Aim: To evaluate the effects of ribavirin on cytokine production of recall antigen and PHA-stimulated PBMC obtained from healthy individuals.
Materials and methods: PBMC were challenged with tetanus toxoid (5μg/mL) and PHA (10 μmL) in absence or presence of ribavirin at different concentrations (1, 10 and 100 (μM). Parallel sets of wells containing PBMC exposed to medium alone were used as negative controls. On day 3 after initiation of the cultures, IL-2, IFN-γ, IL-4, IL-10, TNF-α content were determined in supernatants of PBMC from the different individuals.
Results: The effects of ribavirin on cytokine released by human PBMC in response to PHA and TT showed a great variation among individuals. No significant changes were observed between 1-10μ M concentrations in the production of TNF-α, IFN-γ and IL-10 by both PHA and TT-stimulated PBMC. Ribavirin inhibited TNF-α, IFN-γ, and IL-10 in both PHA and TT-stimulated PBMC at 100 μM (p <0.05). At this concentration, ribavirin induced an increase of 124% in the production of IL-2 by PHA-stimulated PBMC (p <0.05).
Conclusions: The present data suggest that ribavirin may cause diverse effects on immunoregulatory cytokine secretion with changes in the Th1/Th2 balance.
Ribavirin (1-β-D-ribofuranosyl-1, 2, 4-triazole-3 carboxamide) is a synthetic nucleoside discovered in 1970 with a considerable therapeutic potential for the treatment of viral infections in humans.1
Ribavirin inhibits nucleic acid synthesis and replication of ribonucleic and deoxyribonucleic acid viruses by interfering specifically with inosine 5’-monophosphate dehydrogenase, a cellular enzyme required for guanosine 5’-monophosphate biosynthesis.2
This antiviral agent has an inhibitory activity against a broad-spectrum of viral pathogens, including influenza and parainfluenza viruses, bunyaviruses, respiratory syncytial virus,3 and severe acute respiratory syndrome (SARS) virus, a new mutant of coronavirus.4
Ribavirin in combination with alpha interferon is the consensus treatment for chronic hepatitis C, and remains the most frequent indication of this drug. Ribavirun monotherapy in chronic hepatitis C infection normalizes alanino-aminotransferase (ALT) levels leading to a biochemical response in most patients but has minor activity on serum HCV-RNA titers.5 Recent data have shown that a treatment combining ribavirin and alpha interferon is better than interferon alone, with a 38-43% sustained virological response rate.6 Moreover, the combination therapy of ribavirin and pegilated interferons induces sustained virological response in a higher proportion of patients.7
Although the mechanism by which the drug exerts its response on patients with chronic HCV infection is under evaluation, there is evidence that ribavirin is likely to have immunomodulatory and anti-inflammatory effects. Previous studies have shown that ribavirin has immunosuppressive activity, reducing lymphocyte proliferation to mitogens in vitro, prolonging skin allograft survival in rats8,9 and inhibiting mast cell secretory processes.10
We have carried out the present experiment for evaluating the in vitro effects of ribavirin on cytokine production of recall antigens and PHA-stimulated human peripheral blood mononuclear cells PBMC obtained from healthy individuals.
Material and methodsAfter an overnight fasting blood samples were collected from eight healthy adult individuals. This study was performed according to the principles of the Declaration of Helsinki and was approved by the Ethical Committee of the Institution. Written consent was obtained from each volunteer.
Compounds. 1-β-D-ribofuranosyl-1, 2, 4-triazole-3 carboxamide (ribavirin) was purchased from Sigma Chemical Co., St. Louis, USA.
Cell culture. PBMC were isolated from heparinized blood obtained from the healthy volunteers by Ficoll-Hypaque gradient centrifugation. After washing, PBMC were seeded into round-bottom 96 well cultures at 1-2x 105 cell per well in a final volume of 180 μL RPMI’s medium supplemented with antibiotics (penicillin, 100 U/mL and streptomycin, 100 (μgmL), 10% autologous serum, 2mM L-glutamine, 10-5M 2-mercaptoethanol. PBMC were separately challenged with phytohemaglutinin (PHA) (10 (μgmL) and tetanus toxoid (TT) (5 μg/mL) as a recall antigen in absence or presence of ribavirin at different concentrations (1, 10 and 100 μM).
Parallel sets of wells containing PBMC exposed to medium alone were used as negative controls.
Ribavirin in different concentrations, and together with all recall antigens was screened for cytotoxic activity by trypan blue exclusion test.
The cultures were maintained at 37°C in a humidified atmosphere supplemented with 5% CO2. At day 3 and 6 after initiation of the cultures, aliquots (25 μL/well) of supernatant were collected and frozen at –20°C until being assayed for interferon-γ (IFN-γ), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α) content receptively. After completion of different culture periods (3 days for PHA and 6 days for recall antigens-stimulated PBMC), cells were harvested.
Estimation of cytokine in culture supernatantsIFN-γ, IL-2, IL-4, IL-10 and TNF-α were determined by two site capture ELISA using two monoclonal antibodies to IFN-γ, IL-2, IL-4, IL-10 and TNF-α respectively, according to the instructions given by the manufactures (R & D Systems, Minneapolis, MN). Pooled samples from the culture supernatants of 3 wells were assayed to measure the cytokine content. The amount of cytokine was estimated from standard curves generated for each plate by the inclusion of standardized concentrations of the respective recombinant human interleukins.
Statistical methodsResults were expressed as mean ± standard deviation (SD). Student’s or Wilcoxon’s matched paired tests were used to compare continuous variables. A p-value lower than 0.05 was considered statistically significant.
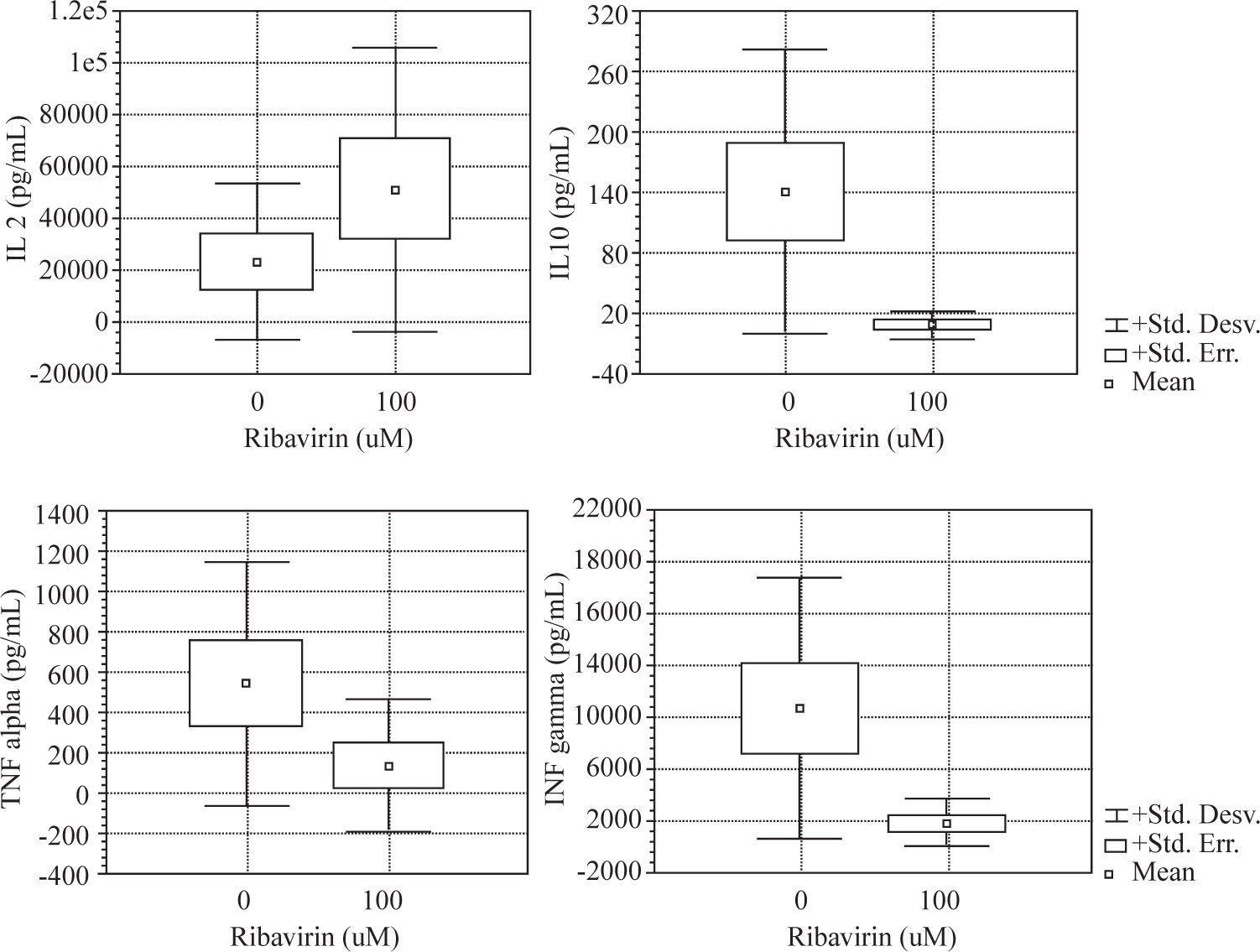
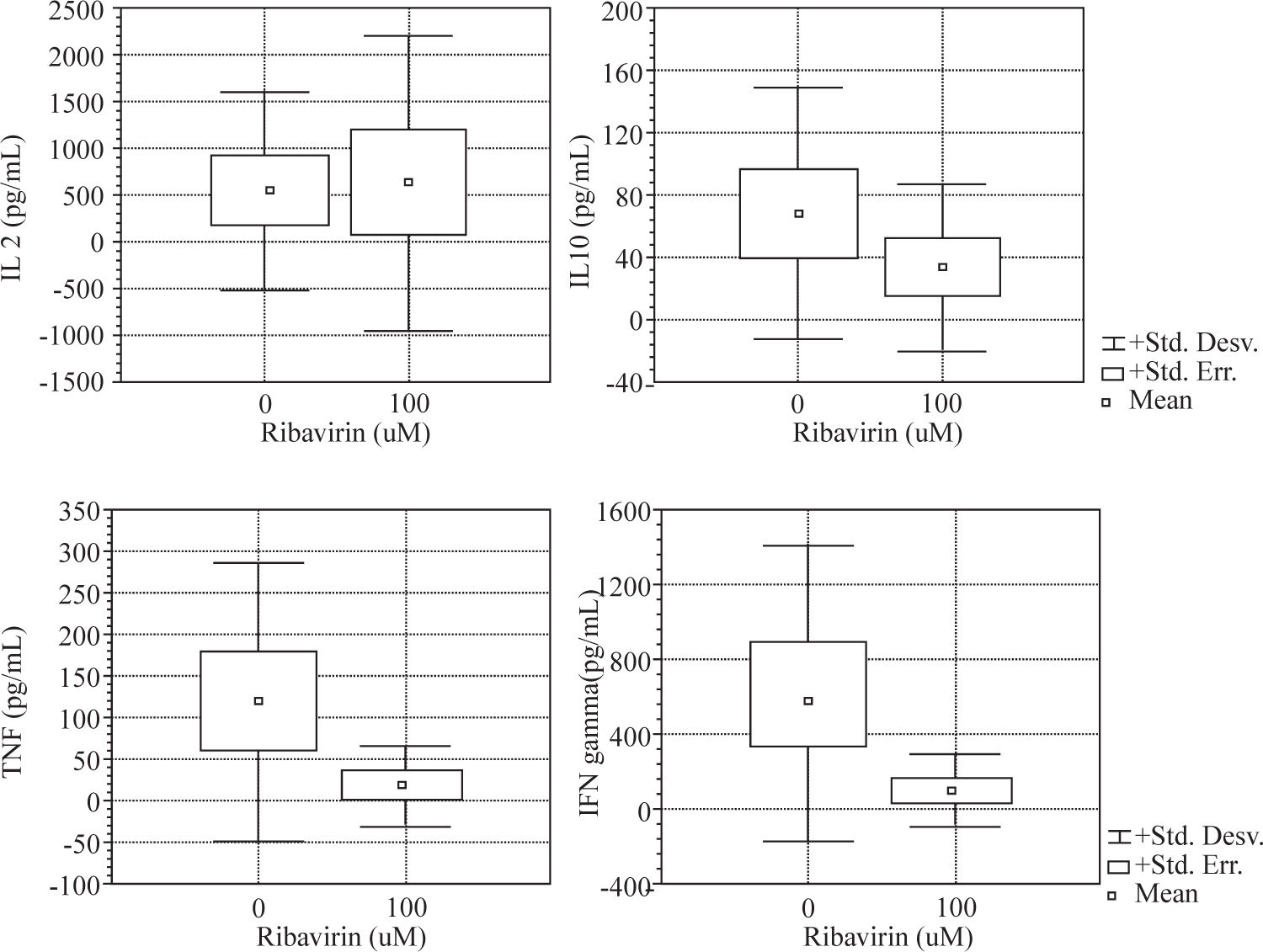
ResultsWe evaluated the effects of ribavirin at 1 μM, 10 μM and 100 μM on cytokine production in human PBMC following polyclonal activation with 10 μg of PHA and following the addition of tetanus toxoid as a recall antigen. Cytokines release (IL-2, IFN-γ, IL-4, IL-10 and TNF-α) was measured in absence or presence of ribavirin (Figures 1and2).
The effects of ribavirin on cytokine released by human PBMC in response to PHA and TT has shown great variation among individuals. No significant changes were observed between 1-10 μM ribavirin in the production of TNF- α, IFN-γ and IL-10 by both PHA and TT-stimulated PBMC.
Ribavirin significantly inhibited TNF-α, IFN-γ, and IL-10 in both PHA and TT-stimulated PBMC at 100 μM. PHA and TT production of TNF-α and IFN-γ by PBMC was reduced by 74 % to 85 % compared to baseline (p <0.05). PHA-induced IL-10 production was reduced in 95 % (p < 0.01) while TT stimulated was reduced in 50% (p < 0.01).
Ribavirin at 100 μM induced an increase of 124% in the production of IL-2 by PHA-stimulated PBMC (p < 0.05).
IL-4 was not detectable in supernatant of PBMC stimulated with PHA or TT in absence or in presence of ribavirin.
DiscussionIn this study we examined the in vitro effects of ribavirin on the cytokine production in human peripheral blood lymphocytes exposed to antigenic and mitogenic stimulation.
We found that ribavirin induced a dose-dependent downregulation in both Th 1 (TNF-α and IFN-γ) and Th 2 (IL-10) cytokine production by mitogen and antigenstimulated PBMC. We showed in our experiment that the drug was capable of inhibiting cytokine secretion in cultured PBMC in a dose-dependent fashion. However, marked inter-individual variation was observed in the response to the drug. Furthermore, we found that the effects of ribavirin on cytokine secretion did not vary on the basis of the inducing stimulus (PHA or tetanus-toxoid) except for IL-2.
The effectiveness of ribavirin given in combination with alpha interferon in the treatment of chronic hepatitis C has been recently reported, however, its mechanism of action remains unknown since the drug appears to exert moderate and transient antiviral effects.11 Even though the beneficial effect of the drug on viral infections has been attributed to its broad-spectrum antiviral activity, ribavirin may also exert immunosuppresive effects since it inhibits proliferation of transformed cells in vitro.12
The mechanism responsible for hepatocellular injury in chronic hepatitis C virus infection is currently unclear. Nevertheless, it has been suggested that immune mediated liver cell damage would play an important role in the pathogenesis of the disease.13 As well as that, cytokines produced by both CD4+ and CD8+ cells play an important role in both inhibiting viral replication and causing liver injury.
In order to explain the mechanism by which ribavirin causes normalization of ALT levels in patients with chronic hepatitis C infection, we hypothesized that the drug may act as immumodulatory and anti-inflammatory agent. Our results showed that ribavirin at high concentrations may induce a significant increase of IL-2 production. It should be noted that a unique feature of lymphocytes is that their activation itself triggers feedback mechanisms that limit their proliferation and differentiation. One of this mechanisms is IL-2-mediated feedback regulation. IL-2 is the prototypical T cell growth factor and functions in a paracrine manner to stimulate clonal expansion of antigen-stimulated lymphocytes and “bystander” cells. Early in a T cell response, when IL-2 concentrations are low, the proliferative effect may be dominant, but when the cytokine accumulates, it may function to terminate the response.14 Therefore, high IL-2 levels enhance FAS-mediated apoptosis of T-cells and other activation feed-back control mechanism that activate termination of T-cell immune response.12 In agreement with our observations, Martin et al. reported that high concentrations of ribavirin markedly increase secretion of IL-2 and suppressed IFN-γ production in cultured PBMC from chronic hepatitis C patients.15
In addition, we found that ribavirin inhibited TNF-α secretion, a pro-inflammatory cytokine. This effect could be another mechanism that would explain ALT normalization in patients receiving the drug.
Ribavirin is a nucleoside analogue with broad antiviral activity. In vitro inhibition of influenza viruses, parainfluenza viruses and respiratory syncytial virus is achieved at concentrations of 3-10 μg/mL. Ribavirin can be given orally with a bioavailability of 40 to 50%, intravenously or as an aerosol. In adult population peak plasma levels of 1.3 μg/mL are obtained after an oral dose of 600 mg. Intravenous administration of 1,000 mg may produce peak plasma levels of 24 μg/mL. After aerosol administration, ribavirin is found in plasma at levels of 0.2 to 1 μg/mL, although concentrations in respiratory secretions can be up to 1000-fold higher.4
Mean serum ribavirin concentration of 3.24 ± 1.35 μg/mL were found in patients with chronic hepatitis C treated with the combination therapy,16,17 and serum concentration of 6 μM following oral administration of 1,000 mg of the drug have been reported.18 Nevertheless, it was shown that ribavirin and its metabolites could be selectively concentrated and retained by specifics cells, such as human red blood cells and perhaps hepatocytes.19,20 Moreover, ribavirin was found in significantly higher concentrations in patients with sustained response.17 We observed that the effects of ribavirin on cytokine production were dose-related since at higher concentrations the drug induced the most remarkable effects. Therefore, at higher concentrations ribavirin may have the capacity to interfere with immune response by termination the T cell immune mediated damage caused by persistent HCV infection.
In conclusion, the present study shows that ribavirin is able to induce diverse effects on immunoregulatory cytokine secretion altering the Th1/Th2 balance. In chronic hepatitis C the drug would be able to reduce the cytokine effect of immune cells via its ability to inhibit their proliferation and production of inflammatory cytokines.