Objetive: Nonalcoholic fatty liver disease is an increasingly recognized condition that may progress to endstage liver disease. We investigated the effects of weight reduction and ursodeoxycholic acid administration in patients with this disease.
Research methods and procedures: A double-blind, placebo-controlled trial.
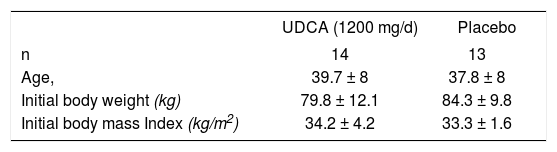
Twenty-seven women with a body mass index of >30 kg/m2 and willing to participate in the diet plan for six weeks were studied were assigned to one of two treatment groups (ursodeoxycholic acid, n = 14: placebo n = 13). Both groups received a normal diet (1,200 kcal/d) plus 1200 mg/d of ursodeoxycholic acid or placebo. Hepatic steatosis, was assessed by abdominal ultrasound. Fasting glucose, cholesterol, triglycerides, and aminotransferases levels were determined before and after treatment.
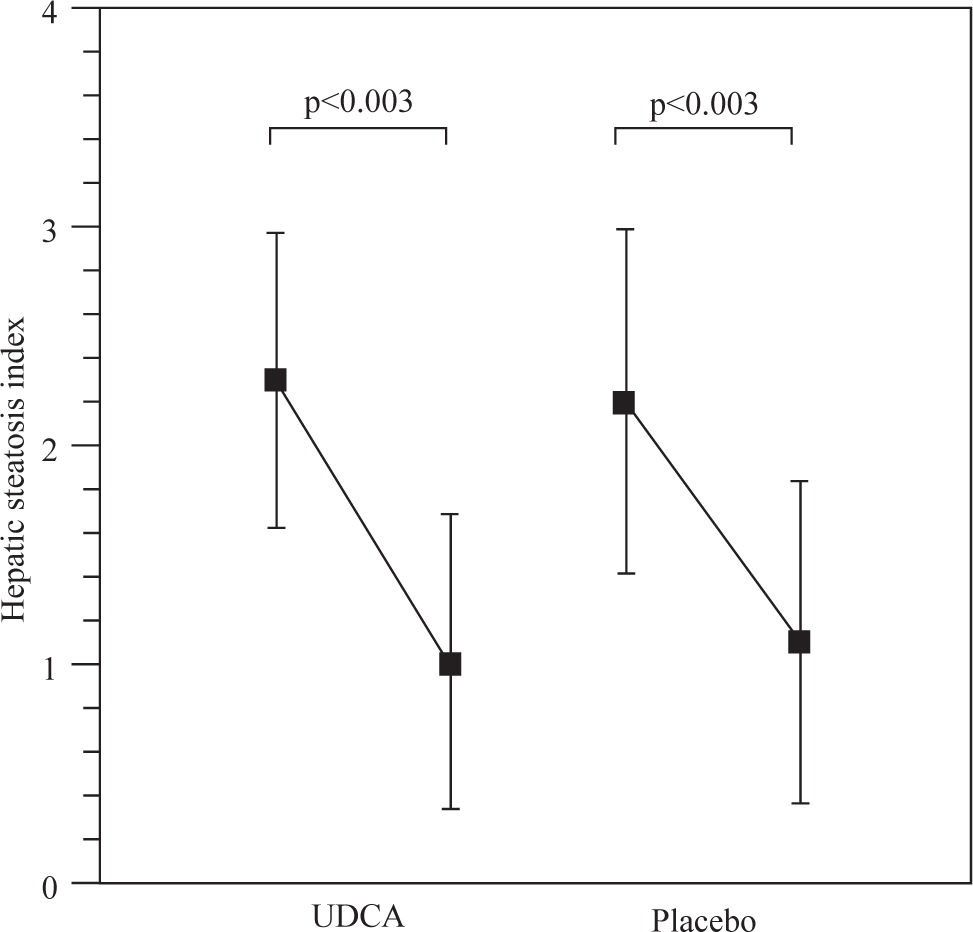
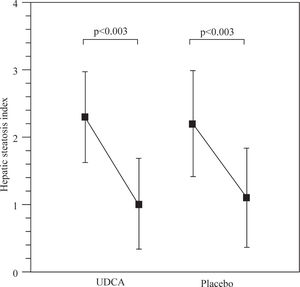
Results: Body mass index decreases significantly from 34.2 ± 4.2 kg/m2 and 33.3 ± 1.6 kg/m2 to 31.8 ± 4.5 kg/m2 and 30.6 ± 2.6 kg/m2 in the ursodeoxycholic acid and placebo groups, p < 0.001. The hepatic steatosis index decreased from 2.3 ± 0.7 to 1.0 ± 0.6 and 2.2 ± 0.7 to 1.1 ± 0.7 in the ursodeoxycholic acid and placebo groups, p<0.003. Serum AST decreased significantly from 41.2 ± 5.6 to 34.5 ± 3.4 in the ursodeoxycholic acid group, p <0.001, and from 43.6 ± 4.2 to 35.3 ± 2.9 in the placebo group, p <0.001. Serum ALT decreased from 62.9 ± 6.5 to 44.0 ± 3.5 in the ursodeoxycholic acid group, p <0.001, and from 63.5 ± 4.5 to 44.0 ± 3.5 in the placebo group. We did not find any differences in all variables studied between groups.
Conclusions:The present study shows beneficial effect of weight reduction, producing improvements in biochemical and imaging markers of liver disease.
Nonalcoholic fatty liver disease (NAFLD) is an increasingly recognized condition that may progress to end-stage liver disease. The pathological picture resembles that of alcohol-induced liver injury, but it occurs in patients who do not abuse alcohol.1 Various terms have been used to describe this entity, including fatty-liver hepatitis, nonalcoholic Laënnec’s disease, diabetes hepatitis, alcohol-like liver disease, and nonalcoholic steatohepatitis.2 Nonalcoholic fatty liver disease is becoming the preferred term, and it refers to a wide spectrum of liver damage, ranging from simple steatosis to steatohepatitis, advanced fibrosis, and cirrhosis. Steatohepatitis (nonalcoholic steatohepatitis) represents only a stage within the spectrum of NAFLD.3
The influence of obesity on liver physiology has been demonstrated in several studies and populations, the analyses showing the effects of weight reduction on biochemical indicators of liver function.4,5 Obesity, or indirectly, body mass index (BMI), is a major risk factor for development of liver disease, and for NAFLD the prevalence increases 4.6-fold in obese people.3,6 Other risk factors associated with NAFLD are waist circumference, hyperinsulinemia, hypertriglyceridemia and impaired glucose tolerance or type 2 diabetes.7,8 These risk factors have also been examined in animal models. For example, studies of genetically obese ob/ob mice and fa/fa rats have provided information about the pathogenesis of obesityrelated fatty liver disease.3,9 The clinical implications of NAFLD are derived mostly from its common occurrence in the general population and its potential to progress to cirrhosis and liver failure. NAFLD should be differentiated from steatosis with or without hepatitis resulting from secondary causes, because these conditions have distinctly different pathogenesis and outcomes. Currently, there is no specific treatment for NAFLD. We hypothesized that weight reduction in patients with NAFLD would be accompanied by a decrease in the degree of hepatic steatosis and a reduction of serum transaminase levels. Furthermore, it has been suggested that ursodeoxycholic acid (UDCA) the epimer of chenodeoxycholic acid and appears to replace endogenous bile acids, some of which may be hepatotoxic, with the nonhepatotoxic UDCA This bile acid has also membrane stabilizing or cytoprotective effects as well as antiapoptotic actions.10 The aim of this study was to investigate the effects of weight reduction and UDCA on patients with NAFLD.
Research Methods and ProceduresSubjectsWe recruited subjects from our medical center in Mexico City (Medica Sur Clinic & Foundation). Obese women were informed of the risk of NAFLD developing according to the degree of hepatic steatosis and were asked whether they would participate in a study of NAFLD treatment. The diagnosis of NAFLD was first suspected in those subjects who had had, for at least six months, abnormal serum aminotransferase levels that were not related to other causes of liver disease, including the hepatitis B and C viruses, autoimmune disorders, alcohol, hemochromatosis, and sonographic findings compatible with hepatic steatosis. To be accepted into this trial, women had to (a) have a BMI of >30 kg/m2, (b) be between 20 and 60 years of age, (c) be willing to participate in the diet plan for six weeks, and (d) have normal serum potassium and calcium levels. Women of childbearing age had to have a negative serum pregnancy test. Women were excluded from the study if they had any one of the following: (1) a history of hypothyroidism or Cushing syndrome, (2) an eating disorder or other psychological problem that would interfere with participation in the diet program, (3) use of oral bile acid preparations, aluminum-based antacids or lithium, and (4) long-term use of nonsteroidal anti-inflammatory agents (including aspirin) or antihyperlipidemic agents (including cholestyramine) within two weeks of entering the trial. Diuretic therapy had to have been discontinued at least one day before trial entry.
Twenty-seven women who met all entry criteria and agreed to participate were enrolled in the trial. The study was approved by the Human Subjects Committee of the Medica Sur Clinic & Foundation, conforming to the ethical guidelines of the 1975 Declaration of Helsinki, and written informed consent was obtained from all participants before entry.
Study designWomen were assigned to one of two treatment groups in a double-blind fashion, with blocking for body weight (BMI >30 kg/m2), according to a table of random numbers. One group received a normal diet (1200 kcal/d) plus 1,200 mg/d of UDCA (Laboratorios Farmasa, S.A. de C.V., Mexico); the second group received a normal diet (1200 kcal/d) plus a placebo. All capsules were identical in appearance and number. Treatment began on the day on which energy restriction began. Compliance was determined by counting unused capsules each week. Women were treated for six weeks.
Weight-control program. The women underwent the Department of Program Control's standard history-taking and physical examination, as well as laboratory tests including a complete blood count, measurement of electrolytes, liver function tests, measurement of fasting lipids, thyroid-function tests, and electrocardiography. They were placed on a food diet (1200 kcal/d) consisting of 20% fat, 60% carbohydrates, 20% protein, and 1 L of water daily. The composition of the diet was carbohydrates 195.6 g/d, total fat 26.4 g/d (saturated fat 7.6 g/d, polyunsaturated fat 2.3 g/d, monounsaturated fat 3.1 g/d, cholesterol 73.8 mg/d), fiber 30.2 g/d, iron 7.1 mg/d, sodium 873.2 mg/d, zinc 9.28 mg/d, and Vitamin C 54.8 mg/d.
Experimental procedures. Real-time ultrasonographic studies were carried out before and after the experimental period. Women fasted overnight before each ultrasound was performed. The protocol used to evaluate the pattern of hepatic steatosis by ultrasound was graded as follows: (0) Normal, (1) Diffuse, homogeneous, (2) Geographic pattern: sharp demarcation between normal and fatty liver, not confined to a lobar distribution and no mass effect on hepatic vessels, (3) Focal: no mass effect, no vessel displacement, segmental or lobar wedge distribution, low attenuation, “earthquake” pattern (scattered irregular low attenuation lines), (4) Focal sparing: pseudotumor (anterior to right portal vein/gallbladder fossa, medial segment of left lobe, porta hepatis), glove pattern (high attenuation finger-like interdigitations of spared parenchyma within a low attenuation background), simulating metastases.11 The severity of hepatic fatty infiltration was graded as follows: grade 0, normal; grade 1, liver attenuation slightly less than spleen; grade 2, a more pronounced difference between liver and spleen, and intrahepatic vessels not seen or with slightly higher attenuation than liver; grade 3, markedly reduced liver attenuation with sharp contrast between liver and intrahepatic vessels.
Analytical methodsPlasma glucose in the fasting state was measured in duplicate with an automated analyzer. The coefficient of variation for a single determination was 1.5%. Cholesterol, HDL-cholesterol, and triglycerides were measured by enzymatic colorimetric methods, using CHOL, HDL-C plus (second generation) and TG assays (Roche Diagnostics Co., Indianapolis, IN). LDL cholesterol concentrations were calculated using the Friedewald formula.12
Statistical analysisDifferences between the groups over time were evaluated by paired Student’s t-test.13 The differences were considered significant when p < 0.05. Values in the text are means ± SD.
ResultsTwenty-seven women were enrolled in the trial. Of these, three withdrew prematurely and were not included in the final analysis. The major reason for early termination was voluntary withdrawal from the program (n = 2 (7.4%)). The percentage of women who voluntarily withdrew from the trial did not differ between the two treatment groups of subjects receiving UDCA, or placebo. Two women, one in each group, were excluded after becoming pregnant during the trial (n = 2 (7.4%)). The clinical characteristics of subjects at the beginning of the diet trial are given in Table I. The initial BMIs were 34.2 ± 4.2 kg/m2 and 33.3 ± 1.6 kg/m2, respectively. BMI did not differ between the two treatment groups before the start of the study.
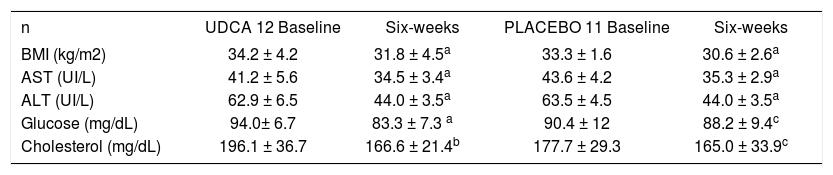
Table II shows the weight loss in both groups. BMI decreased significantly from 34.2 ± 4.2 kg/m2 and 33.3 ± 1.6 kg/m2 to 31.8 ± 4.5 kg/m2 and 30.6 ± 2.6 kg/m2 in the UDCA and placebo groups, respectively, p < 0.001. Serum AST decreased significantly from 41.2 ± 5.6 to 34.5 ± 3.4 in the UDCA group, p <0.001, and from 43.6 ± 4.2 to 35.3 ± 2.9 in the placebo group, p <0.001. Serum ALT decreased from 62.9 ± 6.5 to 44.0 ± 3.5 in the UDCA group, p <0.001, and from 63.5 ± 4.5 to 44.0 ± 3.5 in the placebo group. We did not find any differences in those variables studied between groups. However, cholesterol and glucose decreased significantly only in the UDCA group. Serum triglycerides did not change in either group.
Characteristics of patients at the baseline and at six-weeks of the diet trial.
| n | UDCA 12 Baseline | Six-weeks | PLACEBO 11 Baseline | Six-weeks |
|---|---|---|---|---|
| BMI (kg/m2) | 34.2 ± 4.2 | 31.8 ± 4.5a | 33.3 ± 1.6 | 30.6 ± 2.6a |
| AST (UI/L) | 41.2 ± 5.6 | 34.5 ± 3.4a | 43.6 ± 4.2 | 35.3 ± 2.9a |
| ALT (UI/L) | 62.9 ± 6.5 | 44.0 ± 3.5a | 63.5 ± 4.5 | 44.0 ± 3.5a |
| Glucose (mg/dL) | 94.0± 6.7 | 83.3 ± 7.3 a | 90.4 ± 12 | 88.2 ± 9.4c |
| Cholesterol (mg/dL) | 196.1 ± 36.7 | 166.6 ± 21.4b | 177.7 ± 29.3 | 165.0 ± 33.9c |
Figure 1 shows the hepatic steatosis index. There was a decrease from 2.3 ± 0.7 to 1.0 ± 0.6 and 2.2 ± 0.7 to 1.1 ± 0.7 in the UDCA and placebo groups, p<0.003 respectively.
DiscussionIn this study, we analyzed the effects of weight reduction with or without pharmacological treatment with UDCA. Currently there is no consensus regarding effective therapy for NAFLD; attempts are being made to direct treatment toward avoiding or correcting risk factors, including insulin resistance and decreasing hyperin-sulinemia, and using drugs with potential hepatoprotective effects.14 Our data shows the beneficial effect of diet, especially in body weight reduction, with a decrease of 8% and 7% in the placebo and UDCA groups. In addition, we observed a decrease (16.2%) in the levels of aspartate aminotransferase in both groups, whereas the decrease in alanine aminotransferase (ALT) levels was 30% in UDCA group versus 16.2% in the placebo group. No statistical effect on glucose and cholesterol levels was observed. Schaffner et al.2 showed that a weight reduction of greater than or equal to 10% corrected abnormal hepatic test results, decreased hepatosplenomegaly, and resolved some stigmata of liver disease, while minimal changes in BMI were important in liver function and for every 1% reduction in body weight, alanine aminotransferase activity improved by 8.1%.5 The effects of weight reduction on liver function have been demonstrated in several mammal models.16-18 In cats, the reduction of 25-30 per cent of body weight was associated with significant changes in plasma insulin, cholesterol, triglyceride, and serum glucose concentrations,19 and changes in the metabolism of free fatty acids and hepatic fatty acid synthesis are involved in the development of hepatic lipidosis.20 These data indicate the importance of weight reduction, especially considering that the cat model has similarities with NAFLD in humans.21 One of the most important pathogenic mechanisms involved in NAFLD is insulin resistance. In the cat model, Biourge et al.22 showed that weight reduction was associated with a significant decrease in mean serum insulin concentration and the glucose disappearance coefficient. Similarly in humans, weight reduction has major importance in insulin resistance. Case et al.23 observed that a moderate decrease in weight (6.5%) induced by a very low calorie diet resulted in substantial reductions in glucose (17 mg/dL), triglycerides (94 mg/dL) and total cholesterol (37 mg/dL) after four weeks. According with those observations, we believe that the effects of weight reduction on human liver diseases with similar pathological substrates will have prognostic implications.24 Recently, Hickman et al.25 showed the effect of weight reduction in patients with chronic hepatitis C. They found a decrease in ALT levels and in stellate cell activation and, in some cases, regression of hepatic fibrosis. Weight reduction is also accompanied by diminution of hepatomegaly in obese women with liver steatosis.26 In subjects who underwent gastroplasty for morbid obesity, the weight reduction was associated with morphological liver changes, particularly in the severity of the steatosis seen in liver biopsies.27 In addition, when subjects with non-alcoholic steatohepatitis received a low energy diet for three months, a decrease in the degree of steatosis was observed by tomography.28
On the other hand, a recent study published by Lindor and colleagues29 shows that 2 years of therapy UDCA at a dose of 13 to 15 mg/kg/d was not better than placebo for patients with NASH. These data confirm the previous observation published by Laurin who found no significant changes in the histological grade of inflammation or fibrosis.30 Also Santos et al31 have recently reported that UDCA is able to reduce serum levels of hepatic enzymes in patients with nonalcoholic fatty liver disease, but this effect is not related to modifications in liver fat content.
Finally, the main limitation of our study has been the lack of liver biopsies to assess the histology of the liver, which is the “gold standard” in the diagnosis of NAFLD. Morphologic changes evaluated by ultrasound should be considered with caution, as it is well known that the reproducibility of this technique is related to the operator.
In conclusion, the present results shown a beneficial effect of weight reduction on patients with NAFLD by improving the biochemical and imaging markers of liver disease. This improvement was independent of the quantity of weight reduction. A prospective study with a large population is necessary to determine the histopathology changes associated with weight reduction.
AcknowledgementsGrant support: This work was partly supported by the Medica Sur Clinic & Foundation.
This paper was presented in part at the Annual Meeting of the American Association for the Study of Liver Diseases in Boston, MA, USA, and published in its abstracts (Hepatology 2002;36:412A).