Abnormal serum iron studies are seen in a third or more of patients with chronic hepatitis C infection (HCV), where they have been linked to accelerated fibrosis progression and increased risk of hepatocellular carcinoma and sometimes lead to concern for coexisting hereditary hemochromatosis. The aim of this study was to assess the effect of HCV eradication in patients with abnormal serum iron studies prior to treatment with direct-acting antiviral agents (DAAs).
PatientsHCV-infected subjects with iron studies obtained before and after successful treatment with DAAs were identified (n=27). All had one or more abnormal iron test before treatment.
ResultsFollowing HCV eradication, serum iron, transferrin-iron saturation and ferritin levels decreased significantly (pre- versus post-treatment, p<0.01 for each). Serum iron and/or transferrin-iron saturations normalized in 16/19 subjects and raised ferritin levels returned to the normal range in 14/18 subjects, including several with pretreatment transferrin-iron saturation >90% and/or serum ferritin >1000ng/mL. Elimination of HCV infection was associated with a significant reduction in post-treatment ferritin levels even among subjects whose ferritin levels were within normal limits at baseline. Risk factors for other conditions associated with abnormal iron status were present in the few cases in which iron studies failed to normalize following DAA treatment.
ConclusionsEradication of HCV infection restores normal iron status in most patients with abnormal iron tests, including those whose baseline parameters are suggestive of hemochromatosis.
Abnormalities of serum iron parameters are frequently observed in subjects with chronic hepatitis C (HCV) infection. Shortly after the discovery of HCV, DiBisceglie and colleagues reported that about a third of patients with chronic viral hepatitis had elevations in serum iron, transferrin-iron saturation and/or ferritin levels [1]. Many studies were published in the years that followed, confirming the high prevalence of abnormal serum iron tests in these subjects [2–7]. The elevations in iron tests in HCV-infected patients are usually mild, but they can sometimes be of a magnitude to raise concern for co-existing hereditary hemochromatosis.
The relationship between serum iron tests and hepatic iron deposition was examined in several studies of liver biopsies from HCV patients performed in the interferon era. Some investigators reported correlations between serum ferritin and hepatic iron content, but most found no relationships between serum iron or transferrin-iron saturation and liver iron [6,7]. While the frequency of stainable iron varied among studies, increased hepatic iron content was uniformly less common, reported in 5–20% of cases or less [2,4,8–10]. Further, in most cases, the magnitude of the increase in hepatic iron content was relatively small and could readily be distinguished from hemochromatosis, based on the quantitative measurement then regarded as the gold standard for the diagnosis of hereditary hemochromatosis. These observations demonstrate that hepatic iron deposition can occur in chronic HCV, but bona fide iron overload is relatively uncommon and is not reliably predicted by elevated serum iron parameters.
Despite the variability of these phenomena, the linkage between dysregulation of iron metabolism and HCV infection has been a subject of great interest. The cause or causes of the abnormalities of iron metabolism in this condition remain incompletely understood. Early work in this area suggested that elevations in serum iron parameters might represent release of iron or ferritin from damaged hepatocytes [1]. More recently, several groups have reported dysregulation of the iron-regulatory hormone hepcidin in patients with chronic HCV infection, indicating that this may play a pivotal role in the genesis of these abnormalities [11–13].
The consequences of dysregulated iron metabolism in HCV have also been investigated extensively, with relationships reported between altered iron metabolism and clinical outcomes such as treatment response, fibrosis progression and hepatocellular carcinoma risk [14–24]. Much of this research was predicated on the concept that dysregulation of iron metabolism may contribute to worsening liver injury due to iron-induced oxidative stress [25]. Accordingly, there were numerous studies in the interferon era in which iron reduction was investigated as means of improving treatment response rates and mitigating fibrosis progression and hepatocellular carcinoma risk in HCV patients [26–33] but information concerning the effect of antiviral treatment on disturbances of iron metabolism from this era is scarce [9,34]. Since that time, the treatment of HCV has been revolutionized by the availability of highly effective direct-acting antiviral agents (DAAs). However, the effects of DAA-mediated HCV eradication on altered iron status have not been investigated. The goal of this work was to examine the effect of successful HCV treatment with DAAs on iron status in patients with abnormal pretreatment serum iron studies.
1MethodsFollowing approval by the institutional review board, a master list of HCV-infected veterans of the United States military treated with DAAs between 2014 and 2018 at the Veterans Administration Medical Center affiliated with our institution was obtained. The records of these patients were reviewed to identify those in whom serum iron studies (serum iron, total iron binding capacity, transferrin-iron saturation, serum ferritin) had been obtained before and after HCV treatment. Additional data extracted from the medical records of these individuals included age at treatment, pretreatment FIB-4 score [35], laboratory parameters, results of liver imaging and/or histopathology and relevant comorbidities (body mass index, diabetes, hyperlipidemia, alcohol history, etc.). Pre- and post-treatment serum iron levels, TIBC, transferrin-iron saturation and ferritin levels were compared using paired t-tests; we also compared pre- and post-treatment ferritin levels in the subset of patients with normal pre-treatment ferritin levels (GraphPad Software, San Diego, CA).
2ResultsA total of 29 male subjects met the inclusion criteria. Two of the subjects were not included in the final analysis. One was a man with normal iron studies before HCV treatment who later developed hypoferremia and a raised ferritin post-SVR while hospitalized for an abscess. The second subject had an elevated ferritin level before treatment and developed iron deficiency secondary to gastrointestinal blood loss following SVR. In these subjects, the changes in their iron status post-SVR were deemed unrelated to viral eradication.
The mean age at initiation of treatment of the remaining patients was 62 yrs (range 47–72). Twenty-three patients had HCV genotype 1, 2 had genotype 2 and 2 had genotype 3 infection. Twenty-four were treated with a sofosbuvir-based regimen and 3 with glecaprevir-pibrentasvir; all achieved sustained virological response (SVR). Liver biopsy had been performed in 8 subjects 8–15 yrs before DAA treatment. Hepatocellular iron was present in only 2 (graded as trace in one, 1+ in the other). HFE genotyping had been obtained in 2 patients; mutations were found in neither. 13/27 patients were presumed to have cirrhosis based on imaging findings, liver biopsy and/or pretreatment FIB4 score >3.25 [35].
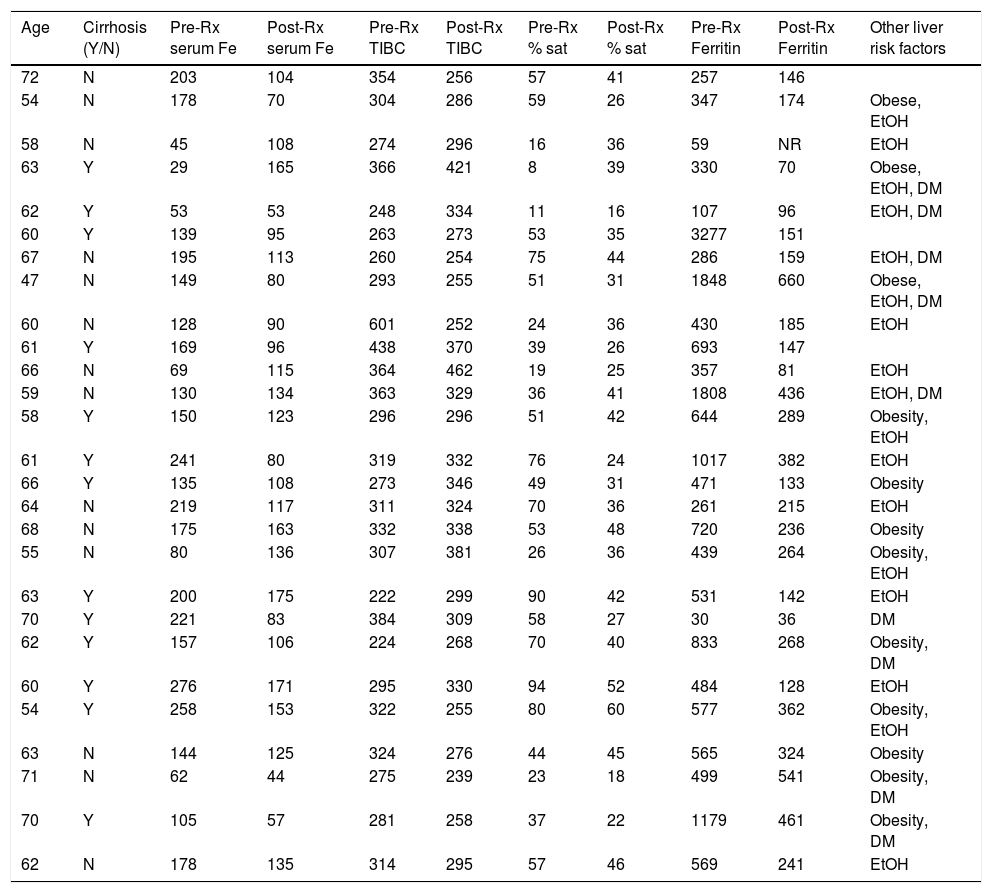
Abnormalities of serum iron levels, transferrin-iron saturation and/or ferritin levels were present in all subjects on pretreatment iron panels (Table 1). 5 patients had raised serum iron (sFe) and/or transferrin-iron saturation (TS), 8 had increased serum ferritin, and 10 had both. Low sFe and/or TS with normal ferritin were present in 4 subjects prior to treatment.
Clinical and laboratory data before and after DAA treatment.
| Age | Cirrhosis (Y/N) | Pre-Rx serum Fe | Post-Rx serum Fe | Pre-Rx TIBC | Post-Rx TIBC | Pre-Rx % sat | Post-Rx % sat | Pre-Rx Ferritin | Post-Rx Ferritin | Other liver risk factors |
|---|---|---|---|---|---|---|---|---|---|---|
| 72 | N | 203 | 104 | 354 | 256 | 57 | 41 | 257 | 146 | |
| 54 | N | 178 | 70 | 304 | 286 | 59 | 26 | 347 | 174 | Obese, EtOH |
| 58 | N | 45 | 108 | 274 | 296 | 16 | 36 | 59 | NR | EtOH |
| 63 | Y | 29 | 165 | 366 | 421 | 8 | 39 | 330 | 70 | Obese, EtOH, DM |
| 62 | Y | 53 | 53 | 248 | 334 | 11 | 16 | 107 | 96 | EtOH, DM |
| 60 | Y | 139 | 95 | 263 | 273 | 53 | 35 | 3277 | 151 | |
| 67 | N | 195 | 113 | 260 | 254 | 75 | 44 | 286 | 159 | EtOH, DM |
| 47 | N | 149 | 80 | 293 | 255 | 51 | 31 | 1848 | 660 | Obese, EtOH, DM |
| 60 | N | 128 | 90 | 601 | 252 | 24 | 36 | 430 | 185 | EtOH |
| 61 | Y | 169 | 96 | 438 | 370 | 39 | 26 | 693 | 147 | |
| 66 | N | 69 | 115 | 364 | 462 | 19 | 25 | 357 | 81 | EtOH |
| 59 | N | 130 | 134 | 363 | 329 | 36 | 41 | 1808 | 436 | EtOH, DM |
| 58 | Y | 150 | 123 | 296 | 296 | 51 | 42 | 644 | 289 | Obesity, EtOH |
| 61 | Y | 241 | 80 | 319 | 332 | 76 | 24 | 1017 | 382 | EtOH |
| 66 | Y | 135 | 108 | 273 | 346 | 49 | 31 | 471 | 133 | Obesity |
| 64 | N | 219 | 117 | 311 | 324 | 70 | 36 | 261 | 215 | EtOH |
| 68 | N | 175 | 163 | 332 | 338 | 53 | 48 | 720 | 236 | Obesity |
| 55 | N | 80 | 136 | 307 | 381 | 26 | 36 | 439 | 264 | Obesity, EtOH |
| 63 | Y | 200 | 175 | 222 | 299 | 90 | 42 | 531 | 142 | EtOH |
| 70 | Y | 221 | 83 | 384 | 309 | 58 | 27 | 30 | 36 | DM |
| 62 | Y | 157 | 106 | 224 | 268 | 70 | 40 | 833 | 268 | Obesity, DM |
| 60 | Y | 276 | 171 | 295 | 330 | 94 | 52 | 484 | 128 | EtOH |
| 54 | Y | 258 | 153 | 322 | 255 | 80 | 60 | 577 | 362 | Obesity, EtOH |
| 63 | N | 144 | 125 | 324 | 276 | 44 | 45 | 565 | 324 | Obesity |
| 71 | N | 62 | 44 | 275 | 239 | 23 | 18 | 499 | 541 | Obesity, DM |
| 70 | Y | 105 | 57 | 281 | 258 | 37 | 22 | 1179 | 461 | Obesity, DM |
| 62 | N | 178 | 135 | 314 | 295 | 57 | 46 | 569 | 241 | EtOH |
Age, age at which DAA treatment was begun.
TIBC, total iron binding capacity.
Reference ranges: serum iron 65–175mg/dL, TIBC 250–420μg/dL, transferrin saturation 20–50%, ferritin 30–400ng/mL.
The diagnosis of cirrhosis was based on biopsy, clinical or imaging findings or FIB4>3.25.
Obesity defined as BMI≥30kg/m2.
EtOH, history of heavy alcohol use.
DM, diabetes mellitus.
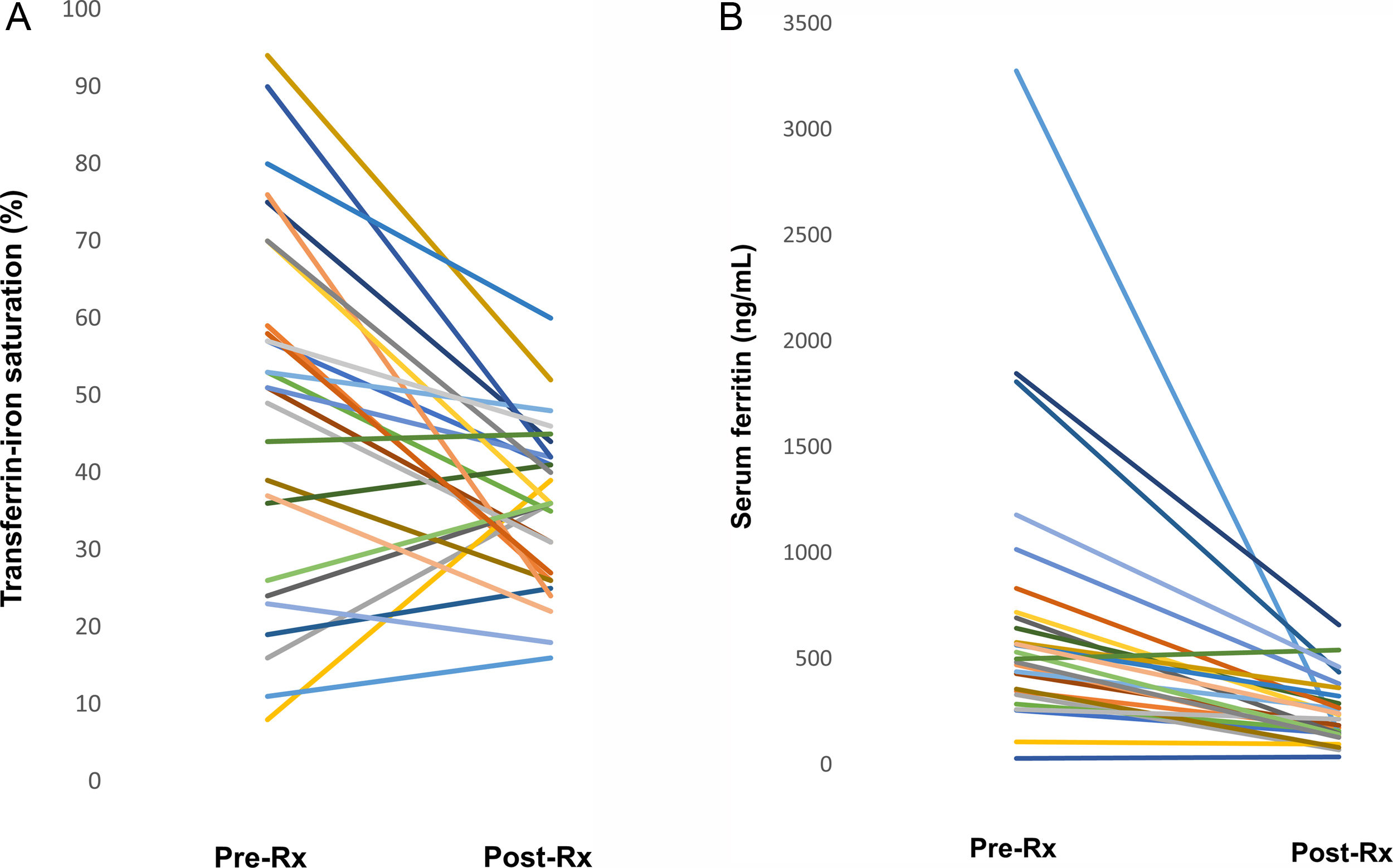
The median interval between completion of treatment and the repeat iron panel was 7 months (range 0–24 months). Serum iron levels decreased significantly after SVR (151±66 vs 111±36, p=0.004), while TIBC was unaffected by SVR. As a result, TS was significantly reduced following treatment in the group overall (49±24 vs 36±10; p=0.002). TS returned to the normal range in 13/15 subjects with elevated pretreatment TS, including several whose initial TS was >90% (Fig. 1A). In 2 patients with ongoing alcohol use and advanced fibrosis, TS decreased but remained slightly above the normal range post-SVR.
(A) Change in transferrin-iron saturation. Lines indicate transferrin-iron saturation before antiviral treatment and following sustained virologic response in individual subjects. Reference range 20–50%. (B) Change in serum ferritin following eradication of HCV. Lines indicate ferritin levels before antiviral treatment and following sustained virologic response in individual subjects. Reference range 30–400ng/mL.
A similar highly significant reduction in ferritin levels in the entire group was observed following SVR (714±682 vs 243±154, p=0.009). Among the individual subjects, ferritin levels returned to the normal range post-treatment in 14, including several whose baseline ferritin was >1000ng/mL (Fig. 1B). In 3 overweight/obese diabetics, post-treatment ferritin was substantially lower than before treatment but remained above the normal range. Interestingly, we noted a significant drop in ferritin levels following viral eradication even among patients whose pre-treatment ferritin levels had been within normal limits (mean 247±118 vs 122±61 after treatment, p=0.013).
3DiscussionThe relationship between HCV infection and dysregulated iron metabolism and the implications of that relationship for progression of liver disease and treatment response have been the subjects of great interest. This is the first study to demonstrate that eradication of HCV infection following treatment with DAAs restores serum iron parameters to normal in most HCV patients with abnormal pretreatment iron tests. The range of time intervals between treatment completion and the second set of iron studies in this cohort suggests that restoration of normal iron parameters occurs soon after antiviral treatment and that the effect is durable. This observation implies that abnormalities of iron metabolism in HCV infection are either a direct effect of the virus or a consequence of viral infection that resolves promptly with elimination of the virus, such as inflammation. Coincident pathology associated with abnormal iron status (i.e., heavy alcohol use, nonalcoholic fatty liver, and chronic kidney disease) likely accounts for the few cases in which iron studies failed to normalize following SVR.
Our findings have direct clinical implications. First and foremost, they suggest that HFE genotyping and/or investigations to assess hepatic iron stores such as liver biopsy or magnetic resonance imaging can be reserved for HCV patients with elevated iron studies that do not normalize following SVR. A second observation of clinical significance is that some patients with HCV have an iron profile that is suggestive of anemia of chronic disease. Attention to the effects of HCV on iron metabolism has focused on the one-third or more of patients with chronic HCV infection who have elevated iron tests. Accordingly, most of the subjects in our study had increased serum iron parameters prior to treatment, but 4 had low sFe and/or TS with normal serum ferritin levels, a pattern that is consistent with anemia of chronic disease. Notwithstanding the fact that anemia of chronic disease has not been previously reported in association with HCV infection, iron studies improved following SVR in 3 of these patients, including one subject whose mild anemia resolved following treatment (pretreatment hemoglobin 11.2g/dL, 13.8g/dL after SVR), supporting the possibility that there was a causal relationship between low sFe and TS and HCV. Further, in one patient with chronic kidney disease, TS and anemia worsened after viral eradication, suggesting that the effects of HCV on iron metabolism may have mitigated the effects of renal failure on his iron status but whether the post-treatment changes were a consequence of SVR versus progression of renal dysfunction cannot be determined.
Interestingly, in addition to the restoration of abnormal iron parameters to normal following treatment, we found that post-SVR serum ferritin levels decreased significantly among subjects whose baseline ferritin was within normal limits. This has not been reported previously and suggests that the effect of HCV on ferritin levels may more pervasive than has been recognized. The rapidity of the change in this laboratory value suggests that pretreatment ferritin levels were more likely reflective of inflammation rather than of increased iron stores in the group overall.
The mechanisms underlying alterations in iron metabolism in HCV remain incompletely understood but are presumed to stem from dysregulated expression of hepcidin. HCV-mediated oxidative stress has been shown to suppress hepcidin expression by hepatoma cells in culture, suggesting that the virus may have direct effects on iron metabolism [36]. Suppression of hepcidin expression would be expected to enhance intestinal iron uptake and mobilization of iron from macrophages, increasing serum iron and ferritin levels and ultimately leading to hepatic iron deposition. The relatively rapid normalization of iron parameters observed following SVR may reflect de-repression of hepcidin expression following elimination of HCV. Consistent with this possibility, Fujita et al. showed that serum hepcidin/ferritin ratios increased from pretreatment levels in 12 patients who achieved SVR following treatment with pegylated interferon and ribavirin; this was a consequence of both increased hepcidin levels and decreased ferritin post-SVR [34]. In addition to direct effects of the virus on hepcidin expression, it is likely that other factors are involved since most patients with chronic HCV have normal serum iron studies and do not develop iron overload even in the face of longstanding infection. Furthermore, dysregulated iron metabolism is frequently encountered in other forms of chronic liver disease such as alcoholic and nonalcoholic fatty liver disease, suggesting that liver injury per se may play a role. This interpretation is broadly consistent with studies that have linked abnormalities of individual iron parameters with specific histopathologic features of chronic HCV infection, such as inflammatory activity, steatosis and fibrosis stage [37–39]. Future research may provide a clearer understanding of the means by which HCV infection modulates iron metabolism.
There are caveats that may limit the generalizability of our results. First, iron studies were not obtained routinely in all subjects prior to HCV treatment. In other cases, iron studies had been obtained prior to treatment, but were not repeated post-SVR. As a result, our data are derived retrospectively from a relatively small number of patients in whom these laboratories were available. Secondly, none of our subjects appear to have had significant hepatic iron accumulation secondary to HCV, so we cannot address the effect of SVR on iron status in patients with significant hemosiderosis. It would be informative to extend this study to a larger group, including subjects with hemosiderosis. Despite these limitations, our data suggest that viral clearance can be expected to normalize abnormal iron studies in most patients with chronic HCV infection, including those with cirrhosis and even those with very elevated iron test results that may raise concern for coexisting hereditary hemochromatosis.AbbreviationsHCV hepatitis C virus sustained virologic response direct-acting antiviral agents serum iron transferrin-iron saturation
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.