Introduction and aim. Patients with liver cirrhosis (LC) and minimal hepatic encephalopaty have a higher accident rate. LC impairs the normal sleep-awake cycle and produces disturbances in behavior, cognition and motor skills. Abnormal melatonin (MT) levels have also been identified in LC. Administration of MT may regulate circadian rhythms and prevent the oxidative damage. We studied the effects of MT on spatial memory acquisition (SMA) and motor skills in a liver fibrosis model (LF)s.
Materials and methods. Forty-five rats, divided into 4 groups. [G1: LF; G2: LF + MT; G3: MT; G4: Healthy control (HC)]. LF was induced by carbon tetrachloride intraperitoneal injection (0.2 mL/kg) for 5 months. MT was administered during 5 weeks (0.4 mg/kg/day). SMA was evaluated by using the Morris Water Maze protocol where the escape latency (EL) and mean speed were measured. Data were registered by SMART®.
Results. The EL measurement analyzed by two way ANOVA: cirrhosis presented a higher EL than controls or those treated with MT suggesting impaired memory acquisition which is rescued by MT treatment. The mean speed analysis revealed that LF presented higher speed than LF+MT or HC, suggesting that LF affects motor skills, which are improved by MT. To discard whether EL is affected by altered motor skills in LF treated with MT, we compared the average EL and speed between days 2 and 6 of the training protocol. Speed was not improved during the trials unlike EL, suggesting that memory acquisition is independent of motor skills.
Conclusion. These findings suggest that MT improves cognition and motor skills in the LF model.
Liver cirrhosis (LC) is one of the highest sanitary burdens worldwide, being responsible for 31 million disability-adjusted life years (DALY) (1.2% of DALY worldwide) and one million deaths (2% of the cause of deaths worldwide).1 In Chile, chronic liver diseases constitutes a large problem to public health, being the third cause of death per specific group.2 In 2008 LC was the fifth specific cause of disability-adjusted life years (DALY).3 LC has a large impact on health-related quality of life (HRQOL), deteriorating as the disease progresses.4,5 Hepatic encephalopathy (HE)6 and sleeping disorders7 are among the pathologies associated to HRQOF deterioration, which can manifest independently or in conjunction.8 LE is often present sub-clinically as a Minimal Hepatic Encephalopathy (MHE) which is commonly sub diagnosed, although it plays an important role in HRQOL as it reduces cognitive and motor capacities of both outpatients and hospitalized patients.9-12 MHE is responsible for increased admittance rates in cirrhotic patients since it is related with an increased risk of falls and deterioration of driving skills.13-15 Additionally, sleep alterations associated to LC can also contribute to the deterioration of driving abilities.16 Studies have shown that the circadian secretion of MT is altered in all stages of HE. Nocturnal MT serum levels are altered during encephalopathy and hepatic damage, especially during more advanced stages. Disengagement of the circadian cycle associated to decreased MT nocturnal levels exist in less severs stages of HE. In more severe cases, MT levels can increase during the day and night, probably due to the increased hepatic damage that impedes MT excretion; which would be related to sleep disorders in cirrhotic patients.17,18 Meanwhile, day-time levels are increased over all stages of encephalopathies, with a larger increase in more severe cases. In non-cirrhotic patients, the use of exogenous MT has proven useful for improving sleep quality and regulating circadian MT levels.19 Additionally, MT has antioxidant properties as a “cleaner” of reactive O2 and N2 of direct action and ample spectrum, and anti-inflammatory properties that inhibit TNF-alpha and pro-inflammatory cytokines.20 This hormone is a regulator of the circadian cycle and a sleep stimulator at the central nervous system (CNS). MT also plays a protector role in the gastrointestinal tract thanks to its antioxidant and anti-inflammatory properties, generating a decrease in bacterial translocation.21,22 To study the possible benefits of MT administration in animals with advanced hepatic fibrosis or compensated cirrhosis, we evaluated its effect on both motor skills and swimming speed, and on cognitive states such as memory acquisition and storage, in rats with liver fibrosis rats (LF). For this, we designed an administration protocol of exogenous doses MT in Sprague-Dawley rats, which were previously induced with LF by intraperitoneal injection of CCl4 during 5 weeks, with the aim of inducing advanced hepatic fibrosis or compensated cirrhosis without reaching the uncompensated cirrhosis stage. Next, we evaluated both spatial memory formation and motor ability using the Morris Water Maze (MWM) method, which allowed us to quantify EL and Swimming Speed (SV). The aim of this study was to evaluate the impact of exogenous MT on spatial memory and motor activity in an animal model of liver advanced fibrosis.
Material and MethodsAn analytic experimental study was performed on 21 day old Sprague Dawley male rats, from the Central Animal Facility of Pontificia Universidad Catolica de Chile. Rats were kept in the animal facilities of the Biomedical Science Department of Universidad Catolica del Norte, at a temperature of 20¯ ± 2¯, and distributed 3 rats/cage. They were assigned by simple random distribution into 4 groups: HC: Healthy Control; LF: Liver Fibrosis; LF + MT: Melatonin Fibrosis; MT: Melatonin, with 6, 9, 15 and 15 rats respectively. All groups had access to food and water ad libitum and consumption was registered during the first 13 weeks. Groups LF and LF + MT had induced liver fibrosis according to the established protocol,23 by intraperitoneal injection twice a week of 0.2 mL of CCl4 (RIEDER®) at 50% during five months. After finishing this stage melatonin (ARAMA®) was administered during the 5 weeks prior to sacrifice. For administration, the four groups were kept under a water consumption regime of 120 mL/cage daily, which was given at 9 am to ensure it was consumed before sleep. The MT was dissolved in ethanol and added to the water with a final concentration of 4 /Ug/mL and 0.01% ethanol.24 Based on the water consumption per animal, the final dose of MT to be administered was calculated; being adjusted to 0.4 mg/kg of weight. The groups that were not treated with MT were submitted to the same water regime with 0.01% ethanol. The Morris Water Maze method (MWM)25 was used to evaluate spatial memory and motor activity we installed a cylinder platform with a 10 cm diameter, submerged under 4 cm of water, with spatial clues on the wall outside to the pool. The MWM was divided into four imaginary quadrants (SW, SE, NW, NE). The MWM was located on a piece implemented with three special marks on the surrounding walls.25 The Morris protocol was applied for 6 days, with three daily sessions during the first three days, separated by a 60 min break the first two days and a 10 min interval between each trial on the third day. Each essay consisted in placing the animal in the pool at a randomly assigned quadrant (different to the quadrant where the platform is located) and leave it in the MWM during 60s or until it reached the platform. The time taken to reach the plata-form is call the escape latency (EL). Independent of the result, after every essay the animal was left on the platform for 15 s. The platform stayed in the same location for the whole procedure. The data for the Time to Platform Arrival (EL) and Swim Speed were registered by a camera, analysed and tabulated by the SMART 3.0 Video Tracking System (Panlab®) and then registered as the group average ± standard error. Animals were sacrificed via an intraperitoneal injection of Chloral Hydrate at a concentration of 0.4 g/kg weight. The right amount of anaesthesia was monitored by blinking and tail movement reflexes. Once asleep, the rat was moved to a metal tray in a diagonal direction, using adhesive tape for later dissection. First the absence or presence of ascites in the peritoneal cavity was observed, then livers were extracted, allowing their macroscopic analysis. The differences between variables were studied using a two-tailed ANOVA and T-Student test. Animal care and use was in compliance with the Ethical Guidelines established by the Ethical Committee from the Faculty of Medicine of Universidad Catolica del Norte. This research was funded with internal resources from the Medical Faculty of Universidad Catolica del Norte and was approved by the ethics committee of this same institution.
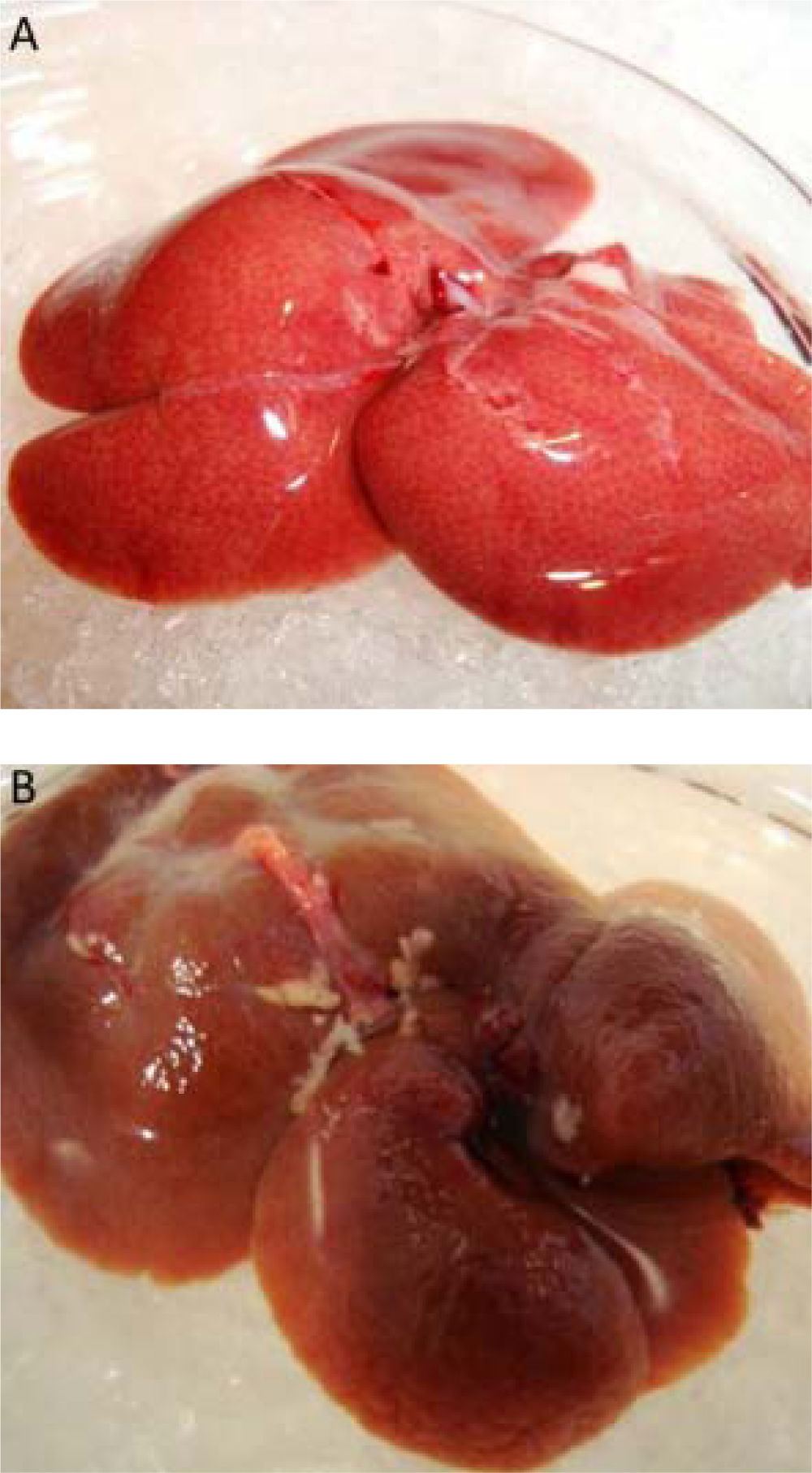
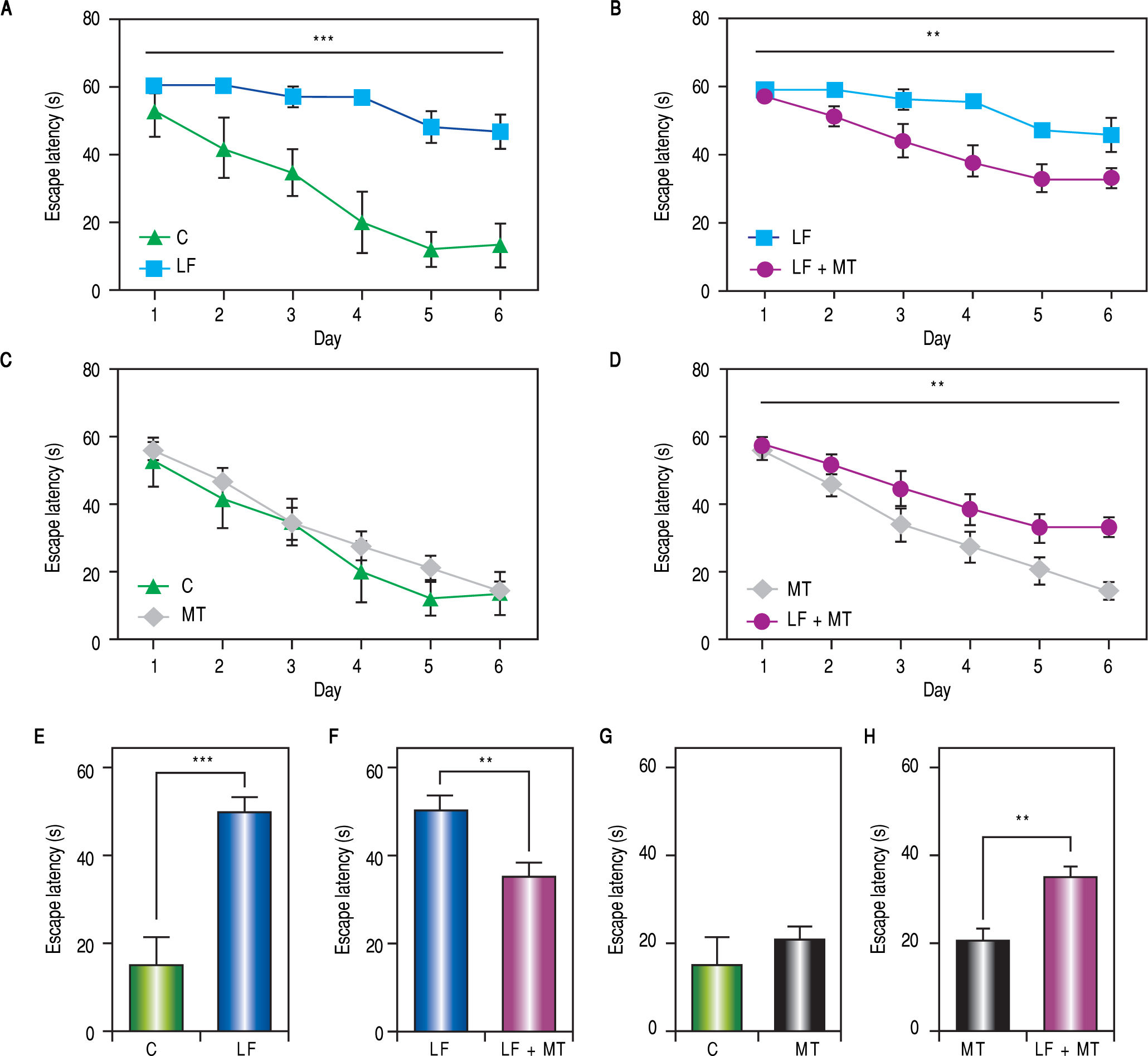
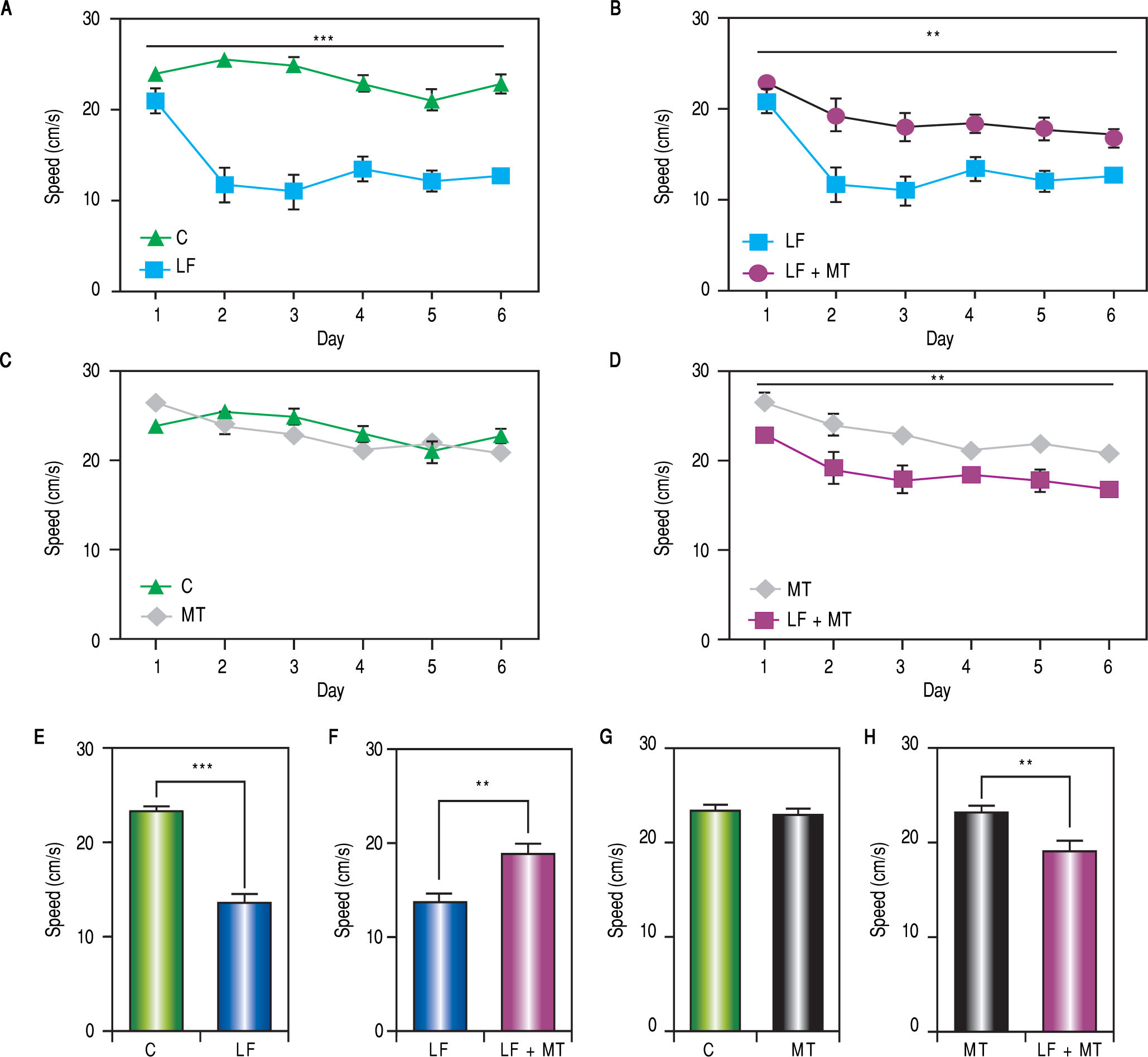
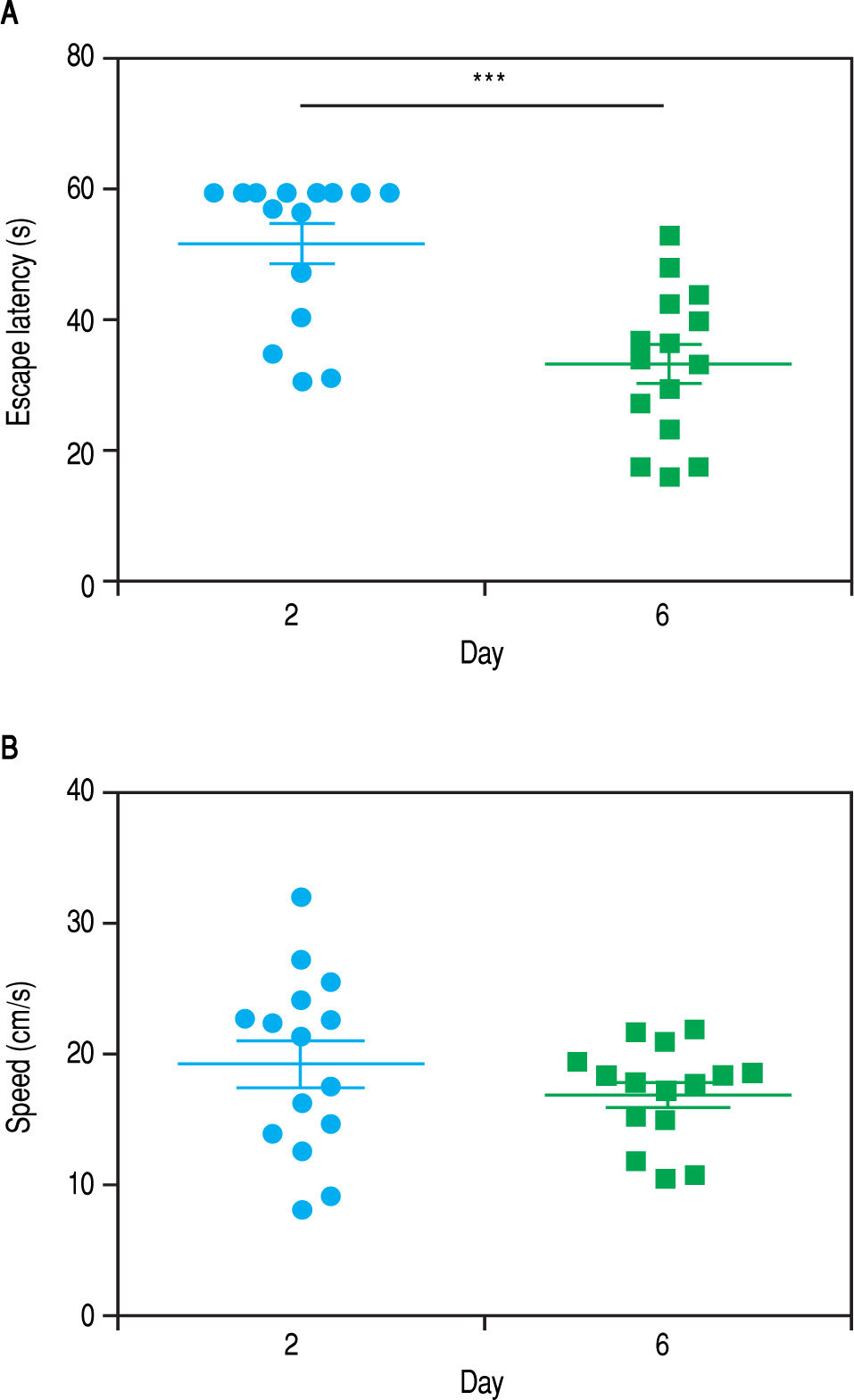
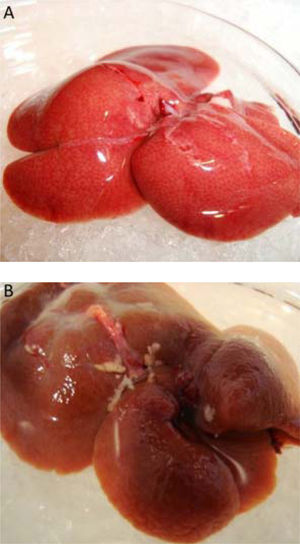
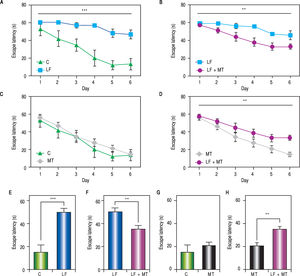
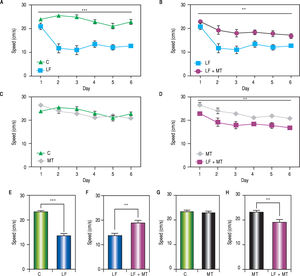
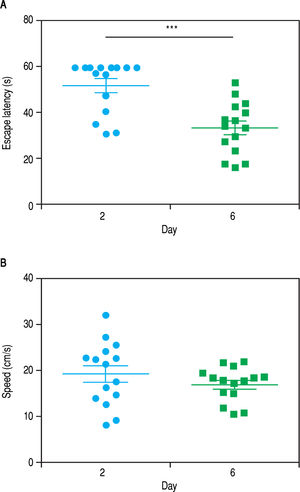
ResultsWhen the rats were sacrificed, their peritoneal cavity and livers were observed. The livers of rats from the CCl4 group were firmer when touched and the majority had a micronodular aspect. A macroscopic micronodular aspect was not observed in any of the remaining livers (Figure 1). Ascites was not observed in any of the studied rats. To evaluate cognitive performance, we analized the EL which describes the time taken to reach the plataform as a measurment of memory, amply used in the memory test in the water maze paradigm. When analysing the EL (Figure 2) we found that LF rats presented a higher latency than controls (HC) with significant differences measured by two way Anova (p < 0.001) (Figure 2A). The average EL over the last three days ofwater maze training was 50.3 ± 3.4 vs. 15.2 ± 6.5 seconds in LF compared to HC (p < 0.001 unpaired t test) (Figure 2E), which is expected, given the deterioration of the cognitive capacities of rats treated with CCl4. We also found a significant difference between the LF vs. LF + MT groups with significant differences measured by two way Anova (p < 0.0028) (Figure 2B). EL over the last three days was 50.3 ± 3.5 x vs. 35.4 ± 3.1 seconds, respectively (p < 0.05) (Figure 2F), revealing a lower EL in cirrhotic rats treated with MT, suggesting that MT improves the cognitive performance of cirrhotic rats. When comparing EL between the HC and MT groups, no significant differences were observed (15.2 ± 6.5 vs. 20.9 ± 2.9 second) (Figure 2F) (p = 0.3610 two way ANOVA (Figure 2C), which indicates that MT does not improve this capacity in healthy individuals. For the swimming speed analysis (Figure 3), significant differences were observed between the HC and LF groups measured by two way ANOVA (p < 0.0001) (Figure 3A). The average speed during MWM training was 23.4 ± 0.5 vs. 13.6 ± 1. cm/s, respectively) (p < 0.0001 unpaired t test) (Figure 3E); which is consistent with previous studies, as expected that rats treated with CCl4 would present decreased motor abilities. No significant differences between groups HC and MT were found (Figures 3B and 3F) (p = 0.6450 ANOVA) 23.4 ± 0.5 vs. 22.9 ± 0.7 cm/s, respectively, unpaired t test. Therefore, MT does not play a role on the improvement of motor capacities of healthy individuals. Significant differences were found between LF vs. LF + MT (p = 0.0045 ANOVA) (Figure 3C); the average speed during MWM training was 13.6 ± 1.1 vs. 18.9 ± 1.1 cm/s) (Figure 3G). These results support our hypothesis, given that melatonin improved the motor abilities of rats with LF. To discard whether improved EL in LF + MT rats is due to the positive effect of MT on swimming speed, we analysed the LF + MT group by comparing the average EL and speed between day 2 and day 6 of training (Figure 4). A statistically significant progression of EL, but not of swimming speed, was found between days 2 to 6. Moreover the Pearson correlation analysis revealed no correlation between both parameters in each group of animals, suggesting that speed improvement does not affect the escape latency. Therefore, MT improves memory acquisition and motor skills, likely through an independent mechanism.
Memory acquisition during the MW protocol. A-D. Escape latency was measured every day in rats with the indicated treatment. The two way ANOVA analysis revealed significant differences between groups in panels A (p < 0,001), B and D (p < 0.01). E-H. Bar graphs show the average escape latency over the last three days of the MWM protocol for each group. White, black, dark and light gray bars represent C, LF, LF + MT and MT groups respectively. Mean ± standard error. Statistical analysis unpaired student t test, ***p < 0.001, **p < 0.01.
Motor skills during the MWM protocol. A-D. Swimming speed was measured every day in rats with the indicated treatment. The two way ANOVA analysis reveals significant differences between groups in panels A (p < 0,001), C and D (p < 0.01). E-H. Bar graphs show the average swimming speed during the MWM protocol for each group. White, black, dark and light gray bars represent C, LF, LF + MT and MT groups respectively. Mean ± standard error. Statistical analysis unpaired student t test, ***p < 0.001, **p < 0.01.
Progression of the escape latency and swimming speed between days 2 and 6 of MWM training in LF + MTgroup. Graphs show the escape latency (A) and speed (B) of each animal in the 2nd and 6th day of training. Points represent each animal and lines represent mean ± standard error. Pared student t test analysis reveals a significant difference in escape latency between day 2 and 6. *** P< 0.001. Pearson correlation analysis suggesting no correlation between escape latency and swimming speed parameters.
As shown by diverse studies, MT levels are dysregulated in LC and could play and important role on cognitive and motor alterations, given by a direct alteration at the brain, either through its antioxidant and anti-inflammatory actions or indirectly through the regulation of its sleep-awake cycle. Patients with chronic hepatic damage present a slight degree of edema and an inhibitory neuronal state as a result of prolonged exposition to ammonium, which generates a series of compensatory mechanisms in astrocytes (secretion of osmolytes such as myo-inositol and taurine, down regulation of glutamate post-synaptic receptors and inactivation of its transporter).26 However, hyperammonemia alone does not explain the alterations observed in MHE nor HE. In a study performed by Shaw-cross, it was shown that the presence of MHE was related to elevated levels of inflammatory markers (white cell count, PCR, IL-6) and not with the degree of hepatic damage nor with the amount of ammonium.27 Exposure to ammonium, inflammatory cytokines, and hyponatremia such as benzodiazepines, increase the production of reactive nitrogen species (RNS) and reactive oxygen species (ROS), a process that is dependent on calcium and NMDA receptor levels. RNS and ROS nitrate tyrosine residues of intracellular proteins which affects blood brain barrier transporters, altering their selectivity and increasing permeability which finally increases cerebral edema.26 When exposure to these injuries is prolonged, alterations to the neurotransmisor pathways are observed in different brain regions. For example, in the Substantia nigra glutamate levels increase (leading to hyperkinesia and increased cognitive damage), meanwhile the GABAergic tone is increased in the cerebellum but decreased in the cortex.28 Also, NMDA receptors modulate learning and memory capacities, where the glutamate nitric oxide (NO) cGMP pathway has an important role. In MHE there is a decrease in this signalling pathway in the hippocampus, leading to deterioration of long term potentiation and spatial learning in the MWM.29 It is believed that the involvement of this pathway in the cerebellum is associated to a decrease in sight learning abilities in rats. Motor activity and coordination are also affected by these same mechanisms. It has been suggested that they would provoke an alteration in neuronal transmission, including dopaminergic, GABAergic and glutamatergic; especially in the thalamus-cortex and brain nodules, which would explain the hyperkinesia observed in these patients and their deterioration in bimanual and visual-motor coordination.28 There are a series of pathways where MT has anti-oxidant abilities. MT is a lipophilic molecule that must be modified by a hydrophilic molecule (6-hydroxymelatonin) in order to be eliminated by the kidneys. This is achieved via the liver by cytochrome P450 (CYP1A1, CYP1A2 and CYP2C19), an extrahepatic pathway by the enzyme CYP1B1, of ubiquitous distribution, and by a non-enzymatic pathway that interacts with RNS/ROS forming 6-hydroxymelatonin. Another hydroxylated metabolite is 2-hydroxymelatonin, which is believed to originate from the interaction of MT with RNS/ROS.20 MT also improves the antioxidant potential of cells, stimulating the synthesis of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and glutathione reductase and increasing glutathione levels.30 The most studied MT action is its effect on the circadian cycle, being part of its main physiological function. This has an extended effect on the CNS due to the ubiquitous location of its M1 and M2 receptors. Improvements to sleep alterations have been amply described, especially in diseases such as chronic renal disease and Parkinson’s disease where the levels of this hormone are decreased by night and increased by day, provoking hypersomnia. Therefore, administrating exogenous MT one or two hours before sleep can regulate circadian levels, stimulating sleep.31-33 Serum MT levels in humans are quite low; however they significantly increase during the night (approximately 80 pg/mL). In sleeping disorders, a dose of 110 mg is used, which exceeds physiological serum levels.34 To have clinical antioxidant effects a supraphysiological doses is required. In clinical studies MT doses within the range 50-100 mg/day are used, which guarantees neuroprotector antioxidant activity.30 MT has higher protector activity against oxidative stress than Vit C and Vit E35 and, although it is used at higher doses, it does not possess significant adverse effects.36 The MT molecule is highly lipophilic, which means it can freely diffuse through cellular membranes, including the hematoencephalic barrier.37 The results from our experiment indicate that exogenous MT, administered previous to sleep, improves some cognitive capacities associated to the formation of spatial memory, shown by the decreased EL. This discovery is important since we can deduce that the improvement to cognitive elements associated to spatial memory and orientation capacity could decrease the risk of accident rates in LC patients. At the same time, the swimming speed results showed that functionality and motor skills of rats treated with MT is significantly improved compared to cirrhotic rats without treatment. Therefore, MT would have a positive impact on the cognitive and motor skill capacities of rats with LC. Finally, considering the performance progression during MWM task of the cirrhotic rats treated with MT (differences in both the Swimming Speed and EL between days 2 and 6), we suggest that mt treatment is not especially associated with improvements of motor capacity, but actually with increasement of cognitive capacities. Therefore we infer that the use of MT allowed EL to be shortened by the sixth day of treatment due to improvement IN THE decision making when choosing the RIGHT pathway, or improvement of spatial learning due to previous experience. We can deduce that learning capacity is improved when this drug is used as a treatment in advanced fibrotic rats. Our study does not allow us to elucidate if the observed improvements are specific to a certain type of hepatic encephalopathy nor the exact mechanisms by which MT induces these positive effects. If HE exists, the anti-inflammatory and antioxidant effects of MT could decrease oxidative stress and reduce insults to both the brain and liver.20 Also, theoretically MT could have an effect on the gastrointestinal tract, reducing inflammation and strengthening the intestinal barrier, decreasing bacterial translocation and the pass of ammonium from the intestine into circulation.21,22 Lastly, in less sever stages of HE, especially in MHE, where nocturnal serum levels of MT decrease, provoking sleeping disorders, administration of MT previous to sleep can favour and reduce the disorder.17 The properties and action mechanism of MT are still being studied and its possible use as an encephaloprotector drug in LC are being encouraged. We believe that our study provides a basis for the study of MT action mechanisms in animal models in the context of improved motor and cognitive abilities in LC. Additionally, after vast experience of using MT in humans, no significant contradictions for its use exist. Studies that evaluate the variables associated to the possible change in HRQOL to the cognitive, motor and/or sleep disorder improvements in these patients should be performed.
Abbreviations- •
EL: escape latency.
- •
HE: hepatic encephalopathy.
- •
LC: liver cirrhosis.
- •
LF: liver fibrosis.
- •
MT: melatonin.
- •
MWM: Morris water maze.
The authors declares that there is no conflict of interest regarding the publication of this article.
AcknowledgeWe thank Juan Madariaga for his help in the histological liver analysis. Floria Pancetti for facilitating the water maze equipment. Diego Rojas for his technical assistance.