disease with metabolic and anthropometric variables by gender.
ResultsThe mean age was 10.64±2.54 years. The frequency of NAFLD in boys was 24.51% and in girls was 11.96% (OR=2.39; 95%CI=1.10–5.19; p=0.025). For girls, NAFLD was significantly associated with triglycerides (p=0.012), homeostatic model assessment of insulin resistance (HOMA-IR) (p=0.048), and the visceral adiposity index (VAI) (p=0.024). The variables related to NAFLD in a gender-specific manner were body mass index (BMI) (p=0.001), waist circumference (WC) (p<0.001), HDL cholesterol (p=0.021), alanine aminotransferase (ALT) (p<0.001), and aspartate aminotransferase (AST) (p=0.002).
ConclusionsIn our study NAFLD is more frequent in boys, only ALT, and no other clinical or metabolic variables, were associated with NAFLD in these patients. HOMA-IR, VAI, triglyceride levels, and ALT were associated with NAFLD only in girls. The ALT cut-off points for the development of NAFLD in our study were 28.5U/L in females and 27.5U/L in males. Our findings showed that NAFLD should be intentionally screened in patients with obesity, particularly in boys.
Non-alcoholic fatty liver disease (NAFLD) is defined by the presence of steatosis in more than 5% of hepatocytes in patients with no history of alcohol consumption, no pharmacological treatment, and no congenital disease [1,2]. NAFLD is the most frequent type of liver disease in the pediatric population, with an overall prevalence of 7.6% and a cumulative prevalence of 34.2% in children with obesity [3]. Epidemiological studies of NAFLD in Mexico show a prevalence of 17.05% in asymptomatic adults and 12.6% in children with a body mass index (BMI) >85th percentile (overweight and obesity) [4,5].
Cross-sectional studies on humans and animal models have reported a higher prevalence of NAFLD in the male gender. In the pediatric population with obesity, the distribution by sex is 35.3% in males and 21.8% in females [3]. Central obesity, insulin resistance, dyslipidemia, Hispanic ethnicity, and the genetic variants COL13A1, ADIPOQ, SAMM50, and PNPLA3 in Mexican Americans and the Mexican-Mestizo population are the variables that are most frequently associated with NAFLD [6–9].
Although a higher prevalence of NAFLD has been reported in boys, gender-specific characteristics of this disorder have rarely been explored. It has been proposed that the gender-specific differences observed in the prevalence of NAFLD may result from the possible protective role of estrogens, which decrease the risk for this disorder among females. Recent findings on the physiopathology of NAFLD, evaluated from a gender-specific perspective, have indicated that lipid metabolism and body fat distribution may play a relevant role [10,11].
Pediatric patients are not frequently screened for NAFLD, and thus, it is rarely identified, primarily because of the complexity of the diagnosis [2,12]. This study aimed to evaluate the gender-specific differences in the clinical and metabolic variables in a pediatric population with NAFLD.
2Methods2.1Study designA cross-sectional comparative study was performed. Eutrophic, overweight, and individuals with obesity and ranging in age from 6–18 years were consecutively included from January 2015 to September 2016. Patients with overweight/obesity from the Childhood Obesity Clinic of the Hospital General de Mexico were invited to participate, whereas eutrophic children were recruited from surrounding schools. All patients included in this study were born and raised in Mexico City and the suburban area. The local ethics committee approved the protocol.
The participants were classified into the following groups, according to the Centers for Disease Control and Prevention guidelines: eutrophic (BMI <85th percentile); overweight (BMI ≥85th and <95th percentile); and obesity (BMI ≥95th percentile).
Patients with obesity that had a genetic or endocrine origin; those with a previously known liver disease, diabetes or other endocrinopathies; patients receiving a pharmacological treatment that was able to modify lipid or carbohydrate metabolism; and those who reported alcohol consumption were excluded.
Informed consent and assent were obtained from the participants and their parent/legal guardian. All sensitive patient data were protected in compliance with the Health Insurance Portability and Accountability Act (HIPAA). A complete medical record was documented for each participant.
The NAFLD diagnosis was determined by a liver ultrasound that was performed by an expert ultrasonographer.
2.2Anthropometric and metabolic evaluationThe clinical variables assessed were age, gender, weight, height, BMI, waist circumference (WC), and sexual development stage.
A mechanical scale and a stadiometer were used to evaluate weight and height. The measuring location for WC was the midway point between the last costal cartilage and the anterosuperior iliac crest. Pediatricians and pediatric endocrinologists appraised the sexual status, according to the Tanner scale [13,14]. Arterial blood pressure was measured from the right brachial artery using a manual sphygmomanometer and special-sized pressure cuffs according to the patient's age, and the measurement was taken twice per patient, with the patient in a sitting position.
We calculated the BMI according to Quetelet's equation [15]. Additionally, using the homeostatic model assessment of insulin resistance (HOMA-IR), we calculated the insulin resistance index [16].
A gender-specific visceral adiposity index (VAI) was calculated using a formula that was previously proposed by our group for pediatric populations, as follows:
Metabolic variables were evaluated by obtaining venous blood after the patients had fasted for 12h. Enzymatic methods were performed to measure glucose, aminotransferases, total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, and triglycerides, using available commercial kits. Insulin was determined through chemiluminescence analysis. Normal values were considered according to the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the Endocrine Society clinical practice guidelines for NAFLD and pediatric obesity, respectively (alanine aminotransferase (ALT) <25U/L in boys and <22U/L for girls, HDL <40mg/dL, and triglycerides in patients 0–9 years <100mg/dL and 10–19 years <130mg/dL) [17,18].
2.3UltrasonographyThe NAFLD diagnosis was performed using a B mode ultrasound and a 1–4mHz curve transducer (Siemens Acuson S2000, Mountain View, CA, USA), according a standardized protocol, by an expert ultrasonographer who was blinded to the clinical and metabolic conditions [19,20]. The kappa coefficient for intra-observer concordance was 0.90. The steatosis condition was categorized as present/not present, according to the hepatic tissue's echogenicity, in contrast to the adjunct right kidney's parenchyma.
2.4Statistical analysesDescriptive and inferential statistics for numerical variables were reported as the mean and standard deviation (SD), and the percentages were used for categorical variables.
The inferential statistical analyses included a two-factor analysis of variance (ANOVA) for all anthropometric and metabolic variables, considering the presence of NAFLD and gender as factor variables, and we ensured that the test assumptions were met. The effect size was calculated using Cohen's f and was considered to be as follows: >0.10 small; >0.25 medium; and >0.40 large [21,22].
Chi-squared tests and Fisher's exact test were used to assess categorical variables. Binary logistic regression evaluated the association between NAFLD and BMI-Pc, ALT, aspartate aminotransferase (AST), WC by gender, and triglycerides by sex [23].
The most relevant variables related to NAFLD were evaluated using the receiver operating characteristics (ROC) curve analysis, according to gender. The cut-off points, sensitivity, and specificity were obtained for each variable [24,25].
All analyses were performed using the statistical software IBM SPSS Statistics version 22.0 (IBM, Armonk, NY, USA). A p value of <0.05 was considered to be statistically significant.
3Results3.1Descriptive statisticsA total of 194 patients (102 males and 92 females) were recruited: 44 eutrophic, 38 overweight, and 112 with obesity. The mean age of the participants was 10.64±2.54 years.
The overall NAFLD frequency in our study was 18.55%; it was 24.51% in boys and 11.96% in girls, which was significantly different (OR=2.39; 95%CI=1.10–5.19; p=0.025).
In the obesity group, the frequency was 29.46%: 32.8% in males and 24.4% in females (OR=1.51; 95%CI=0.65–3.54; p=0.340). We found that 91.7% of the patients with a NAFLD were obese.
The participants’ demographic and anthropometric characteristics (by gender) are shown in Table 1.
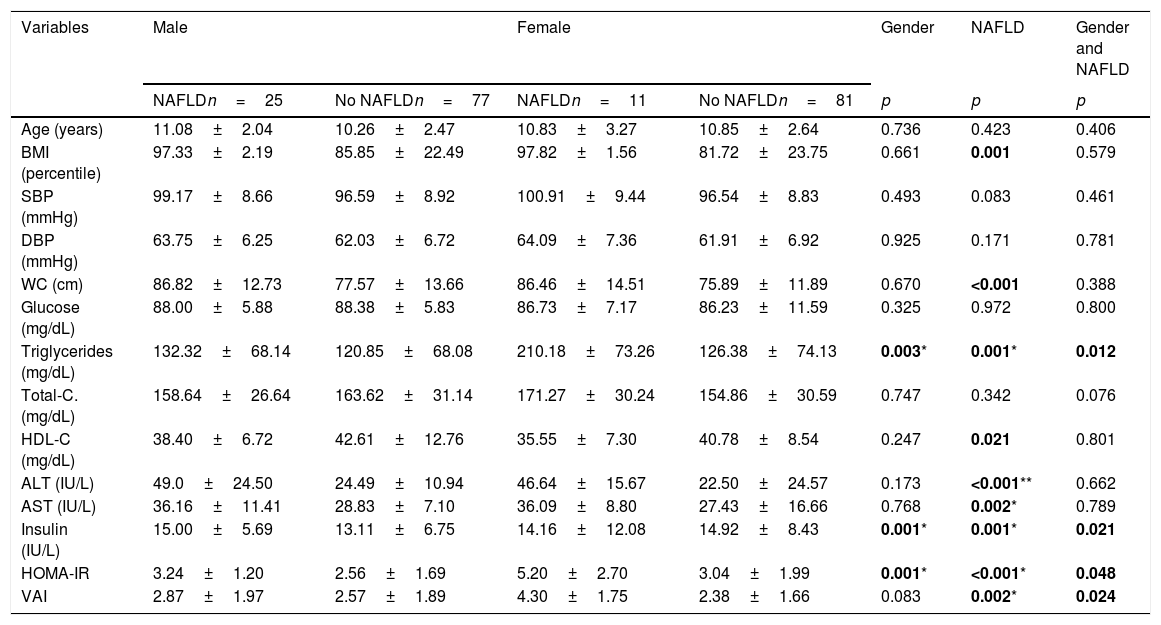
Clinical and metabolic variables associated to NAFLD by gender in Mexican children.
| Variables | Male | Female | Gender | NAFLD | Gender and NAFLD | ||
|---|---|---|---|---|---|---|---|
| NAFLDn=25 | No NAFLDn=77 | NAFLDn=11 | No NAFLDn=81 | p | p | p | |
| Age (years) | 11.08±2.04 | 10.26±2.47 | 10.83±3.27 | 10.85±2.64 | 0.736 | 0.423 | 0.406 |
| BMI (percentile) | 97.33±2.19 | 85.85±22.49 | 97.82±1.56 | 81.72±23.75 | 0.661 | 0.001 | 0.579 |
| SBP (mmHg) | 99.17±8.66 | 96.59±8.92 | 100.91±9.44 | 96.54±8.83 | 0.493 | 0.083 | 0.461 |
| DBP (mmHg) | 63.75±6.25 | 62.03±6.72 | 64.09±7.36 | 61.91±6.92 | 0.925 | 0.171 | 0.781 |
| WC (cm) | 86.82±12.73 | 77.57±13.66 | 86.46±14.51 | 75.89±11.89 | 0.670 | <0.001 | 0.388 |
| Glucose (mg/dL) | 88.00±5.88 | 88.38±5.83 | 86.73±7.17 | 86.23±11.59 | 0.325 | 0.972 | 0.800 |
| Triglycerides (mg/dL) | 132.32±68.14 | 120.85±68.08 | 210.18±73.26 | 126.38±74.13 | 0.003* | 0.001* | 0.012 |
| Total-C. (mg/dL) | 158.64±26.64 | 163.62±31.14 | 171.27±30.24 | 154.86±30.59 | 0.747 | 0.342 | 0.076 |
| HDL-C (mg/dL) | 38.40±6.72 | 42.61±12.76 | 35.55±7.30 | 40.78±8.54 | 0.247 | 0.021 | 0.801 |
| ALT (IU/L) | 49.0±24.50 | 24.49±10.94 | 46.64±15.67 | 22.50±24.57 | 0.173 | <0.001** | 0.662 |
| AST (IU/L) | 36.16±11.41 | 28.83±7.10 | 36.09±8.80 | 27.43±16.66 | 0.768 | 0.002* | 0.789 |
| Insulin (IU/L) | 15.00±5.69 | 13.11±6.75 | 14.16±12.08 | 14.92±8.43 | 0.001* | 0.001* | 0.021 |
| HOMA-IR | 3.24±1.20 | 2.56±1.69 | 5.20±2.70 | 3.04±1.99 | 0.001* | <0.001* | 0.048 |
| VAI | 2.87±1.97 | 2.57±1.89 | 4.30±1.75 | 2.38±1.66 | 0.083 | 0.002* | 0.024 |
Values expressed as means±standard deviation.
NAFLD: non-alcoholic fatty liver disease; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; C: cholesterol; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HOMA-IR: homeostatic model assessment; VAI: visceral adiposity index.
Effect size (f)=*0.10–0.24; **0.25–0.39.
Bold values arepvalues <0.05.
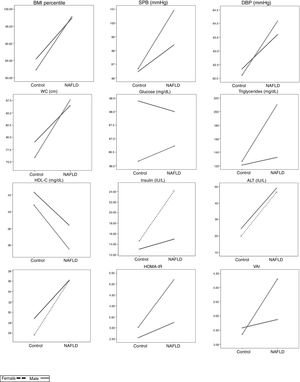
Differences in anthropometric and metabolic variables by gender and the presence of NAFLD were assessed using two-factor ANOVAs.
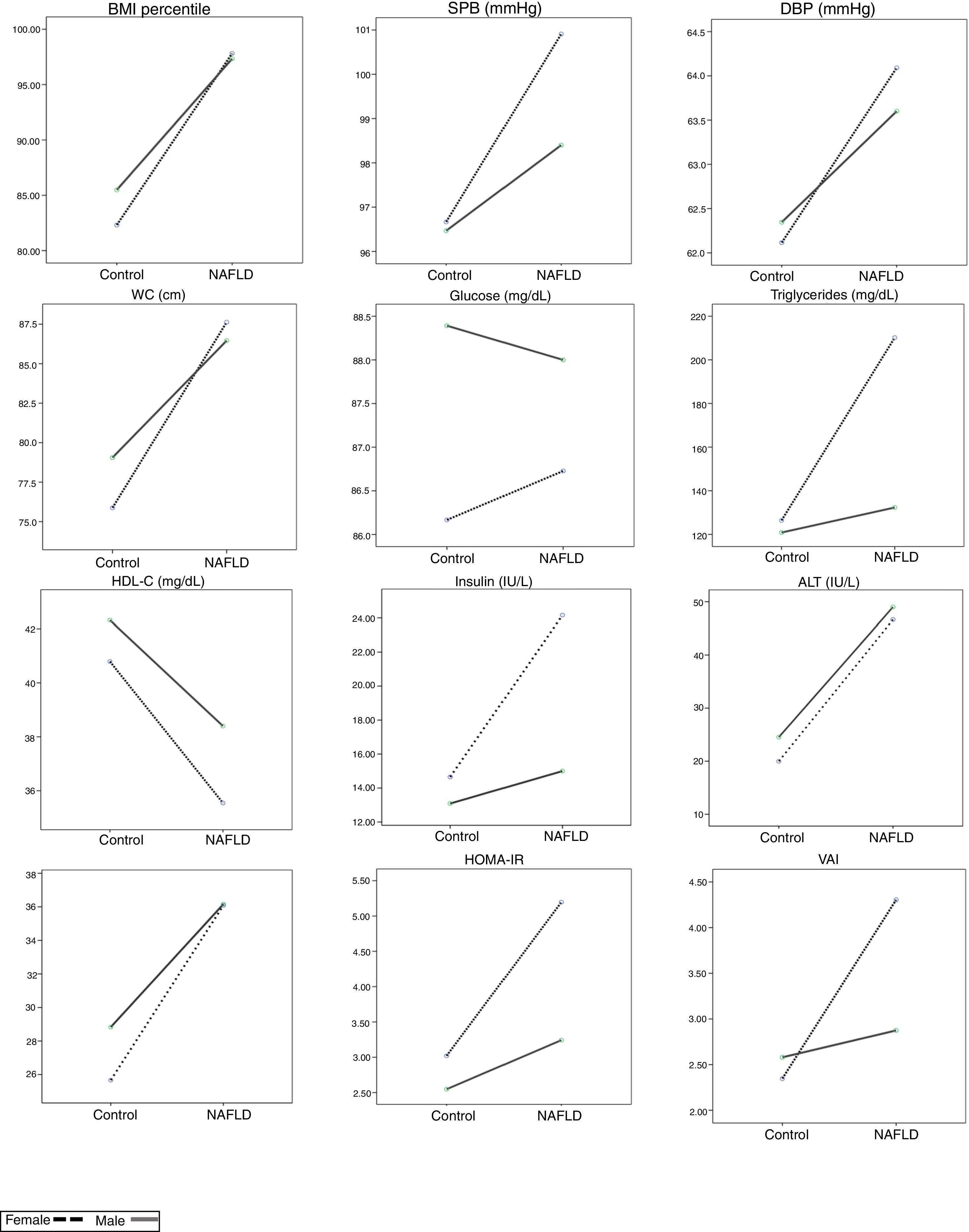
We found statistically significant differences between gender and the presence of NAFLD for the following variables: triglycerides (F(1,185)=6.618, p=0.012, f=0.0895); insulin (F(1,174)=6.197, p=0.021, f=0.087); HOMA-IR (F(1,174)=4.28, p=0.048, f=0.0895), and VAI (F(1,185)=5.47, p=0.02, f=0.0959). The combined effect of the presence of NAFLD and gender was also evaluated for all significant ANOVAs. The mean triglyceride concentration in males with and without NAFLD and in females with and without NAFLD were 132.32±68.14, 120.85±68.08, 210.18±73.26, and 126.38±74.13mg/dL, respectively. When comparing differences in triglyceride concentrations among the male and female patients with and without this disease, we found that the mean triglyceride concentration in female NAFLD patients was 77.86mg/dL higher than that in males. Similarly, in the group without NAFLD, the triglyceride levels were higher in females compared to males, with a mean difference of 5.53mg/dL. The difference between the differences was 70.993 (95%CI, 15.966–126.02, p=0.012).
For insulin concentrations, the mean levels in males with and without NAFLD and in females with and without this disorder were 15.00±5.69, 13.11±6.75, 14.16±12.08, and 14.92±8.43 UI/L, respectively. In a mean comparison by gender and the presence of NAFLD, male NAFLD patients had higher insulin concentrations compared to females, with a mean difference of 0.84 UI/L, whereas among patients without NAFLD, females had higher insulin concentrations, with a mean difference of 1.81 UI/L. The difference between the differences was 7.357 (95%CI, 1.145–13.569, p=0.021).
The mean HOMA-IR levels in males with and without NAFLD and in females with and without NAFLD were 3.24±1.20, 2.56±1.69, 5.20±2.70, and 3.04±1.99, respectively. In a mean comparison by gender and the presence of NAFLD, in patients with NAFLD, females had a higher mean HOMA-IR level compared to males, with a mean difference of 1.96 points. Similarly, in patients without NAFLD, the HOMA-IR level was also higher in females than in males, with a smaller mean difference of 0.48 points. The difference between the differences was 1.471 (95%CI, 0.013–2.928, p=0.048).
For VAI scores, the mean values in males with and without NAFLD and in females with and without NAFLD were 2.87±1.97, 2.57±1.89, 4.30±1.75, and 2.38±1.66, respectively. In a mean comparison by gender and the presence of NAFLD, in NAFLD patients, the VAI scores were higher in females compared to males, with a mean difference of 1.43 points. Similarly, among the participants without NAFLD, females had higher VAI scores than males, with a mean difference of 0.19 points. The difference between the differences was 1.62 (95%CI, 0.215–3.024, p=0.024) (Table 1 and Fig. 1).
Clinical and metabolic variables adjusted by gender and NAFLD condition in Mexican children.
On X axis NAFLD condition is expressed. The values on Y axis express the estimated marginal means.
NAFLD: non-alcoholic fatty liver disease; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; WC: waist circumference; Chol.: cholesterol; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HOMA-IR: homeostatic model assessment – insulin resistance; VAI: visceral adiposity index.
Female:
; Male: .For the variables that did not demonstrate significant interactions in factorial ANOVAs, the main effects were evaluated. The following significant main effects from NAFLD were observed: for BMI-Percentile (Pc) the mean was 97.48±2.01 for patients with NAFLD vs. 83.87±22.52 for those without NAFLD (p=0.001); for WC, the mean was 86.82±12.73 with NAFLD vs. 77.43±13.47 without NAFLD (p<0.001); for HDL cholesterol, the mean was 37.53±6.93 with NAFLD vs. 41.54±10.21 without NAFLD (p=0.018); for ALT, the mean was 48.28±21.98 with NAFLD vs. 22.22±9.81 without NAFLD (p<0.001); and for AST, the mean was 36.14±10.55 with NAFLD vs. 27.22±6.58 without NAFLD (p=0.002; Table 1 and Fig. 1).
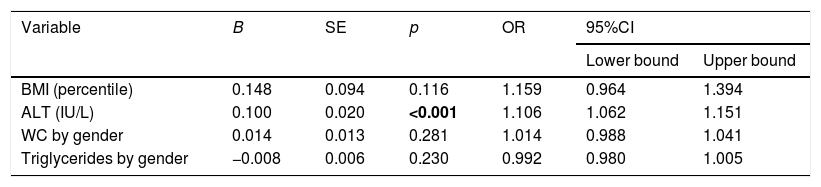
A binomial logistic regression was performed to ascertain the effects of BMI percentile, ALT, WC by gender, and triglycerides by gender on the likelihood that participants would have NAFLD. The logistic regression model was statistically significant (χ2(6)=79.47, p<0.0001). The model explained 54.9% (Nagelkerke's R2) of the variance in NAFLD and correctly classified 81.2% of the cases. The sensitivity of the model was 79%, and specificity was 90%; the positive predictive value was 53%, and the negative predictive value was 97%. The model was then internally validated using the ROC curve. The AUC value was 0.922 (95%CI=0.877–0.966, p<0.0001). The only statistically significant variable was ALT (Table 2).
Logistic regression predicting likelihood of NAFLD in Mexican children based on BMI, ALT, WC by gender and triglycerides by gender.
| Variable | B | SE | p | OR | 95%CI | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| BMI (percentile) | 0.148 | 0.094 | 0.116 | 1.159 | 0.964 | 1.394 |
| ALT (IU/L) | 0.100 | 0.020 | <0.001 | 1.106 | 1.062 | 1.151 |
| WC by gender | 0.014 | 0.013 | 0.281 | 1.014 | 0.988 | 1.041 |
| Triglycerides by gender | −0.008 | 0.006 | 0.230 | 0.992 | 0.980 | 1.005 |
NAFLD: non-alcoholic fatty liver disease; BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; WC: waist circumference; SE: standard error; OR: Odds Ratio; CI: confidence interval. Bold values are p values <0.05.
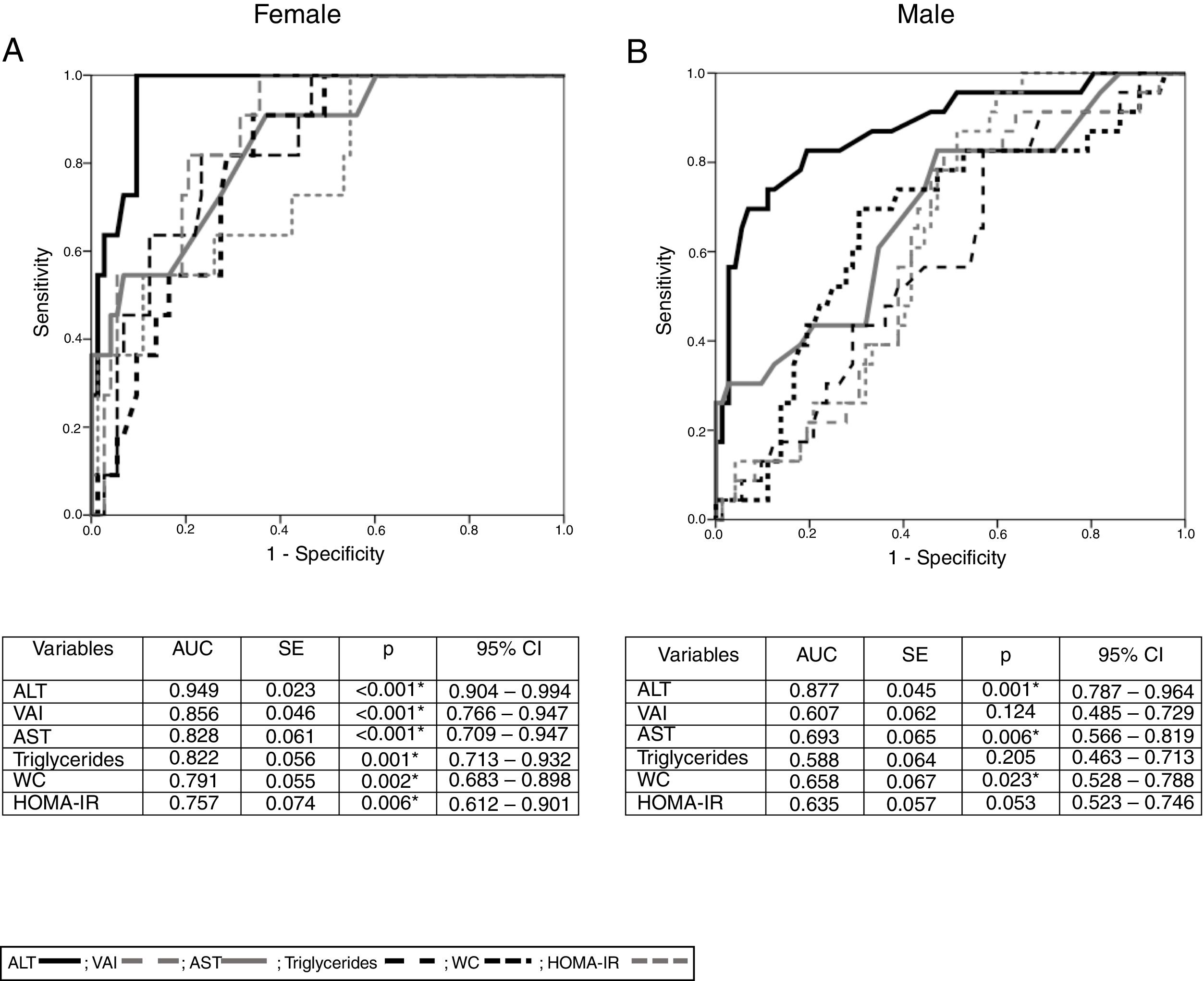
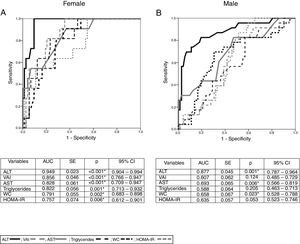
With the aim of evaluating the accuracy of the variables associated with NAFLD by gender, we performed receiver-operating characteristics (ROC) curve discrimination. For female participants with NAFLD, we found a significant AUC value for ALT, AST, WC, HOMA-IR, triglycerides, and VAI values. Based on these results, and by calculating the Youden index, we obtained the cut-off points for these variables in males and females. In females, the optimal cut-off points were as follows: ALT, 28.5U/L (sensitivity 100%, specificity 89.2%); AST, 27.5U/L (sensitivity 90.90%, specificity 62.20%); WC, 79.5cm (sensitivity 90.90%, specificity 64.90%); HOMA-IR, 2.08 (sensitivity 100%, specificity 44.6%); triglycerides, 128mg/dL (sensitivity 81.8%, specificity 69%); and VAI score, 2.33 (sensitivity 100%, specificity 63.5%).
In male patients, the only significant AUC value was found for ALT concentrations, and the cut-off point was 27.5U/L (sensitivity 82.60%, specificity 80.60%; Fig. 2).
ROC curves for NAFLD by gender and ALT, AST, WC, VAI, HOMA-IR and triglycerides.
(A) Figure and table for female ROC curve. (B) Figure and table for male ROC curve.
NAFLD: non-alcoholic fatty liver disease; ALT: alanine aminotransferase; AST: aspartate aminotransferase; WC: waist circumference; VAI: visceral adiposity index; HOMA-IR: homeostatic model assessment – insulin resistance; AUC: area under curve; SE: standard error; CI: confidence interval; *p<0.05.
ALT:
; VAI: ; AST: ; triglycerides: ; WC: ; HOMA-IR: .The presence of metabolic syndrome (MS) in females with and without NAFLD was statistically significant, as shown by Fisher's exact test (OR=9.019; 95%CI=2.30–35.30; p=0.002). However, in males with and without NAFLD, there was no significant difference (OR=1.125; 95%CI=0.36–3.51; p=0.84).
4DiscussionOur study results revealed gender-specific differences in metabolic and anthropometric variables related to NAFLD. Moreover, we found evidence that the variables that were positively associated with insulin resistance, such as insulinemia, HOMA-IR, triglyceride concentration, and VAI scores, were also associated with NAFLD in a gender-specific manner. For example, VAI, HOMA-IR, and triglycerides were significantly higher in female patients with NAFLD compared to males. However, in the regression analysis, the only variable that significantly predicted the presence of NAFLD was ALT, which increased the risk of NAFLD by 10% for each unit increment for both genders.
Mexico currently has one of the highest proportions people with obesity, and consistent with the prevalence of NAFLD, the frequency of NAFLD in our obesity group was 29.46%. The development of NAFLD requires a complex interaction of multifactorial features such as genetic susceptibility and environmental factors. Ramos-López et al. analyzed the pattern of dietary intake in a Mexican population with and without liver disease, and they found that both have a diet high in fat and cholesterol that lacks polyunsaturated fatty acids and some micronutrients with antioxidant properties that have been associated with the development of liver damage in the general population. The previous statements could explain the relationship between pediatric patients and the development of NAFLD through the influence of genetic variants of risk, a sedentary lifestyle, and the Mexican obesogenic diet [26].
In our study, the frequency of NAFLD by gender was higher in boys compared to girls, with a 2:1 ratio. Previous studies that were included in an epidemiological meta-analysis of NAFLD showed results similar to ours. Although the diagnostic methodologies used in the studies included in this meta-analysis were heterogeneous, the most frequently used method for diagnosing NAFLD was ultrasonography [3,11,27].
NAFLD is associated with various anthropometric and metabolic factors, such as high BMI, ethnic group (with a higher prevalence in Asian and Hispanic patients), male gender, insulin resistance, and dyslipidemia. BMI has also been related to increased visceral and profound subcutaneous adiposity, which are also considered to be risk factors for NAFLD [28]. In our study, univariate analyses performed by gender and the presence of NAFLD showed that in males, the only variables that were significantly related to NAFLD were ALT and those that indirectly reflect visceral adiposity (BMI and WC). However, in females, NAFLD was also associated with a higher BMI, WC, and ALT, as well as higher insulin, HOMA-IR, and VAI. Although in previous studies the variables involved in NAFLD and insulin resistance are frequently reported, we found that there is a gender-specific association between these variables and the female population [2,6,7].
Our results also showed a significant association between NAFLD and the BMI-Pc, WC, HDL-cholesterol, and ALT concentration, with no differences observed between the genders. This finding is in accordance with that of Lonardo et al. and Singh et al., who reported that BMI, WC, waist-to-height ratio, and waist-to-hip ratio are gender-independent predicting factors for NAFLD in adults [29,30].
ROC curve discrimination analysis by gender showed a significant association between NAFLD and AST, ALT, triglycerides, HOMA-IR, insulin, and VAI only in girls. In boys, the only variable that behaved as a possible diagnostic biomarker was ALT, confirming the gender-specific behavior. The means for ALT in both genders from our study are aligned with the cut-off points that are recommended by the SAFETY study and the Council for the Treatment of Child Obesity published by the Endocrine Society [18,31].
In the last two decades, researchers worldwide have described NAFLD as a condition with sexual dimorphism. Given the complex multifactorial pathophysiology of NAFLD, research on the sexual dimorphism inherent to this disease may lead us to a better understanding of the pathophysiological processes that are involved [11,29].
Women tend to have more subcutaneous adipose tissue and higher leptin levels, which, combined with estrogen production, protects them from the accumulation of fatty tissue in the visceral compartment. In contrast, the distribution of adipose tissue in males is primarily visceral, a condition that is associated with insulin resistance and a greater influx of free fatty acids into the portal venous system, thereby promoting NAFLD [10,11,32]. In our study, we did not observe an association between NAFLD and any of the factors related to insulin resistance in boys.
The influence of sex steroids on metabolic disturbances remains an unanswered question because hyperandrogenic women as well as men with androgen deficiency tend to develop abdominal obesity, insulin resistance, type 2 diabetes, and NAFLD, and they have a higher cardiovascular risk [33].
Moreover, women with polycystic ovarian syndrome (PCOS) have an increased prevalence of NAFLD compared to age and BMI-paired controls (OR=2.54; 95%CI=2.19–2.95), and NAFLD-PCOS women have an androgen bioavailability that increases independently of BMI and insulin resistance [34–36]. Conversely, androgen deficiency in men has been related to NAFLD, independent of other known risk factors such as insulin resistance and visceral adiposity [37]. These studies show that female androgen excess and male androgen deficiency have similar metabolic phenotypes, showing the complexity of androgens’ role in metabolism.
The variables in our study that were associated with NAFLD in females are consistent with those observed in previous reports, and these include insulin resistance, hypertriglyceridemia, and visceral adiposity (evaluated as VAI and WC in our study). Given that we did not find an association between these variables and NAFLD in males, we believe that it is possible that the pathophysiological mechanisms in this population may differ from those involved in insulin resistance.
For lipid metabolism, studies performed in humans and in animal models have shown an increased ability in females to metabolize free fatty acids by lipolysis. However, males can have prolonged de novo lipogenesis, which may condition them for a longer period of free fatty acid exposure and their associated damage. These changes could help us to understand why men are more likely to develop NAFLD [38].
A study performed in the United Kingdom evaluated fatty acid metabolism according to gender, and it showed that the beta-oxidation during fasting in men is less efficient compared to women, and it also has longer lasting de novo lipogenesis. Both phenomena may promote esterification and storage of free fatty acids in the liver, and consequently, stimulate the development of NAFLD in the male population [38].
Experimental models have often shown that androgens and estrogens are balanced in the modulation of hepatic lipid metabolism. Zhang et al. suggested that the combined administration of selective androgen and estrogen receptor modulators could be a potential treatment for patients with NAFLD [39]. Considering that there is a fluctuation of estrogens or androgens in teenagers, we attempted to evaluate the effect of these hormones by pubertal stage; however, there were no differences between pubertal and prepubertal patients.
The Metabolic syndrome (MS) is a critical factor that is related to NAFLD, to the extent that NAFLD has been considered by many to be the hepatic manifestation of this syndrome [40,41]. Concerning the association of this disorder with MS, it is relevant to say that in our study, a statistically significant relationship was observed between NAFLD and MS in females but not in males. The frequency of NAFLD in patients with fewer than three criteria for MS in our study was 63.9%. These results are clinically relevant, considering that in children with obesity, the presence of MS is believed to be the most important risk factor for the development of other comorbidities [42,43]. Our results contribute to the evidence that supports considering each component of the MS as a risk factor for NAFLD, particularly hypertriglyceridemia (>110mg/dL) [44]. Srinivas et al. studied, from a gender perspective, the association of NAFLD with MS component risk factors in an urban cohort, and they found that the components related to this disorder in women were hyperglycemia and hypertriglyceridemia, whereas in men, only BMI was related (used as a criterion for MS instead of WC) [27]. In our study, the only risk component among males was the indirect measure of adiposity (WC), while in females, it was low HDL levels and hypertriglyceridemia.
This study has some limitations. The most relevant limitation is that the diagnosis of NAFLD was not performed using the gold standard (liver biopsy), because of the inherent risks related to the procedure and ethical issues. We evaluated NAFLD using ultrasonography because of accessibility in our population.
According to our study, ALT, AST, WC, triglycerides, HOMA-IR, and VAI were only related to NAFLD in the female population. Logistic regression showed that only ALT was predictive of NAFLD in both genders. The ALT cut-off points for the development of NAFLD in our study were 28.5U/L (sensitivity 100%, specificity 89.2%) in females and 27.5U/L (82.60% sensitivity, 80.60% specificity) in males. Although ALT was the only predictor for NAFLD in boys, it does not have the same diagnostic efficiency as it does in females.
5ConclusionsWe demonstrated that, although NAFLD is more frequent in boys, only ALT, and no other clinical or metabolic variables, were associated with NAFLD in these patients. HOMA-IR, VAI, triglycerides levels, and ALT were associated with NAFLD only in girls.
The ALT cut-off points for the development of NAFLD in our study were 28.5U/L in females and 27.5U/L in males. Our results suggest that we may consider each MS criterion individually as a risk factor for developing NAFLD. Our findings showed that NAFLD should be strongly suspected and that patients with obesity should be intentionally screened for NAFLD, particularly boys.
Further research is required using non-invasive methods and biomarkers that can help us to predict NAFLD in children and teenagers.AbbreviationsNAFLD non-alcoholic fatty liver disease homeostatic model assessment of insulin resistance visceral adiposity index body mass index waist circumference high density lipoprotein alanine aminotransferase aspartate aminotransferase Health Insurance Portability and Accountability Act low density lipoprotein standard deviation analysis of variance receiver operating characteristics area under curve polycystic ovarian syndrome free androgen index
Informed consent and assent were obtained from the participants and their parent/legal guardian. All sensitive patient data were protected in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
FundingThis study was funded by grant CONACYT-SALUD-2013-1-202499.
Author contributionsVOE and GNN conceived the idea and evaluate the patients.
VOE, GNN, LAJC and EG contributed to the interpretation of the results.
GHMJ, QG and CCS contribute to patients recruitment and collected the data.
HRA performed the ultrasonographic evaluation.
LAJC and LSE performed the statistical analysis of the data.
All authors discussed the results and contribute to the final manuscript.
Conflict of interestThe authors have no conflicts of interest to declare.
The authors are grateful to all the patients for their collaboration in this study.