The fibrosis score 4 (FIB-4) has been identified as a biochemical surrogate for histological fibrogenesis and fibrosis in cirrhosis. This study investigates the impact of preoperative FIB-4 on postoperative liver failure of patients with hepatocellular carcinoma (HCC).
Materials and methodsData from 205 patients who underwent curative resection for HCC were retrospectively analyzed. The receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff value of the FIB-4. Univariate analysis and multivariate analysis were performed to identify risk factors for postoperative liver failure. The clinical outcomes were compared between patients with high FIB-4 and low FIB-4.
ResultsThe optimal cutoff value of the FIB-4 was set at 5.92 for postoperative liver failure according to ROC curve. By univariate and multivariate analysis, the number of resected segments, FIB-4, and model for end-stage liver disease score were identified as independent risk factors for postoperative liver failure. Patients with preoperative FIB-4>5.92 had poorer liver function and higher occurrence of postoperative liver failure.
ConclusionsPreoperative FIB-4 was associated with postoperative liver failure. Patients with preoperative FIB-4>5.92 carry a high risk of postoperative liver failure.
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the third leading cause of cancer-related death worldwide [1]. So far, surgical resection of HCC has become one of the most effective treatments. Most patients with HCC have chronic liver disease, such as hepatitis and cirrhosis, liver resection may lead to postoperative liver failure in these patients [2,3]. Although various methods, such as Child–Pugh (CP) score, have been developed for preoperative assessment of liver function, accurate predicting the postoperative outcome remains difficult [4].
According to previous studies, active hepatitis and hepatic fibrosis were identified as negative factors for liver regeneration and postoperative liver failure [5–7]. The gold standard for assessing degree of active hepatitis and hepatic fibrosis remains liver biopsy. However, due to the invasiveness and complications of biopsy, it is rarely performed in all candidates for liver surgery. Recently, the Fibrosis score 4 (FIB-4), which is an index calculated from age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count (PLT), has been identified as a biochemical marker to predict hepatic fibrosis and cirrhosis with high accuracy [8,9]. However, it is unclear whether preoperative FIB-4 could serve as a predictor of postoperative liver failure in patients with HCC. In this study, we investigated the role of preoperative FIB-4 in predicting the postoperative liver failure of HCC patients.
2Materials and methods2.1PatientsThis retrospective study was approved by the Institutional Review Board of Changzhou First People's Hospital. All participants gave written informed consent for their clinical records to be used in this study. A total of 281 patients who received curative surgery for HCC from January 2009 to August 2017 at the Changzhou First People's Hospital were retrospectively reviewed from our department prospective surgical database. To be enrolled, patients had to satisfy the following inclusion criteria: (1) initial HCC diagnosis; (2) combined with hepatitis and cirrhosis; (3) Child–Pugh grade A or B. Patients were excluded from the study if they (1) had another malignancy before hepatectomy; (2) received preoperative cancer treatment such as radio- or chemotherapy or transcatheter arterial chemoembolization; (3) had underwent hepatectomy previously; (4) had coexistent hematologic disorders; (5) received a PLT transfusion before surgery, or (6) lacked entire set of laboratory data. In total, 205 patients were finally included and evaluated.
2.2Definitions used in subsequent analysesThe FIB-4 was calculated based on laboratory data at the time of HCC diagnosis, which was within 1 weeks before hepatectomy, as: FIB-4=(age [years]×AST [U/L])/(PLT [109/L]×ALT [U/L]1/2). The aspartate aminotransferase/PLT count ratio index (APRI) was calculated by dividing AST [U/L] by PLT [109/L]. Forn's index was calculated using the following formula: 7.811−3.131×ln (PLT [109/L])+0.781×ln (GGT [U/L])+3.467×ln (age [years])−0.014×(cholesterol [mmol/L]). The model for end-stage liver disease (MELD) score was calculated using the following formula: 11.2×ln (international normalized ratio)+9.57×ln (creatinine [mg/dl])+3.78×ln (bilirubin [mg/dl])+6.43×(etiology—0 if cholestatic or alcoholic, 1 otherwise) [10].
The severity of liver disease was assessed by the Child–Pugh class and score, and the MELD score. Posthepatectomy liver failure was defined based on the “50-50 criteria”, which means a total serum bilirubin value>50μmol/l and a prothrombin time index<50% on postoperative day 5 or thereafter [11]. Besides, posthepatectomy liver failure was determined based on the International Study Group of Liver Surgery (ISGLS) classification [12]. Depending on the level of treatment needed, severity of postoperative liver failure was also differentiated into three different grades from A to C [13]. Grade A posthepatectomy liver failure requires no change of the patient's clinical management. The clinical management of patients with grade B posthepatectomy liver failure deviates from the regular course but does not require invasive therapy. The need for invasive treatment defines grade C posthepatectomy liver failure. In this study, grade B and C was defined as postoperative liver failure.
Specimens of resected liver tissue were cut serially into tissue blocks 5mm thick, fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. The degree of hepatic fibrosis was classified as follows: F0, no fbrosis; F1, portal fibrous expansion; F2, presence of portal–portal septa without architectural distortion; F3, portocentral septa with architectural distortion; F4, cirrhosis [14].
2.3Statistical analysisAll statistical analyses were carried out using the SPSS v 25.0 software (Chicago, IL, USA). Receiver operating characteristic (ROC) curve was generated to determine the optimal cutoff of FIB-4 in predicting the posthepatectomy liver failure. Based on the ROC curves, the cut-off values were determined by seeking the maximal sum of sensitivity and specificity. Kaplan–Meier analysis was used to analyze the cumulative incidental rate of posthepatectomy liver failure. The measurement data were expressed as the mean±SD. The χ2 test or Fisher's exact test was used, as appropriate, for categorical data, whereas continuous variables were compared with Student t tests or the Mann–Whiney U test. Hazard ratio was used to estimate the relative risk of posthepatectomy liver failure. The Cox proportional hazards model was employed for univariate and multivariate analyses. Variables that proved significant in univariate analysis were then tested by multivariate analyses using the Cox regression mode. The correlation between the FIB-4 and clinical characteristics was determined by Spearman's rank correlation. Statistical significance was defined as a P-value<0.05.
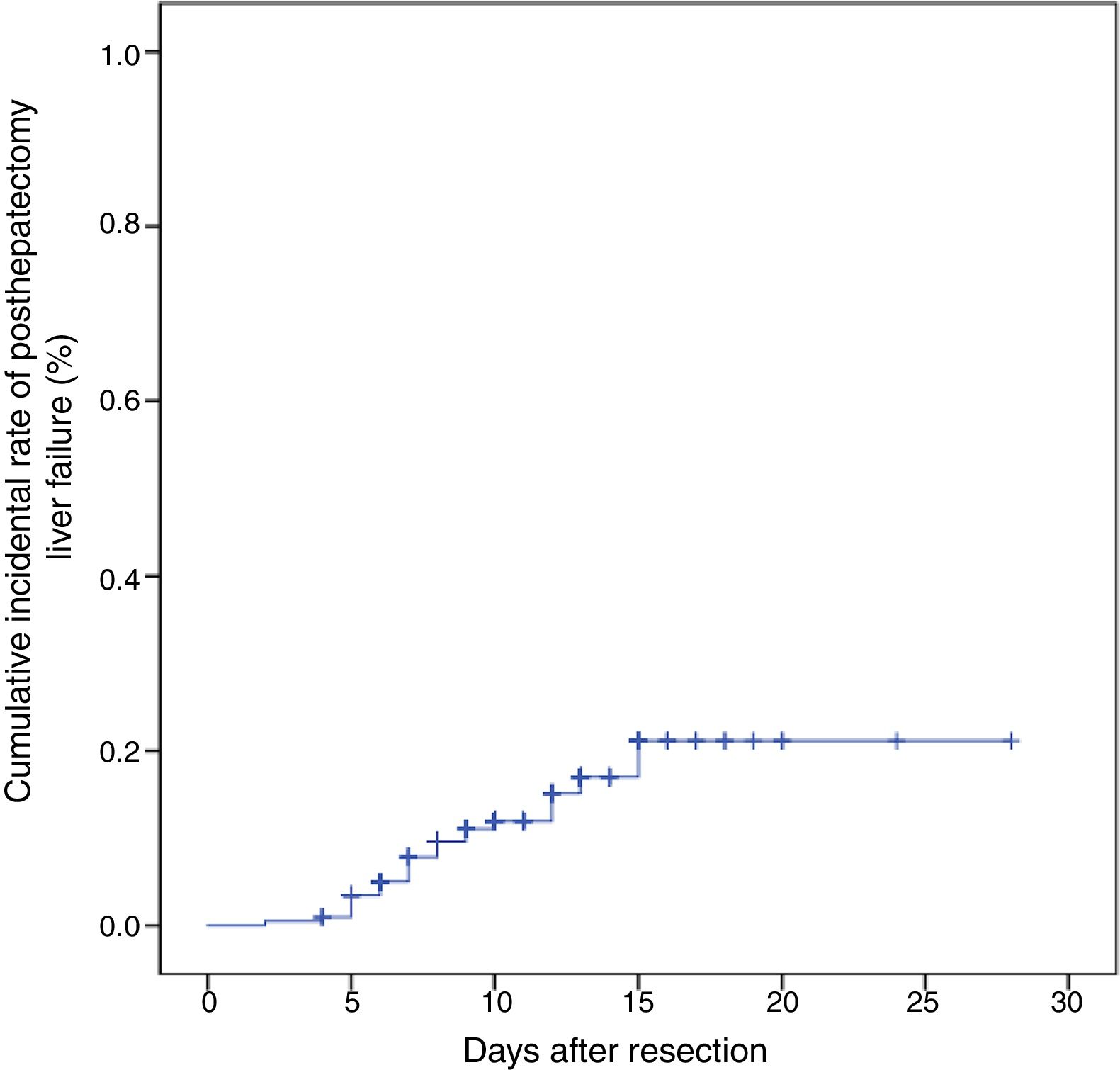
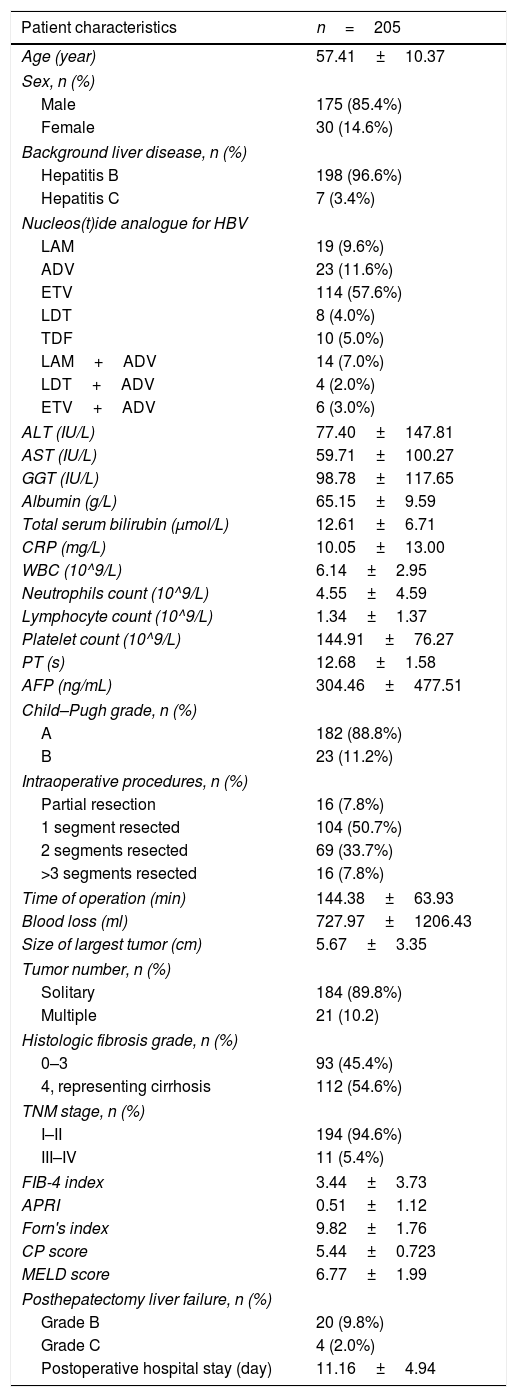
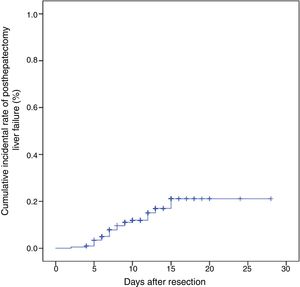
3Results3.1Patient demographicsA total of 205 HCC patients were included in this study. This population comprised 175 males and 30 females with a mean age of 57.41±10.37 years. Hepatitis B was detected during surveillance at our institution in 198 patients, and 7 patients were Hepatitis C. One hundred and ninety-eight patients with hepatitis B infection were all treated with nucleos(t)ide analogues for anti-viral therapy. Open hepatectomy was performed in all patients, partial hepatectomy was performed in 16 cases, liver resection of one segment was performed in 104 cases, liver resection of two segments was performed in 69 cases, and liver resection of more than or equal to three segments was performed in 16 cases. Twenty-four patients experienced postoperative liver failure, with 20 patients in Grade B and 4 patients in Grade C. The cumulative incidental rate of posthepatectomy liver failure was 21.1% (Fig. 1). The perioperative mortality of patients with posthepatectomy liver failure grades B and C was 5.0%, and 50.0%, respectively. Details of these features are shown in Table 1.
Baseline characteristics.
| Patient characteristics | n=205 |
|---|---|
| Age (year) | 57.41±10.37 |
| Sex, n (%) | |
| Male | 175 (85.4%) |
| Female | 30 (14.6%) |
| Background liver disease, n (%) | |
| Hepatitis B | 198 (96.6%) |
| Hepatitis C | 7 (3.4%) |
| Nucleos(t)ide analogue for HBV | |
| LAM | 19 (9.6%) |
| ADV | 23 (11.6%) |
| ETV | 114 (57.6%) |
| LDT | 8 (4.0%) |
| TDF | 10 (5.0%) |
| LAM+ADV | 14 (7.0%) |
| LDT+ADV | 4 (2.0%) |
| ETV+ADV | 6 (3.0%) |
| ALT (IU/L) | 77.40±147.81 |
| AST (IU/L) | 59.71±100.27 |
| GGT (IU/L) | 98.78±117.65 |
| Albumin (g/L) | 65.15±9.59 |
| Total serum bilirubin (μmol/L) | 12.61±6.71 |
| CRP (mg/L) | 10.05±13.00 |
| WBC (10^9/L) | 6.14±2.95 |
| Neutrophils count (10^9/L) | 4.55±4.59 |
| Lymphocyte count (10^9/L) | 1.34±1.37 |
| Platelet count (10^9/L) | 144.91±76.27 |
| PT (s) | 12.68±1.58 |
| AFP (ng/mL) | 304.46±477.51 |
| Child–Pugh grade, n (%) | |
| A | 182 (88.8%) |
| B | 23 (11.2%) |
| Intraoperative procedures, n (%) | |
| Partial resection | 16 (7.8%) |
| 1 segment resected | 104 (50.7%) |
| 2 segments resected | 69 (33.7%) |
| >3 segments resected | 16 (7.8%) |
| Time of operation (min) | 144.38±63.93 |
| Blood loss (ml) | 727.97±1206.43 |
| Size of largest tumor (cm) | 5.67±3.35 |
| Tumor number, n (%) | |
| Solitary | 184 (89.8%) |
| Multiple | 21 (10.2) |
| Histologic fibrosis grade, n (%) | |
| 0–3 | 93 (45.4%) |
| 4, representing cirrhosis | 112 (54.6%) |
| TNM stage, n (%) | |
| I–II | 194 (94.6%) |
| III–IV | 11 (5.4%) |
| FIB-4 index | 3.44±3.73 |
| APRI | 0.51±1.12 |
| Forn's index | 9.82±1.76 |
| CP score | 5.44±0.723 |
| MELD score | 6.77±1.99 |
| Posthepatectomy liver failure, n (%) | |
| Grade B | 20 (9.8%) |
| Grade C | 4 (2.0%) |
| Postoperative hospital stay (day) | 11.16±4.94 |
Continuous data were presented as mean±standard deviation, LAM, Lamivudine; ADV, Adefovir; ETV, Entecavir; LDT, Telbivudine; TDF, Telbivudine; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; CRP, C-reactive protein; WBC, white cell count; PLT, platelet count; PT, prothrombin time; AFP, alfa-fetoprotein; FIB-4, index fibrosis score 4; APRI, aspartate aminotransferase/platelet count ratio index; CP score, Child–Pugh score; MELD score, model for end-stage liver disease score.
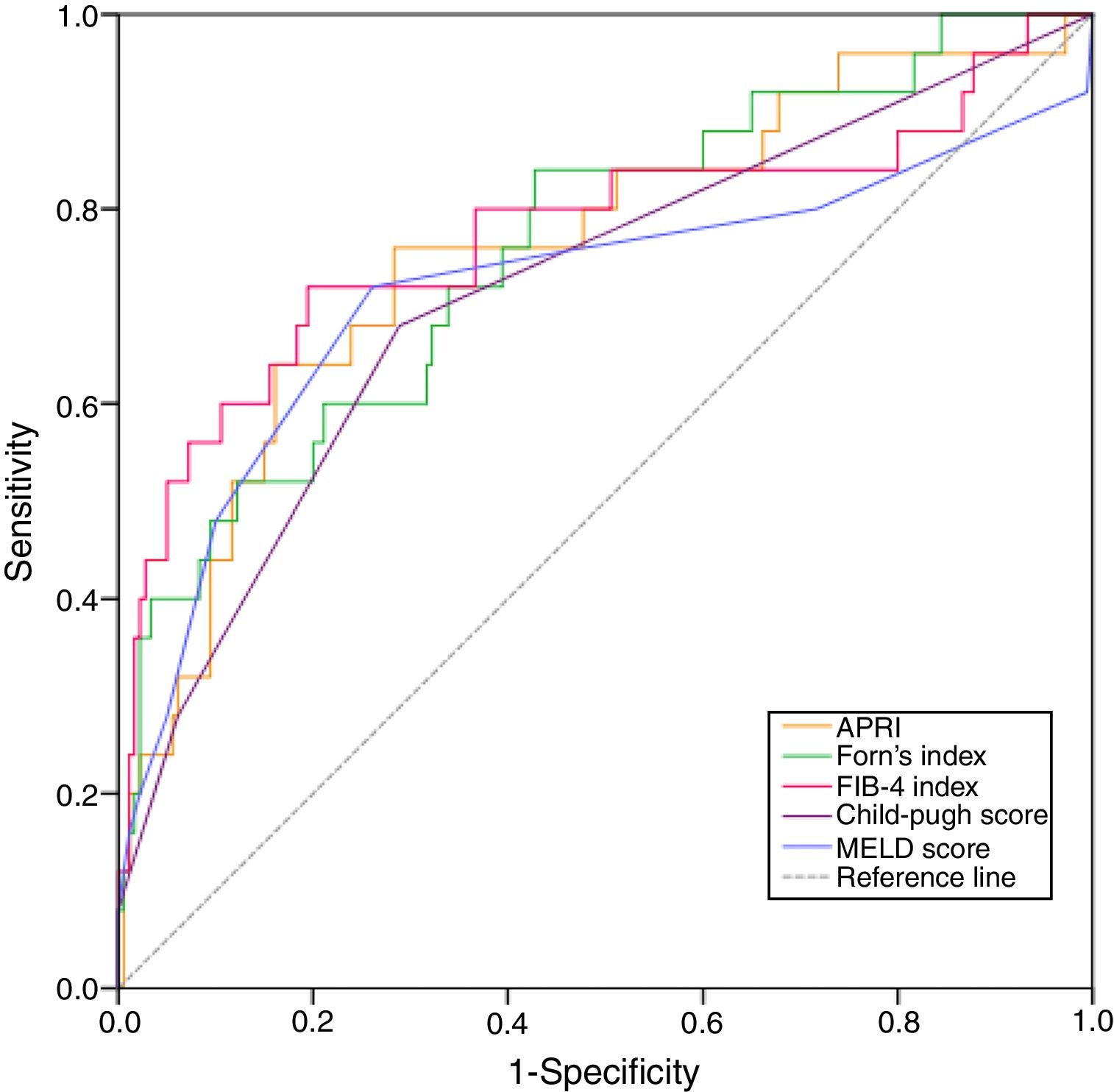
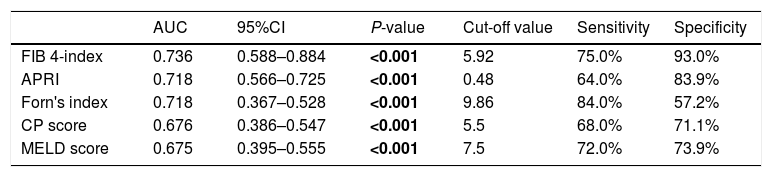
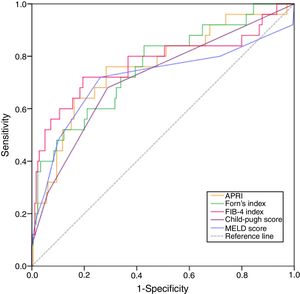
We determined the best cut-off values for indices by the ROC curve analysis for predicting posthepatectomy liver failure. The ROC curve for indices in relation to posthepatectomy liver failure is shown in Fig. 2. FIB-4 showed a significant positive correlation with posthepatectomy liver failure. The calculated cut-off value for FIB-4 was 5.92, with sensitivity of 75.0%, specificity of 93.0% in the prediction of posthepatectomy liver failure. And the area under the curve was 0.736 (95% CI: 0.588–0.884; P=0.001). Similarly, optimal cut-off values were set as 0.48, 9.86, 5.5 and 7.5 for APRI, Forn's index, CP score and MELD score, respectively (Table 2). Thus, all the patients were divided into high FIB-4 (FIB-4>5.92) group and low FIB-4 (FIB-4<5.92) group, high APRI (APRI>0.48) group and low APRI (APRI<0.48) group, high Forn's index (Forn's index>9.86) group and low Forn's index (Forn's index<9.86) group, high CP score (CP score>5.5) group and low CP score (CP score<5.5) group, high MELD score (MELD score>7.5) group and low MELD score (MELD score<7.5) group.
Comparison of the AUC between different scores.
| AUC | 95%CI | P-value | Cut-off value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| FIB 4-index | 0.736 | 0.588–0.884 | <0.001 | 5.92 | 75.0% | 93.0% |
| APRI | 0.718 | 0.566–0.725 | <0.001 | 0.48 | 64.0% | 83.9% |
| Forn's index | 0.718 | 0.367–0.528 | <0.001 | 9.86 | 84.0% | 57.2% |
| CP score | 0.676 | 0.386–0.547 | <0.001 | 5.5 | 68.0% | 71.1% |
| MELD score | 0.675 | 0.395–0.555 | <0.001 | 7.5 | 72.0% | 73.9% |
AUC, area under the curve; FIB-4, index fibrosis score 4; APRI, aspartate aminotransferase/platelet count ratio index; CP, score Child–Pugh score; MELD score, model for end-stage liver disease score.
Bold values indicate that P<0.05 and have statistical significance.
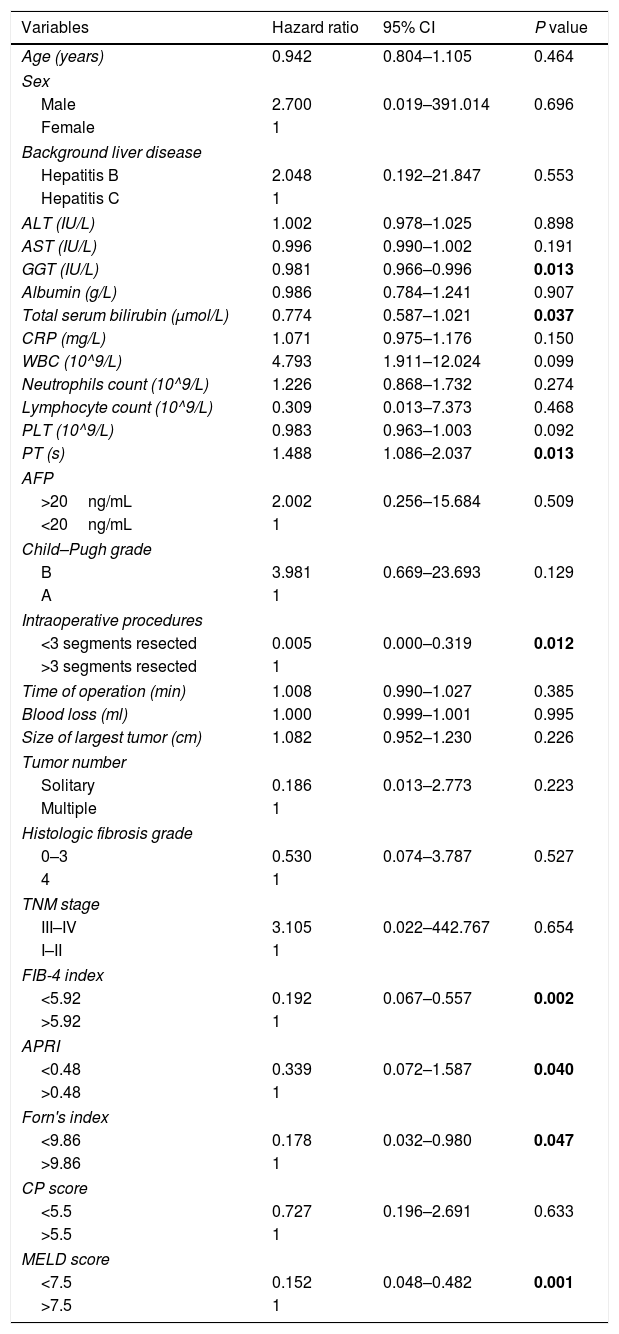
Univariate analysis was carried out to determine risk factors for posthepatectomy liver failure. As shown in Table 3, GGT, total serum bilirubin, PT, number of resected segments, FIB-4, APRI, Forn's index and MELD score were risk factors for posthepatectomy liver failure.
Univariate analysis of risk factors for posthepatectomy liver failure.
| Variables | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age (years) | 0.942 | 0.804–1.105 | 0.464 |
| Sex | |||
| Male | 2.700 | 0.019–391.014 | 0.696 |
| Female | 1 | ||
| Background liver disease | |||
| Hepatitis B | 2.048 | 0.192–21.847 | 0.553 |
| Hepatitis C | 1 | ||
| ALT (IU/L) | 1.002 | 0.978–1.025 | 0.898 |
| AST (IU/L) | 0.996 | 0.990–1.002 | 0.191 |
| GGT (IU/L) | 0.981 | 0.966–0.996 | 0.013 |
| Albumin (g/L) | 0.986 | 0.784–1.241 | 0.907 |
| Total serum bilirubin (μmol/L) | 0.774 | 0.587–1.021 | 0.037 |
| CRP (mg/L) | 1.071 | 0.975–1.176 | 0.150 |
| WBC (10^9/L) | 4.793 | 1.911–12.024 | 0.099 |
| Neutrophils count (10^9/L) | 1.226 | 0.868–1.732 | 0.274 |
| Lymphocyte count (10^9/L) | 0.309 | 0.013–7.373 | 0.468 |
| PLT (10^9/L) | 0.983 | 0.963–1.003 | 0.092 |
| PT (s) | 1.488 | 1.086–2.037 | 0.013 |
| AFP | |||
| >20ng/mL | 2.002 | 0.256–15.684 | 0.509 |
| <20ng/mL | 1 | ||
| Child–Pugh grade | |||
| B | 3.981 | 0.669–23.693 | 0.129 |
| A | 1 | ||
| Intraoperative procedures | |||
| <3 segments resected | 0.005 | 0.000–0.319 | 0.012 |
| >3 segments resected | 1 | ||
| Time of operation (min) | 1.008 | 0.990–1.027 | 0.385 |
| Blood loss (ml) | 1.000 | 0.999–1.001 | 0.995 |
| Size of largest tumor (cm) | 1.082 | 0.952–1.230 | 0.226 |
| Tumor number | |||
| Solitary | 0.186 | 0.013–2.773 | 0.223 |
| Multiple | 1 | ||
| Histologic fibrosis grade | |||
| 0–3 | 0.530 | 0.074–3.787 | 0.527 |
| 4 | 1 | ||
| TNM stage | |||
| III–IV | 3.105 | 0.022–442.767 | 0.654 |
| I–II | 1 | ||
| FIB-4 index | |||
| <5.92 | 0.192 | 0.067–0.557 | 0.002 |
| >5.92 | 1 | ||
| APRI | |||
| <0.48 | 0.339 | 0.072–1.587 | 0.040 |
| >0.48 | 1 | ||
| Forn's index | |||
| <9.86 | 0.178 | 0.032–0.980 | 0.047 |
| >9.86 | 1 | ||
| CP score | |||
| <5.5 | 0.727 | 0.196–2.691 | 0.633 |
| >5.5 | 1 | ||
| MELD score | |||
| <7.5 | 0.152 | 0.048–0.482 | 0.001 |
| >7.5 | 1 | ||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; CRP, C-reactive protein; WBC, white cell count; PLT, platelet count; PT, prothrombin time; AFP, alfa-fetoprotein; FIB-4, index fibrosis score 4; APRI, aspartate aminotransferase/platelet count ratio index; CP, score Child–Pugh score; MELD score, model for end-stage liver disease score.
Bold values indicate that P<0.05 and have statistical significance.
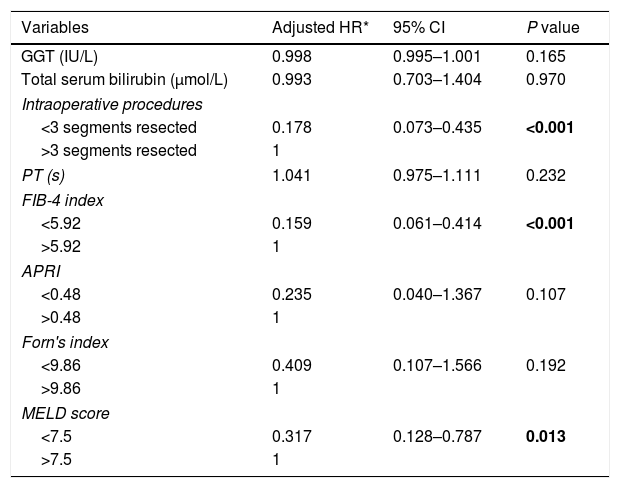
As shown in Table 4, independent predictors of postoperative liver failure were identified by multivariate analysis. Less than 3 segments resected (Adjusted HR=0.178; 95% CI:0.073–0.435; P<0.001), FIB-4<5.92 (Adjusted HR=0.159; 95% CI:0.061–0.414; P<0.001), and MELD score<7.5 (Adjusted HR=0.317; 95% CI:0.128–0.787; P=0.013) were the independent predictors after controlling the potential confounding factors.
Multivariate analysis of risk factors for posthepatectomy liver failure.
| Variables | Adjusted HR* | 95% CI | P value |
|---|---|---|---|
| GGT (IU/L) | 0.998 | 0.995–1.001 | 0.165 |
| Total serum bilirubin (μmol/L) | 0.993 | 0.703–1.404 | 0.970 |
| Intraoperative procedures | |||
| <3 segments resected | 0.178 | 0.073–0.435 | <0.001 |
| >3 segments resected | 1 | ||
| PT (s) | 1.041 | 0.975–1.111 | 0.232 |
| FIB-4 index | |||
| <5.92 | 0.159 | 0.061–0.414 | <0.001 |
| >5.92 | 1 | ||
| APRI | |||
| <0.48 | 0.235 | 0.040–1.367 | 0.107 |
| >0.48 | 1 | ||
| Forn's index | |||
| <9.86 | 0.409 | 0.107–1.566 | 0.192 |
| >9.86 | 1 | ||
| MELD score | |||
| <7.5 | 0.317 | 0.128–0.787 | 0.013 |
| >7.5 | 1 | ||
HR, Hazard ratio; GGT, γ-glutamyl transferase; PT, prothrombin time; FIB-4, index fibrosis score 4; APRI, aspartate aminotransferase/platelet count ratio index; MELD score, model for end-stage liver disease score.
All HRs were adjusted for age (years), sex, PLT (10^9/L), TNM stage, Child–Pugh grade, time of operation.
Bold values indicate that P<0.05 and have statistical significance.
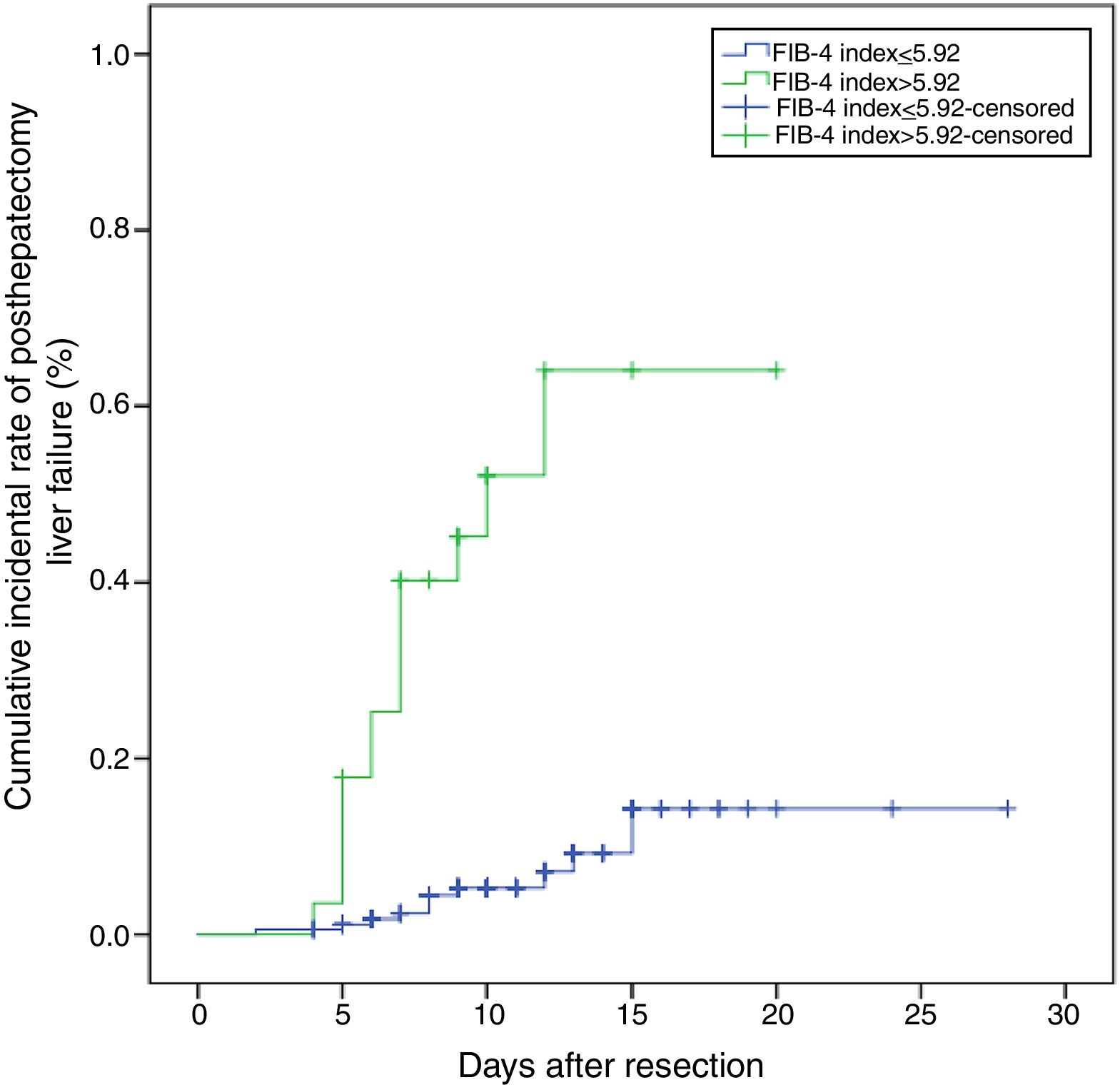
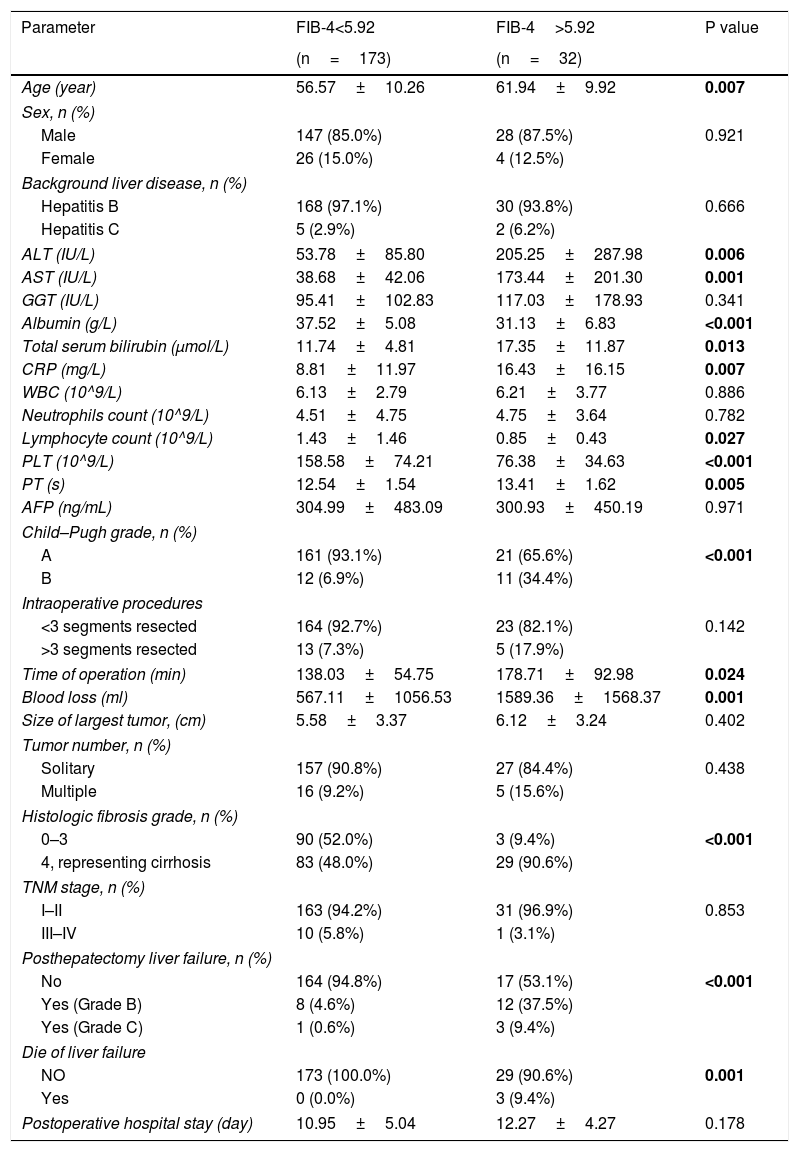
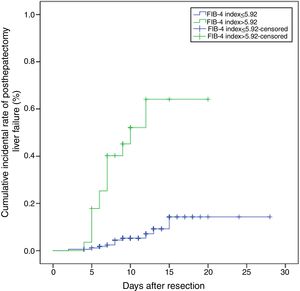
We then compared the clinicopathological characteristics between patients with low FIB-4 (<5.92) and patients with high FIB-4 (>5.92) (Table 5). Patients with high FIB-4 had higher Child-Pugh grade and higher histologic fibrosis grade (P<0.001). Preoperative levels of ALT, AST, total serum bilirubin, CRP, PT were higher in the high FIB-4 group than the low FIB-4 group (P<0.005). However, the preoperative levels of albumin, lymphocyte count, PLT were lower in the high FIB-4 group (P<0.005). Although there was no statistical difference in the number of resected segments, size of tumor and tumor number between two groups, the low FIB-4 group had more time of operation and more blood loss (P<0.005). In addition, patients with high FIB-4 developed a high proportion of liver failure and died of liver failure (P<0.005). The cumulative incidental rate of posthepatectomy liver failure in the high FIB-4 group was 64.1%, which was significantly higher than 14.3% in the low FIB-4 group (P<0.001) (Fig. 3).
Baseline Comparison between patients with FIB-4>5.92 and <5.92.
| Parameter | FIB-4<5.92 | FIB-4>5.92 | P value |
|---|---|---|---|
| (n=173) | (n=32) | ||
| Age (year) | 56.57±10.26 | 61.94±9.92 | 0.007 |
| Sex, n (%) | |||
| Male | 147 (85.0%) | 28 (87.5%) | 0.921 |
| Female | 26 (15.0%) | 4 (12.5%) | |
| Background liver disease, n (%) | |||
| Hepatitis B | 168 (97.1%) | 30 (93.8%) | 0.666 |
| Hepatitis C | 5 (2.9%) | 2 (6.2%) | |
| ALT (IU/L) | 53.78±85.80 | 205.25±287.98 | 0.006 |
| AST (IU/L) | 38.68±42.06 | 173.44±201.30 | 0.001 |
| GGT (IU/L) | 95.41±102.83 | 117.03±178.93 | 0.341 |
| Albumin (g/L) | 37.52±5.08 | 31.13±6.83 | <0.001 |
| Total serum bilirubin (μmol/L) | 11.74±4.81 | 17.35±11.87 | 0.013 |
| CRP (mg/L) | 8.81±11.97 | 16.43±16.15 | 0.007 |
| WBC (10^9/L) | 6.13±2.79 | 6.21±3.77 | 0.886 |
| Neutrophils count (10^9/L) | 4.51±4.75 | 4.75±3.64 | 0.782 |
| Lymphocyte count (10^9/L) | 1.43±1.46 | 0.85±0.43 | 0.027 |
| PLT (10^9/L) | 158.58±74.21 | 76.38±34.63 | <0.001 |
| PT (s) | 12.54±1.54 | 13.41±1.62 | 0.005 |
| AFP (ng/mL) | 304.99±483.09 | 300.93±450.19 | 0.971 |
| Child–Pugh grade, n (%) | |||
| A | 161 (93.1%) | 21 (65.6%) | <0.001 |
| B | 12 (6.9%) | 11 (34.4%) | |
| Intraoperative procedures | |||
| <3 segments resected | 164 (92.7%) | 23 (82.1%) | 0.142 |
| >3 segments resected | 13 (7.3%) | 5 (17.9%) | |
| Time of operation (min) | 138.03±54.75 | 178.71±92.98 | 0.024 |
| Blood loss (ml) | 567.11±1056.53 | 1589.36±1568.37 | 0.001 |
| Size of largest tumor, (cm) | 5.58±3.37 | 6.12±3.24 | 0.402 |
| Tumor number, n (%) | |||
| Solitary | 157 (90.8%) | 27 (84.4%) | 0.438 |
| Multiple | 16 (9.2%) | 5 (15.6%) | |
| Histologic fibrosis grade, n (%) | |||
| 0–3 | 90 (52.0%) | 3 (9.4%) | <0.001 |
| 4, representing cirrhosis | 83 (48.0%) | 29 (90.6%) | |
| TNM stage, n (%) | |||
| I–II | 163 (94.2%) | 31 (96.9%) | 0.853 |
| III–IV | 10 (5.8%) | 1 (3.1%) | |
| Posthepatectomy liver failure, n (%) | |||
| No | 164 (94.8%) | 17 (53.1%) | <0.001 |
| Yes (Grade B) | 8 (4.6%) | 12 (37.5%) | |
| Yes (Grade C) | 1 (0.6%) | 3 (9.4%) | |
| Die of liver failure | |||
| NO | 173 (100.0%) | 29 (90.6%) | 0.001 |
| Yes | 0 (0.0%) | 3 (9.4%) | |
| Postoperative hospital stay (day) | 10.95±5.04 | 12.27±4.27 | 0.178 |
Continuous data were presented as mean±Standard Deviation, ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; CRP, C-reactive protein; WBC, white cell count; PLT, platelet count; PT, prothrombin time; AFP, alfa-fetoprotein; FIB-4, index fibrosis score 4.
Bold values indicate that P<0.05 and have statistical significance.
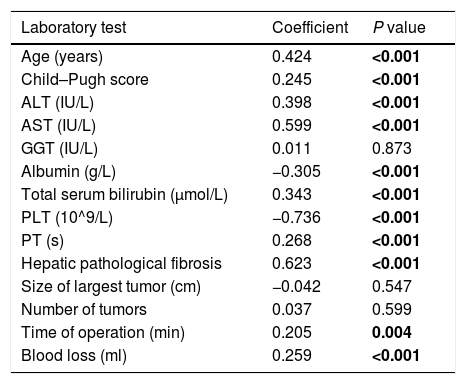
Furthermore, the Spearman correlation analysis showed that there was a positive correlation between FIB-4 and age, Childe-Pugh score, ALT, AST, total serum bilirubin, PT, hepatic pathological fibrosis, time of operation, blood loss (all P<0.05). These variables gradually increased with the increase in FIB-4 (Table 6). Furthermore, there is a negative correlation between FIB-4 and albumin, PLT (all P<0.001). These variables gradually increased with the decrease in FIB-4 (Table 6).
Spearman's correlation analysis between FIB-4 and clinical characteristics.
| Laboratory test | Coefficient | P value |
|---|---|---|
| Age (years) | 0.424 | <0.001 |
| Child–Pugh score | 0.245 | <0.001 |
| ALT (IU/L) | 0.398 | <0.001 |
| AST (IU/L) | 0.599 | <0.001 |
| GGT (IU/L) | 0.011 | 0.873 |
| Albumin (g/L) | −0.305 | <0.001 |
| Total serum bilirubin (μmol/L) | 0.343 | <0.001 |
| PLT (10^9/L) | −0.736 | <0.001 |
| PT (s) | 0.268 | <0.001 |
| Hepatic pathological fibrosis | 0.623 | <0.001 |
| Size of largest tumor (cm) | −0.042 | 0.547 |
| Number of tumors | 0.037 | 0.599 |
| Time of operation (min) | 0.205 | 0.004 |
| Blood loss (ml) | 0.259 | <0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; PLT, platelet count; PT, prothrombin time.
Bold values indicate that P<0.05 and have statistical significance.
The main cause of HCC and cirrhosis is chronic hepatitis B, a disease caused by hepatitis B virus (HBV) [15]. In China, approximately 80% of HCC patients have either cirrhosis or different degree of liver fibrosis [16]. The severity of liver fibrosis and cirrhosis is closely related to postoperative regeneration of remnant liver volume and function. Despite the significant advancement in surgical technique, the incidence of postoperative liver failure remains high, especially in HCC patients with chronic liver disease [5,12,17]. Postoperative liver failure was not only related to intraoperative liver injury, such as inflow occlusion, blood loss and massive liver removed volume, but also related to preoperative liver function.
Several studies have proven that cirrhosis is the risk factor for postoperative liver failure [2,3,18]. It is worth noting that degree of active hepatitis and hepatic fibrosis are adverse factors for liver regeneration and liver function recovery after hepatectomy, moreover, hepatic fibrosis also influences the duration of postoperative liver failure [5–7]. As a non-invasive method with high reproducibility and reliability, liver stiffness measurement (LSM) using transient elastography has been used to evaluate the degree of liver fibrosis [19]. Considering expensive costs and difficult operations, LSM is not routinely used for assessing HCC preoperatively. For reasons of safety and other issues, biopsy specimens of noncancerous hepatic tissue could not obtain preoperatively from most patients. Therefore, non-invasive markers which associated with histologic degree of hepatic fibrosis are critical.
Recently, the FIB-4, which is calculated from age, AST, ALT and PLT, was reported to have relatively high diagnostic value in detecting liver fibrosis [20,21]. In addition, the FIB-4 was also a predictor for long-term outcomes in HCC patients who underwent radical hepatectomy [22,23]. Our results showed preoperative FIB-4 positively correlated with histologic fibrosis grade in patients with HCC.
As shown in univariate and multivariate analyses, FIB-4 was an independent risk factor for postoperative liver failure. In our study, patients in the high FIB-4 group were more likely to develop liver failure and die of liver failure, further demonstrating that increase in the degree of liver fibrosis and cirrhosis did lead to poor prognosis. According to the linear correlation analysis, a higher FIB-4 was significantly associated with an older age, a higher level of Childe-Pugh grade; ALT; AST; total serum bilirubin and PT, while a lower FIB-4 was significantly associated the higher level of albumin and PLT. This finding suggested that higher FIB-4 is associated with poorer liver function which may derive from HBV or HCV infection. As we all know, poor liver function is the risk factor for postoperative liver failure [24]. Recently, the role of PLT in promoting liver regeneration after hepatectomy has been proven by experimental studies. PLT-derived serotonin has been proven to be involved in initiating liver regeneration [25]. Studies also proved that PLT accumulate in the liver immediately and promote liver regeneration after hepatectomy [26,27]. In addition, low preoperative PLT count was associated with increased risk of posthepatectomy grade A liver failure in right lobe donors for liver transplantation [28]. Therefore, calculating the FIB-4 preoperatively not only inform the surgeon about severity of active hepatitis and degree of fibrosis, but also provide a measurement of the risk of postoperative liver failure.
Other risk factors, such as APRI, Forn's index and MELD score, have proven to be associated with posthepatectomy liver failure on univariate analysis. But the area under the curve was all smaller than FIB-4, which suggest FIB-4 is more predictive in posthepatectomy liver failure when compared with these factors.
Interestingly, Wang et al. [29] found that the immediate postoperative FIB-4 could predict the posthepatectomy liver failure for patients with HCC undergoing curative surgery. However, the immediate postoperative PLT, AST and ALT were not only affected by hemodilution or hemoconcentration, but also by intraoperative bleeding and blood transfusion. Furthermore, postoperative drugs may also have an impact on liver function. Therefore, the value of postoperative FIB-4 may be less accurate than preoperative FIB-4.
Because these variables could be easily obtained in clinical practice, this model could help surgeons to predict the risk of posthepatectomy liver failure preoperatively and take positive measures to prevent liver failure. For example, preoperative PLT transfusion, and administration of thrombopoietin and serotonin may helpful in decreasing the liver failure risk for patients with low PLT count. In addition, for elderly patients with liver dysfunction, abstinence from potential liver injury drugs and actively adjust liver function to the best level is also an effective way to reduce FIB-4.
There are several limitations in our study. First, our study cohort a retrospective study, and all data were collected from a single medical center. Second, the optimal cutoff used in this study for FIB-4 was based on calculations in our own patients and have not been validated in an independent cohort. Third, the number of patients who underwent major resection (removal of 3 or more segments) was less frequent in our series, indicating that hepatectomy was performed on selected patients. Therefore, future multi-center prospective studies with larger sample size are needed to verify whether the value of FIB-4 is effective for patients with major liver resection.
In conclusion, this retrospective analysis showed that preoperative FIB-4 was correlated with posthepatectomy liver failure. Patients with preoperative FIB-4>5.92 carry a high risk of postoperative liver failure.AbbreviationsFIB-4 fibrosis score 4 hepatocellular carcinoma receiver operating characteristic Child–Pugh aspartate aminotransferase alanine aminotransferase platelet count model for end-stage liver disease hepatitis B virus liver stiffness measurement
Bao-Qiang Wu took charge of conceiving and designing the study; Jia-Wei Feng was responsible for collecting, analyzing and interpreting the data, and took charge of writing the manuscript; Dong-Lin Sun was responsible for providing critical revisions; approving the final version of the manuscript was in charge of Yong Jiang and Zhen Qu.
Financial supportNone.
Conflict of interestsNone.
Compliance with ethical standardsThis manuscript has not been published nor submitted for publication elsewhere. All authors have contributed significantly, and agree with the content of the manuscript. Informed consent was obtained from all individual participants included in the study.