Cryoglobulinemia is one of the most frequent extrahepatic manifestations of chronic hepatitis C virus (HCV) infection and it may evolve to cryoglobulinemic vasculitis (CryoVas) which is a systemic vasculitis that affects small-sized vessels. The objective of this study was to evaluate the prevalence of cryoglobulinemia and CryoVas in HCV patients in São Paulo, Brazil.
Materials and methodsA cross-sectional study was conducted and included sixty-eight viremic HCV patients, without HIV or hepatitis B coinfection. A thorough clinical and laboratory evaluation was performed including the detection of serum cryoglobulins and measurement of serum complement components. The classification criteria for CryoVas were applied.
ResultsThe study population comprised mainly women (61.8%) with long term HCV infection (median 11.0 years). Advanced hepatic fibrosis was detected in 20.6% (14/68) of cases. Cryoglobulins were detected in 48.5% (33/68) of HCV-patients with type III cryoglobulinemia being the most frequent. CryoVas was present in 10.3% (7/68) and the main manifestations were peripheral neuropathy (85.7%), palpable purpura (42.8%), arthralgias (42.8%) and renal involvement (42.8%). Life-threatening manifestations were rare. Low hemolytic C2, C4 and total hemolytic complement (CH100) levels were common findings in the cryoglobulinemia group. Low C4 levels were independently associated with the development of CryoVas.
ConclusionA high prevalence of cryoglobulinemia and CryoVas was found in Brazilian HCV-patients. CryoVas patients mostly presented non-life-threatening manifestations, especially peripheral neuropathy. Complement abnormalities were common in patients with cryoglobulinemia and low serum C4 levels were associated with CryoVas.
It has been estimated that 130–170 million people worldwide are chronically infected by the hepatitis C virus (HCV), leading to almost 500,000 deaths per year [1]. Up to two-third of HCV patients will experience extrahepatic manifestations, that include lymphoproliferative disorders and autoimmune diseases [2]. Cryoglobulinemia is one of the most frequent extrahepatic manifestations in HCV chronic infection [2–6]. Cryoglobulins are immunoglobulins that precipitate in vitro in temperatures below 37°C and resolubilize upon rewarming. The term cryoglobulinemia refers to the presence of circulating cryoglobulins [7].
The cryoglobulinemic vasculitis (CryoVas) is a systemic vasculitis that affects predominantly small-vessels, especially in the skin, joints, peripheral nerves and kidneys [8]. Cryoglobulinemia is the consequence of the chronic proliferation of B-cells, that produces pathogenic IgG and IgM immunoglobulin (Ig) isotypes with rheumatoid factor activity [9–11]. Almost 80% of CryoVas cases are secondary to chronic HCV infection, but lymphoproliferative disorders, autoimmune diseases and other infections are also possible etiologies for cryoglobulinemia and CryoVas [4]. CryoVas has a variable clinical expression, ranging from mild symptoms such as fatigue, palpable purpura, peripheral polyneuropathy and arthralgia to fulminant life-threatening manifestations including rapidly progressive glomerulonephritis, pulmonary capillaritis, gastrointestinal and central nervous system vasculitis [12].
After the discovery of the HCV as the main etiologic agent of CryoVas, an opportunity to control CryoVas manifestations with antiviral therapy emerged [13]. Despite the initial success observed with interferon alpha (IFNα) based antiviral treatment, HCV-associated CryoVas still remained a severe disease with a 5-year mortality rate of 25% [14]. Even with the advent of novel therapies for hepatitis C with interferon-free agents, CryoVas is still a problem that may persist after the sustained virological response [15].
The global prevalence of cryoglobulinemia and CryoVas remains unknown [16] and the reasons for this include the lack of standardization of laboratory techniques to detect and measure serum cryoglobulins, the heterogeneity of clinical manifestations of CryoVas, geographic differences in the development of cryoglobulinemia in HCV-positive individuals and differences in disease phenotypes of CryoVas [17].
According to the World Health Organization (WHO), there are 7 million people living with chronic HCV infection in the Americas, with an incidence rate of 6.4/100,000 in 2015. The WHO's Global Health Sector Strategy on viral hepatitis estimates that only 20% of all HCV infections were diagnosed in 2015 and an even smaller fraction of these individuals (i.e. 7% of those diagnosed with HCV or approximately 1.1 million people) had started curative treatment in that year [18]. It is estimated that 700,000 individuals in Brazil are living with hepatitis C [19]. Although the CryoVas is considered a formal indication of antiviral therapy [20], the prevalence of cryoglobulinemia is still not known in Brazilian HCV patients.
This study aims to evaluate the frequency of cryoglobulinemia and CryoVas in HCV-infected patients followed in an outpatient clinic specialized in hepatitis management in Brazil, between 2016 and 2017. In addition, disease features and associations with CryoVas were analyzed in this cohort of Brazilian HCV patients.
2Patients and methods2.1PatientsA cross-sectional study was conducted at the outpatient clinic of hepatitis at the Gastroenterology Division of Universidade Federal de São Paulo – Escola Paulista de Medicina (Unifesp-EPM), between March 2016 and May 2017. One hundred HCV patients were initially enrolled in the study, according to the following inclusion criteria: chronic viremic HCV infection defined by a positive HCV PCR-RNA, age>18 years and written informed consent. The exclusion criteria were current antiviral treatment for HCV infection, coinfection with the Human Immunodeficiency Virus (HIV) or with the Hepatitis B Virus (HBV) and the diagnosis of an autoimmune rheumatic disease or a lymphoproliferative disease. This study was approved by the Institutional Ethics Committee and was in accordance with the Declaration of Helsinki.
Among 100 patients initially included in the study, only 68 HCV patients completed the study's protocol. Thirty patients did not come for collecting blood for laboratory tests and two other patients were excluded because they had developed type 1 autoimmune hepatitis and psoriatic arthritis.
2.2Clinical features and laboratory studiesThe patients were carefully evaluated for manifestations suggestive of CryoVas such as fatigue, arthritis and/or arthralgia, palpable purpura and cutaneous ulcers, tingling, numbness, paresthesia and muscle weakness. Indirect evidence of renal disease was also pursued and included peripheral edema, foamy urine and hypertension. Suspicious vasculitic cutaneous lesions were evaluated by a dermatologist who performed skin biopsies to confirm the diagnosis of cutaneous vasculitis. Whenever peripheral nerve involvement was suspected, HCV patients underwent electroneuromyography. Patients meeting the classification criteria for CryoVas were included in the CryoVas group. The criteria for CryoVas have been validated in a large cohort of cases and controls from both European and non-European countries and showed 88.5% sensitivity and 93.6% specificity [21].
Qualitative serum cryoglobulin detection was performed using previously described technique [22]. Blood samples were collected under appropriated conditions and stored at 4°C for fourteen days. The composition of cryoglobulins was determined by serum protein electrophoresis technique. We used Brouet's classification [22], where type I cryoglobulins are single monoclonal immunoglobulins, type II comprises mixed cryoglobulins with polyclonal IgG and monoclonal IgM with rheumatoid factoractivity, and type III are mixed cryoglobulins with polyclonal IgG and polyclonal IgM with rheumatoid factor activity. Cryoglobulin positivity was considered when cryoglobulins were detected on at least two occasions at least 12 weeks apart [21]. Information about the cryoglobulin types was available for 14 patients with cryoglobulinemia.
The quantification of HCV-RNA levels was made by real time polymerase chain reaction (PCR) with the threshold of 12IU/mL. Measurement of creatinine (mg/dL), serum sodium, prothrombin time (PT/INR) and serum bilirubin levels were a part of patient care and were performed using standard procedures. Measurement of serum hemolytic C2 (C2h) and total hemolytic complement activity (CH100) was performed by the immune hemolysis technique using sheep erythrocytes. Serum C3 and C4 levels were measured by nephelometry. Serum IgM rheumatoid factor (RF) was measured by the latex technique and considered positive when titer≥40UI/mL. Serum complement level was considered abnormal if patients presented CH100<75%, C2h<75%, C3<90mg/dL or if C4<10mg/dL. Urinalysis was also performed in HCV patients and hematuria was defined as the presence of more than 10,000 red blood cells per mL of urine. If proteinuria was detected in random specimens, a quantitative 24h-urinary protein excretion was also performed and considered abnormal when >0.30g/24h. Data about previous liver biopsies were retrieved from patients’ medical records from the last 3 years. Histologic examination of liver biopsies included qualitative and quantitative analysis of hepatic lesions, using the METAVIR scoring system [23]. In patients without biopsy, data about liver ultrasonography (US)-based transient elastography were collected from medical records. This method allows a noninvasive assessment of tissue mechanical properties and has been thoroughly researched and validated for diagnosis of liver fibrosis in large cohort studies [24]. Fifty-four patients had liver biopsy, six patients had US-based transient elastography and eight patients had no information about liver biopsy status.
2.3Statistical analysisStatistical analysis was performed using the IBM SPSS for Windows version 20.0 (Armonk, USA) and with GraphPad Prism version 6.00 for Windows (La Jolla, USA). Continuous variables were presented as mean and standard deviation or as median and interquartile range. The Shapiro–Wilki test was used to assess normality of continuous variables. Categorical variables were presented as total number and percentage. Comparisons between two groups regarding continuous variables were performed by the Student's t test or by Mann–Whitney's U test, whereas the one-way ANOVA or the Kruskal–Wallis test were used to analyze 3 or more groups. The following post hoc tests were used: Tukey's test and Mann–Whitney's U test, respectively. Categorical variables were assessed by the Chi-square or by the Fisher's exact tests accordingly. Univariate and multivariate models of logistic regression were built to analyze associations with CryoVas. Backward stepwise conditional was the method used for multivariate analysis. Results were expressed as odds ratio (OR) and 95% confidence intervals (95CI). Significance level accepted was 5% (p<0.05) [25].
3ResultsTable 1 describes demographic data and features of hepatitis C in study participants. The study population was mainly composed by middle-aged women with long-term known HCV infection (i.e. roughly one decade of known HCV infection). Presence of genotype 1 was observed in fifty (73.5%) HCV-positive patients and most of our patients who underwent liver biopsy presented none or mild to moderate liver fibrosis by the METAVIR scoring system. Almost 60% of HCV patients had never received antiviral therapy.
Clinical and demographic features of HCV-positive patients.
| Variables | Results |
|---|---|
| Sex | |
| Female, n (%) | 42 (61.8) |
| Male, n (%) | 26 (38.2) |
| Age, years | 59.0 (44.0–65.0) |
| HCV infection duration, years | 11.0 (6.0–16.8) |
| HCV-genotype | |
| 1, n (%) | 50 (73.5) |
| 2 and 3, n (%) | 14 (20.6) |
| Unknown, n (%) | 4 (5.9) |
| Liver biopsy and/or liver US-elastography results | |
| F0, F1 and F2, n (%) | 46 (67.6) |
| F3 and F4, n (%) | 14 (20.6) |
| No information, n (%) | 8 (11.8) |
| Previous antiviral treatment | |
| None, n (%) | 40 (58.8) |
| IFNα+RIB, n (%) | 25 (36.8) |
| IFNα+RIB+PI, n (%) | 3 (4.4) |
F, fibrosis, Metavir score; IFNα, interferon alpha; HCV, Hepatitis C virus; RIB, ribavirin; PI, Proteasis inhibitor: telaprevir or boceprevir; US, ultrasonography; n, number of patients. Quantitative variables were described by the median and the interquartile range.
The frequency of cryoglobulinemia in HCV positive patients was 48.5% (33/68) and cryoglobulinemia was classified as type III in 71.4% (10/14) and as type II in 28.6% (4/14). Unfortunately, it was not possible to classify cryoglobulinemia in 19 patients. Amongst HCV patients, 10.3% (7/68) fulfilled the classification criteria for CryoVas. Table 2 displays the comparisons between HCV patients with and without cryoglobulinemia regarding demographic data and clinical features. HCV patients with cryoglobulinemia had a significantly higher frequency of liver fibrosis categories F3 and F4, and of low serum complement levels including CH100, C2h, C3 and C4 when compared with HCV patients without cryoglobulinemia. There were no statistical differences between HCV related variables such as time since the diagnosis of hepatitis C, HCV genotype 1, and the number of HCV–RNA copies. Nonetheless, no differences regarding the presence of cryoglobulins were found between previously treated and untreated HCV patients (57.1% vs. 42.5%, p=0.234). The frequency of rheumatoid factor positivity, proteinuria and altered urinalysis were similar regarding the presence or absence of cryoglobulinemia.
Comparison between HCV-positive patients with and without cryoglobulinaemia.
| Variables | Cryoglobulinemia (n=33) | No cryoglobulinemia (n=35) | p |
|---|---|---|---|
| Age, years | 56.4±12.8 | 55.3±12.7 | 0.700 |
| Females, n (%) | 22 (66.7) | 20 (57.1) | 0.419 |
| Time since HC diagnosis, years | 12.0 (6.5–18.0) | 9.0 (5.0–16.0) | 0.212 |
| Genotype 1, n (%) | 22 (73.3) | 28 (82.4) | 0.384 |
| HCV-RNA copies | 210,841.00 (65,770.5–1,254,465.0) | 540,181.00 (268,996.0–2,730,864.0) | 0.129 |
| Previous therapy for HC, n (%) | 16 (48.5) | 12 (34.3) | 0.234 |
| F3 or F4 at liver histopathology, n (%) | 10 (34.5) | 4 (12.9) | 0.048 |
| Positive rheumatoid factor, n (%) | 8 (25.0) | 4 (11.4) | 0.148 |
| CH100, % | 0.00 (0.00–72.50) | 101.00 (88.00–108.00) | <0.0001 |
| C2h, % | 0.00 (0.00–86.00) | 95.00 (80.00–106.00) | <0.0001 |
| C3, mg/dL | 102.00 (87.25–117.50) | 114.00 (98.50–124.00) | 0.038 |
| C4, mg/dL | 14.63±7.71 | 25.27±9.65 | <0.0001 |
| Low CH100 level, n (%) | 22 (66.7) | 6 (17.1) | <0.0001 |
| Low C2h level, n (%) | 23 (69.7) | 5 (14.3) | <0.0001 |
| Low C3 level, n (%) | 11 (36.7) | 2 (6.1) | 0.004 |
| Low C4 level, n (%) | 7 (23.3) | 1 (3.0) | 0.022 |
| Proteinuria, n (%) | 5 (15.6) | 5 (14.7) | 0.917 |
| Hematuria, n (%) | 6 (18.8) | 2 (5.9) | 0.143 |
| Serum creatinine, mg/dL | 0.80 (0.70–0.91) | 0.77 (0.69–0.88) | 0.523 |
C2h, C2 hemolytic; CH100, total hemolytic complement; HC, hepatitis C; HCV, hepatitis C virus; n, number of patients. Quantitative variables were described by the mean±standard deviation and median and the interquartile range.
The peripheral nervous system was involved in the majority of HCV CryoVas patients, mainly manifested as distal sensory polyneuropathy in the lower limbs. The central nervous system was involved by the CryoVas in only one patient. In order of frequency, neurological involvement was followed by palpable purpura, arthralgias and glomerulonephritis. Renal involvement was characterized by mild glomerulonephritis with minor abnormalities in urinary sediment. Dysmorphic hematuria and subnephrotic proteinuria were common findings of renal involvement in HCV CryoVas patients, but without overt manifestations such as edema and hypertension or renal impairment. Thus, renal biopsies were not indicated to investigate glomerulonephritis. The gastrointestinal tract and the heart were not involved in HCV CryoVas patients (Table 3). All but one HCV CryoVas patient presented mild to moderate manifestations of CryoVas and the central nervous system vasculitis was the only life-threatening manifestation that affected one patient.
Clinical and laboratorial manifestations of patients with cryoglobulinemic vasculitis.
| Variables | Results (n=7) |
|---|---|
| Age, years | 58.1±11.9 |
| Females, n (%) | 6 (85.7) |
| Clinical features | |
| Cutaneous involvement,n(%) | 6 (85.7) |
| Palpable purpura, n | 3 |
| Raynaud's phenomenon, n | 1 |
| Skin ulcers, n | 2 |
| Joints (arthralgias),n(%) | 3 (42.8) |
| Neurological involvement,n(%) | 6 (85.7) |
| Peripheral nervous system, n | 6 |
| Peripheral polyneuropathy, n | 4 |
| Mononeuritis multiplex, n | 2 |
| Central nervous system, n | 1 |
| Kidneys,n(%) | 3 (42.8) |
| Gastrointestinal tract,n(%) | – |
| Heart disease,n(%) | – |
n, number of patients. The continuous variable was described as mean±standard deviation.
When comparing HCV CryoVas patients with HCV patients with cryoglobulinemia and without cryoglobulinemia no statistically significant differences were found regarding age, frequency of females, serum creatinine levels, proteinuria, hematuria, HCV genotype or HCV viral load (Table 4). However, the frequency of positive rheumatoid factor was higher in HCV CryoVas patients than HCV patients without cryoglobulinemia (p=0.014), whereas no significant differences were found between HCV CryoVas patients and HCV patients with cryoglobulinemia (p=0.052) nor between HCV patients with and without cryoglobulinemia (p=0.700).
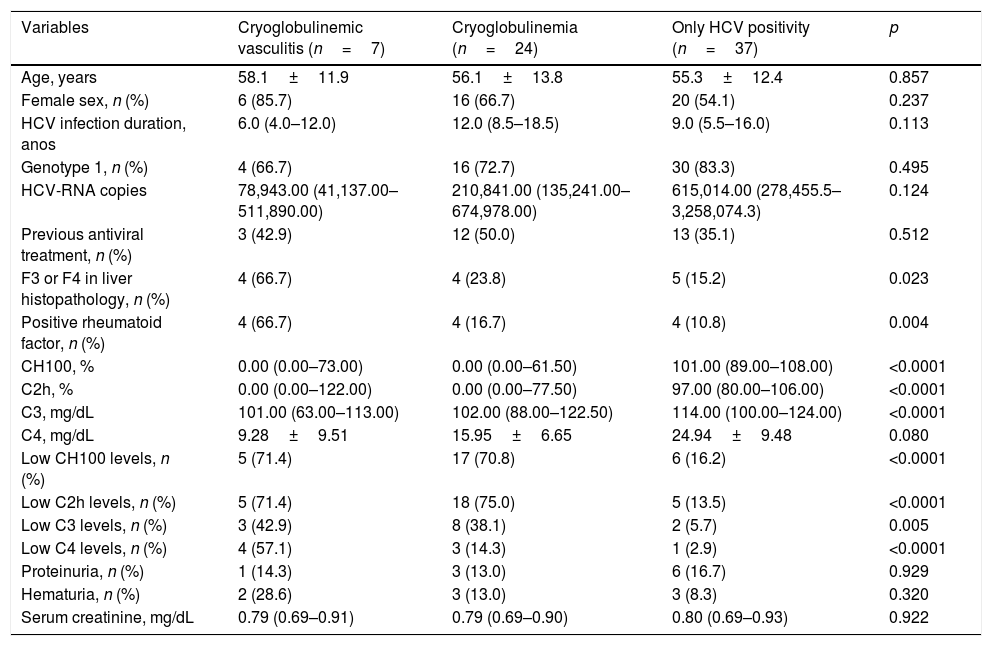
Comparision between the groups with only HCV infection, HCV with isolated cryoglobulins and HCV-related CryoVas.
| Variables | Cryoglobulinemic vasculitis (n=7) | Cryoglobulinemia (n=24) | Only HCV positivity (n=37) | p |
|---|---|---|---|---|
| Age, years | 58.1±11.9 | 56.1±13.8 | 55.3±12.4 | 0.857 |
| Female sex, n (%) | 6 (85.7) | 16 (66.7) | 20 (54.1) | 0.237 |
| HCV infection duration, anos | 6.0 (4.0–12.0) | 12.0 (8.5–18.5) | 9.0 (5.5–16.0) | 0.113 |
| Genotype 1, n (%) | 4 (66.7) | 16 (72.7) | 30 (83.3) | 0.495 |
| HCV-RNA copies | 78,943.00 (41,137.00–511,890.00) | 210,841.00 (135,241.00–674,978.00) | 615,014.00 (278,455.5–3,258,074.3) | 0.124 |
| Previous antiviral treatment, n (%) | 3 (42.9) | 12 (50.0) | 13 (35.1) | 0.512 |
| F3 or F4 in liver histopathology, n (%) | 4 (66.7) | 4 (23.8) | 5 (15.2) | 0.023 |
| Positive rheumatoid factor, n (%) | 4 (66.7) | 4 (16.7) | 4 (10.8) | 0.004 |
| CH100, % | 0.00 (0.00–73.00) | 0.00 (0.00–61.50) | 101.00 (89.00–108.00) | <0.0001 |
| C2h, % | 0.00 (0.00–122.00) | 0.00 (0.00–77.50) | 97.00 (80.00–106.00) | <0.0001 |
| C3, mg/dL | 101.00 (63.00–113.00) | 102.00 (88.00–122.50) | 114.00 (100.00–124.00) | <0.0001 |
| C4, mg/dL | 9.28±9.51 | 15.95±6.65 | 24.94±9.48 | 0.080 |
| Low CH100 levels, n (%) | 5 (71.4) | 17 (70.8) | 6 (16.2) | <0.0001 |
| Low C2h levels, n (%) | 5 (71.4) | 18 (75.0) | 5 (13.5) | <0.0001 |
| Low C3 levels, n (%) | 3 (42.9) | 8 (38.1) | 2 (5.7) | 0.005 |
| Low C4 levels, n (%) | 4 (57.1) | 3 (14.3) | 1 (2.9) | <0.0001 |
| Proteinuria, n (%) | 1 (14.3) | 3 (13.0) | 6 (16.7) | 0.929 |
| Hematuria, n (%) | 2 (28.6) | 3 (13.0) | 3 (8.3) | 0.320 |
| Serum creatinine, mg/dL | 0.79 (0.69–0.91) | 0.79 (0.69–0.90) | 0.80 (0.69–0.93) | 0.922 |
C2h, C2 hemolytic; CH100, total hemolytic complement; HC, hepatitis C; HCV, hepatitis C virus; n, number of patients. Quantitative variables were described by the mean±standard deviation and median and the interquartile range.
The frequency of serum complement abnormalities was higher in HCV CryoVas patients and in HCV patients with cryoglobulinemia than in HCV patients without cryoglobulinemia (p<0.05) for low serum levels of CH100, C2h and C3 while no significant differences were found between HCV CryoVas patients and HCV patients with cryoglobulinemia concerning these three complement tests. Low serum C4 was specifically associated with HCV CryoVas, since the frequency of abnormal serum C4 levels was higher in HCV CryoVas patients than in HCV patients with cryoglobulinemia (57.1% vs. 14.3%, p=0.029) and also higher than in HCV patients without cryoglobulinemia (57.1% vs. 2.9%, p=0.0012). No differences were found between HCV patients with and without cryoglobulinemia for low serum C4 levels (14.3% vs. 2.9%, p=0.290).
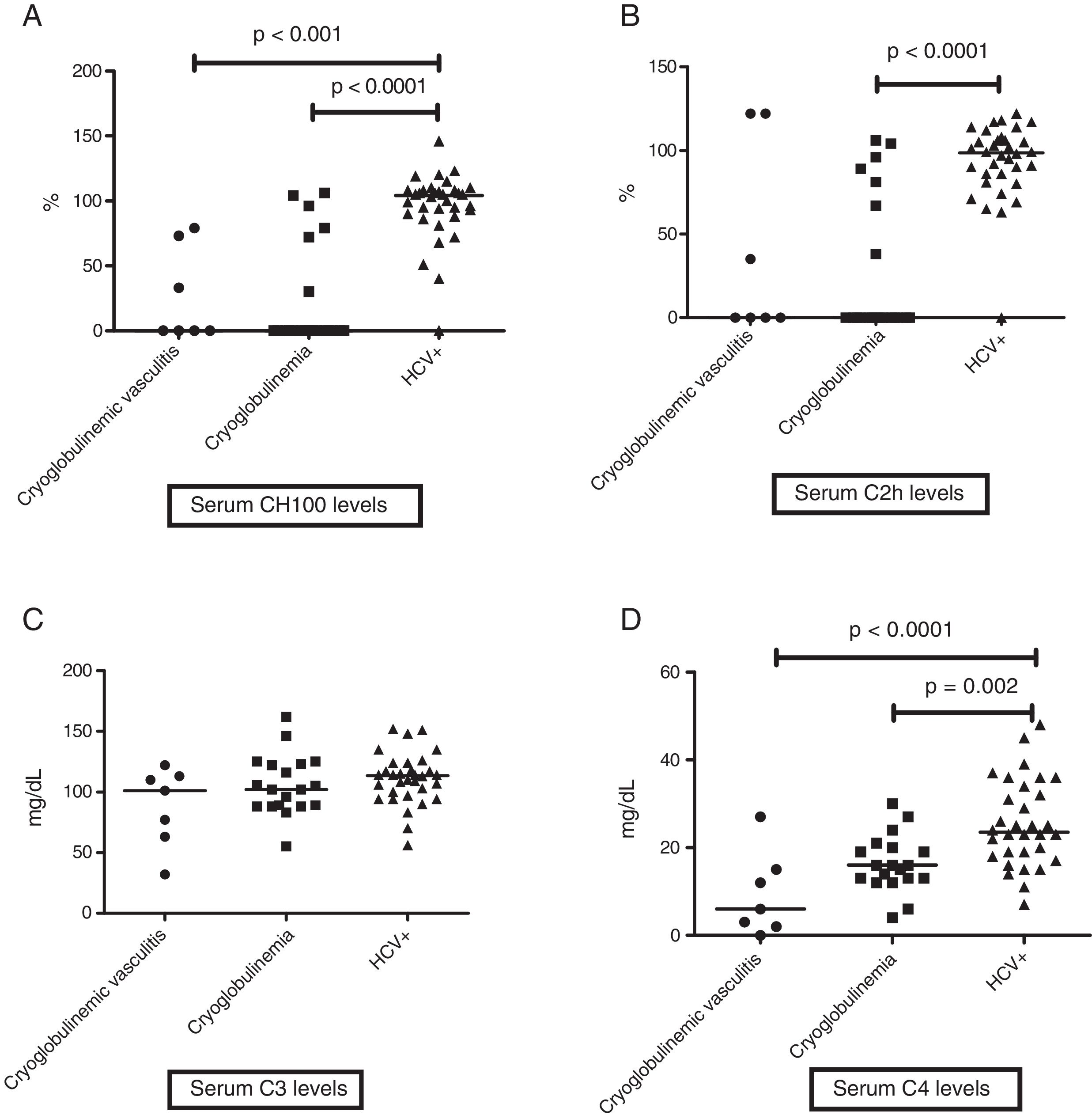
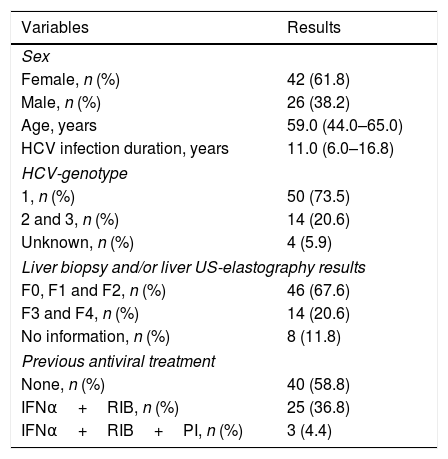
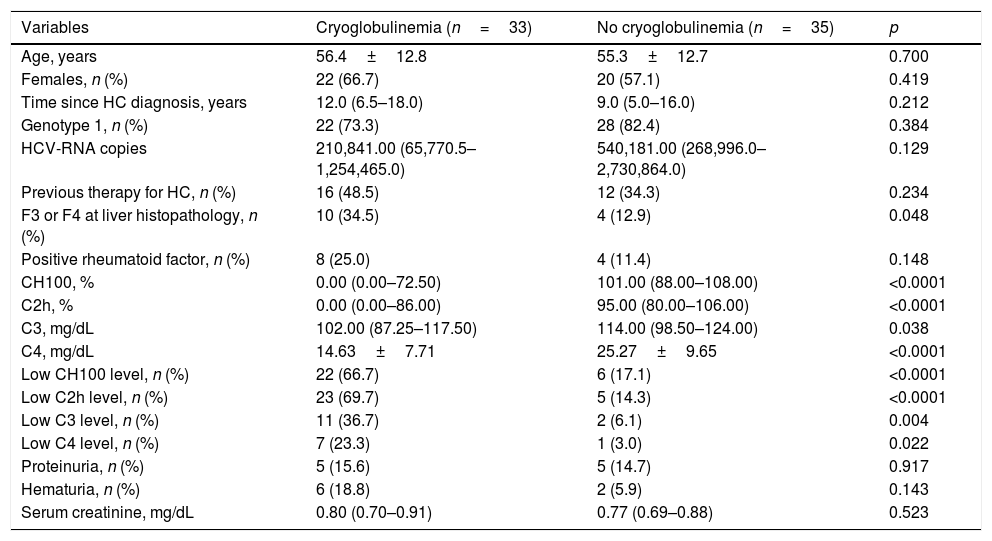
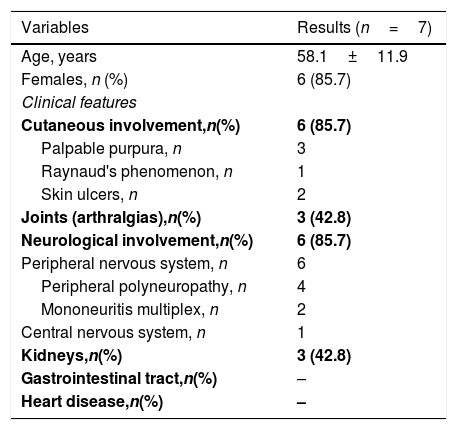
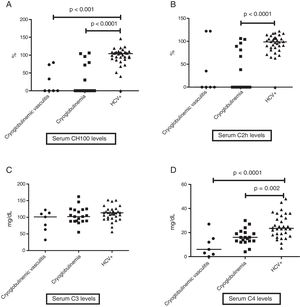
When evaluating levels of serum complement, HCV patients without cryoglobulinemia had significantly higher serum levels of CH100 and C4 than HCV CryoVas patients (p<0.0001 and p=0.002, respectively) and HCV patients with cryoglobulinemia (p<0.0001 and p<0.0001), whereas serum C2h was significantly higher only in HCV patients without cryoglobulinemia than HCV patients with cryoglobulinemia (p<0.0001). No differences regarding serum C3 levels were observed amongst the three HCV groups (Table 4 and Fig. 1A–D). The frequency of F3 and F4 categories was higher in HCV CryoVas patients compared with HCV patients without cryoglobulinemia (p=0.034) but not higher than HCV patients with cryoglobulinemia (p=0.052).
Comparison of serum complement levels in groups of HCV patients. Charts display the comparison among HCV patients without cryoglobulinemia, HCV patients with cryoglobulinemia and HCV patients with CryoVas. Serum CH100 levels were lower in CryoVas and in cryoglobulinemia ptients compared with HCV patients without these complications (A), serum C2h levels were lower in patients with cryoglobulinemia than in HCV patients without this complication (B), serum C3 levels were similar among HCV patient groups (C) and serum C4 levels were significantly lower in CryoVas patients in comparison with patients presenting cryoglobulinemia and HCV patients without these complications (D).
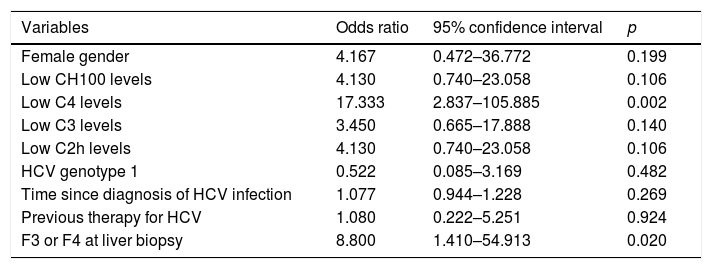
Univariate and multivariate logistic regression models were built to analyze associations with the development of CryoVas. In univariate models, low C4 levels and F3 or F4 at liver biopsy were significantly associated with the development of CryoVas (Table 5). In the multivariate analysis, the independent variables were female gender, time since the diagnosis of HCV infection, low C3 levels, low C4 levels, F3 or F4 at liver biopsy and previous therapy for HCV. Only low C4 levels remained independently associated with the development of CryoVas in HCV patients (OR: 24.00; 95CI: 2.83–203.13; p=0.004).
Univariate analysis to analyze associations with the development of CryoVas in HCV-positive patients.
| Variables | Odds ratio | 95% confidence interval | p |
|---|---|---|---|
| Female gender | 4.167 | 0.472–36.772 | 0.199 |
| Low CH100 levels | 4.130 | 0.740–23.058 | 0.106 |
| Low C4 levels | 17.333 | 2.837–105.885 | 0.002 |
| Low C3 levels | 3.450 | 0.665–17.888 | 0.140 |
| Low C2h levels | 4.130 | 0.740–23.058 | 0.106 |
| HCV genotype 1 | 0.522 | 0.085–3.169 | 0.482 |
| Time since diagnosis of HCV infection | 1.077 | 0.944–1.228 | 0.269 |
| Previous therapy for HCV | 1.080 | 0.222–5.251 | 0.924 |
| F3 or F4 at liver biopsy | 8.800 | 1.410–54.913 | 0.020 |
C2h, C2 hemolytic; CH100, total hemolytic complement; HCV, hepatitis C virus.
This study evaluated the prevalence of cryoglobulinemia and CryoVas in HCV patients in outpatient clinic of hepatitis in Brazil. Although 40% of our patients had already received an IFNα-based therapy with ribavirin and/or a first-generation protease inhibitor, the frequency of both cryoglobulinemia and CryoVas was still high. Most Brazilian HCV patients presented type III cryoglobulinemia. The main disease manifestations of CryoVas were peripheral polyneuropathy followed by palpable purpura, arthralgias and mild glomerulonephritis.
Clinical and biological manifestations of cryoglobulinemia were described before the discovery of HCV [7,22,26]. The first study that evidenced anti-HCV antibodies in patients with type II cryoglobulinemia was published in 1990 [27] and subsequent studies confirmed this finding and showed that HCV viral RNA was selectively concentrated in cryoprecipitates [28–32].
After the discovery of HCV role in the pathogenesis of cryoglobulinemia, large-population surveys found anti-HCV antibodies in 43–85% of selected patients with mixed cryoglobulinemia [29,30,32,33]. Initial studies showed a high prevalence of cryoglobulinemia mainly in Mediterranean countries, but all patients were of Italian descent and many of them also had serological evidence of HBV infection. These studies included only few cases of CryoVas [29,33].
The first prospective study performed in France in 1994 found 54% of cryoglobulinemia in 127 HCV patients, which was associated with cirrhosis and a prolonged history of hepatitis C. French patients with HCV-associated cryoglobulinemia were younger than Brazilian patients (median age 50.4 vs. 59.0 years) and had a higher frequency of cirrhosis (33.8%) [34]. Another French study published in the same year, analyzed 115 patients with cryoglobulinemia and found anti-HCV antibodies in 52% of cases [28]. Patients with HCV-related cryoglobulinemia are considered to present a more severe disease course when compared with patients with non-HCV-related cryoglobulinemia and this was confirmed by Pawlotsky et al. [35] when comparing patients with HCV, HBV and hepatic disease of non-infectious etiologies. Cryoglobulinemia was found in 36% of the HCV patients, four of whom had dermatological and/or neurological manifestations of CryoVas.
Regarding the cryoglobulinemia in HCV patients from other continents, the prevalence reported in the United States, China, Japan and India are as follows: 19%, 44%, 37% and 32%, respectively [17]. To date, five studies [36–40] have assessed cryoglobulinemia in Brazilian HCV patients and the range of the reported prevalence was very wide (i.e. 1.0–59.6%), whereas the prevalence found in our study was 48.5%. The reasons for this heterogeneity may be due to the lack of standardization of the technique used to detect cryoglobulins. In two studies [36,37], cryoglobulins were investigated using gel cryoprecipitation. In another study, the methodology used to detect cryoglobulins was not explained [38]. There was only one Brazilian study that looked for clinical manifestations of CryoVas [39], but it included Sjögren syndrome and lymphoma as manifestations of the “cryoglobulinemic syndrome”. As CryoVas classification criteria were validated only in 2014, this is the first time that Brazilian HCV-patients with cryoglobulinemia were systematically evaluated regarding the presence of vasculitis.
The reasons for geographic differences in the prevalence of cryoglobulinemia in HCV patients are not clear and, besides factors related to the techniques used to detect serum cryoglobulins, climate variations, degree of hepatic damage, genetic background of HCV patients and access to antiviral treatment may all contribute to the wide variation in the prevalence of cryoglobulinemia. As an evidence of the genetic influence in the development of cryoglobulinemia in HCV patients, a study showed that the homozygosity of the T allele of the BAFF promoter was associated with the development of mixed cryoglobulinemia in HCV patients [41].
The frequency of most clinical features of CryoVas in Brazilian patients was similar to previous reports from other countries. The onset of CryoVas manifestations is usually between 45 and 65 years, being more common in women [8,42,43]. Like other studies, cutaneous manifestations were one of the most frequent manifestations of CryoVas [22,39,44–46], especially palpable purpura. On the other hand, the frequency of Raynaud's phenomenon was low in our patients with CryoVas (14.2%) and this may be justified in part by the warm weather in the Southeast of Brazil. Peripheral neuropathy was much more common in our patients with CryoVas (85.7%) than the frequency previously reported by other studies which ranged from 8.3% to 55.1% [5,22,39,45,46]. This may indicate a unique feature of CryoVas phenotype in our patients. Only one patient with CryoVas had the involvement of central nervous system, manifested as right hemiparesis, with complete recovery after plasmapheresis sessions and intravenous cyclophosphamide. Stroke-like syndromes have been observed in CryoVas and it is attributed to brain ischemia or hemorrhage [47].
The prevalence of renal involvement amongst our patients with CryoVas falls within the 21% to 55% previously reported by other studies [22,26,28,45,46]. Dysmorphic hematuria and subnephrotic proteinuria without relevant renal impairment were the main findings in our patients, indicating mild glomerulonephritis. Histopathology of renal biopsies in CryoVas shows membranoproliferative glomerulonephritis with sub-endothelial deposits in 70–80% of the cases [7].
In this study, the presence of cryoglobulins was associated with lower serum levels of all complement components evaluated (i.e. C2h, C3, C4 and CH100) even in the absence of CryoVas manifestations. When comparing HCV patients presenting cryoglobulinemia with those presenting CryoVas, low serum C4 levels were more frequently found in the latter. In addition, low C4 levels were independently associated with CryoVas. Indeed, the hypocomplementemia is a common finding in cryoglobulinemia and it is described in 70–90% of cases [8]. A striking feature of patients with active CryoVas is the remarkable decrease in the serum levels of complement C4 and, to a lesser extent, of CH50 and C3 [11]. The complement activation through the classic pathway due to cryoglobulins precipitation and tissue deposits of immunocomplexes is thought to be the cause of complement consumption even in asymptomatic patients [17], but the exact mechanism responsible for this selective depression of C4 remains unclear.
A high frequency of positive rheumatoid factor was another relevant laboratory finding in our CryoVas patients. Rheumatoid factor positivity is observed especially in patients with mixed cryoglobulinemia, in which a monoclonal IgM component generates large immune complexes with IgG and complement fractions. The presence of RF has also been reported as a very frequent finding even in serum of HCV positive patients without vasculitis and it could be influenced by antiviral treatment [48].
In this study, we found type III cryoglobulinemia as the most common subtype. Despite the classic description that type II cryoglobulinemia being more associated with chronic HCV infection [9], a previous study has also found type III as the most frequent cryoglobulinemia amongst HCV-patients [42], with type II being more related with symptomatic disease [39]. Nonetheless, the serum protein electrophoresis technique that we used to classify cryoglobulins has some limitations, since it allows a complete cryoglobulin identification in only 28% of the cases, against immunofixation and immunoblotting, which identify 54% and 98% of cryoglobulins subtypes, respectively [12].
Although there were no significant associations between age, female gender, longer time since diagnosis of HCV infection, HCV genotype or HCV viral load and the presence of serum cryoglobulins or CryoVas, previous studies described associations between the female gender and genotypes 2 and 3 [42,49] with HCV-related cryoglobulinemia. This is the first time that extensive hepatic fibrosis was found to associated with CryoVas. Previous studies had only described associations between higher METAVIR scores with the presence of serum cryoglobulins [34,39,40,42], but not with CryoVas. Higher METAVIR scores are also predictors of poor prognosis in CryoVas patients [13].
Limitations of this study include the relatively low number of HCV patients evaluated, the detection of cryoglobulins by a qualitative method and, in some cases, the lack of renal and/or nerve biopsies to confirm the kidney and the peripheral nervous system involvement by CryoVas. In addition, the time elapsed between the assessment of liver biopsy and the evaluation of cryoglobulinemia in this study may have had an impact on the analysis of associations between both variables.
In conclusion, this study showed a high frequency of cryoglobulinemia and of CryoVas in Brazilian HCV-patients and it is the first time that these patients were systematically evaluated regarding the presence of vasculitis through validated classification criteria. Complement abnormalities are frequent in HCV patients with cryoglobulinemia compared with HCV patients without this complication and low serum C4 levels are independently associated with CryoVas. In Brazilian patients who developed CryoVas, the skin and the peripheral nervous system are frequently affected, whereas severe manifestations are relatively rare. The high prevalence of cryoglobulinemia and CryoVas in HCV patients from Brazil makes it crucial to actively investigate both entities in HCV patients and this requires a high clinical suspicion associated with careful physical examination and adequate laboratory investigation.AbbreviationsHCV hepatitis C virus cryoglobulinemic vasculitis total hemolytic complement immunoglobulin interferon alpha World Health Organization Universidade Federal de São Paulo Escola Paulista de Medicina polymerase chain reaction ribonucleic acid human immunodeficiency virus hepatitis B virus prothrombin time international normalized ratio rheumatoid factor serum hemolytic C2 International Business Machines Statistical Package for the Social Sciences odds ratio number of patients hepatitis C
Mariana F. Aguiar, Anna Larissa F. Janes, Gabriela I.G. Brandes and Christini T. Emori were involved in the acquisition of data, assessed patients, analyzed data and drafted the manuscript. Alexandre W.S. de Souza, Luis Eduardo C. Andrade and Maria Lúcia G. Ferraz conceived the study, analyzed the data and corrected the manuscript.
Confirmation that informed patient consent was obtained for publication of case detailsAll procedures performed in this study were in accordance with the ethical standards of institutional research committee and complied with the 1994 Helsinki Declaration. The patients were recruited in this study after written informed consent.
Financial supportThis study was developed without any financial support.
Conflict of interestThe authors have no potential conflicts of interest.