Introduction and aim. We assessed the characteristics of virological response to a combination treatment of ombitasvir, paritaprevir, and ritonavir in hepatitis C virus genotype 1-infected elderly Japanese patients.
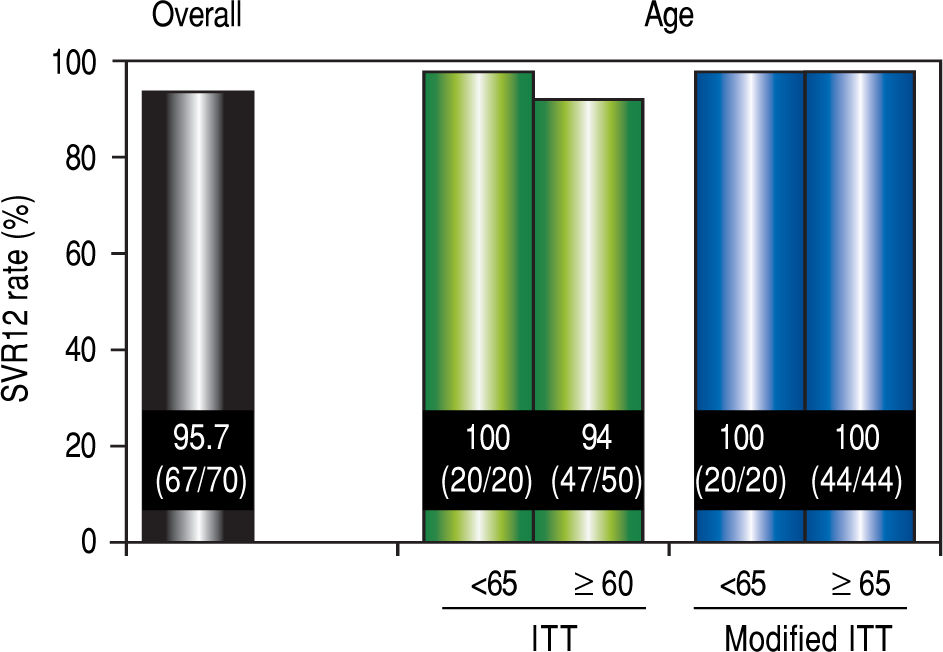
Material and Methods. This multicenter prospective study was conducted at six locations in Japan. Seventy patients with chronic hepatitis C virus genotype 1b infection were orally administered ombitasvir/paritaprevir/ritonavir once daily for 12 weeks. The primary endpoint was the proportion of elderly patients with sustained virological response (SVR) 12 weeks after the completion of treatment. Adverse events were also recorded to evaluate drug safety and tolerability during the trial period. SVR in elderly patients (age > 65; 94% [47 / 50]) was lower than that in younger patients (100% [20 / 20]).
Results. No significant differences in SVR 12 weeks after the completion of treatment were observed between the age groups (P = 0.153). Adverse events were observed in 16 patients (23.3%). Multivariate analysis confirmed that the change or discontinuation of concomitant drugs owing to drug interactions was independent of risk factors for adverse events associated with this drug combination (P = 0.015; odds ratio, 15.9; 95% confidence interval, 1.79 - 148). Ombitasvir/paritaprevir/ritonavir combination treatment was highly effective in elderly patients.
Conclusion. Tolerability should be monitored in older patients for whom concomitant medications are discontinued or changed because of drug interactions.
Hepatitis C virus (HCV) is the most important causative agent of chronic liver disease and cirrhosis.1-4 The treatment of patients with HCV is complex, and there have been changes in treatment methods after the development of direct-acting antiviral agents (DAAs).5-10 The introduction of DAAs has improved sustained virological response (SVR) rates and enabled successful outcomes without interferon (IFN) therapy6,7 In particular, an IFN-free regimen with DAAs can be effective in elderly patients because an IFN-based regimen may produce marked adverse events in this difficult-to-treat population.8,9
A phase III study in Japan demonstrated that a 12-week combined regimen of ombitasvir (OBV), paritaprevir (PTV), and ritonavir (r) was highly effective in Japanese patients with HCV genotype 1b infections.10 OBV inhibits NS5A, which plays a key role in HCV RNA replication.7 PTV is an inhibitor of an HCV protease complex containing NS3 / 4A protease.6 Ritonavir is a potent inhibitor of protease that increases the serum concentrations of PTV.10 This regimen is expected to show beneficial effects in specific populations, such as HCV-infected elderly Japanese patients with insufficient renal function. However, regarding the problems associated with IFN-free regimens in the elderly, few studies demonstrate whether aged patients can achieve significant rates of SVR or influence the rates of adverse side effects. The efficacy of the combination regimen of OBV/PTV/r in patients aged 65 years or older was uncertain. In the present study, we assessed the virological response to a 12-week OBV/PTV/r treatment for HCV genotype 1 in elderly Japanese patients.
Material and MethodsEthicsThis study was approved by the Institutional Review Board Ethics Committee of Tokushukai Medical Group (Number: TGE00575-024) and Kitasato University School of Medicine (Number: C16-947). Informed consent was obtained from patients and their families prior to commencement of the study. This study is registered in the UMIN Clinical Trials Registry (Number: UMIN000021123).
Study design and patientsA multicenter prospective study was conducted at six locations in Japan. Enrollment commenced in February 2016 and concluded in October 2017. Patients with chronic HCV genotype 1b infection received OBV/PTV/r for 12 weeks. The OBV/PTV/r regimen was administered orally once daily at doses of 25/150/100 mg, respectively.
Clinical characteristics, including general characteristics, demographic information, and baseline laboratory data, were evaluated at each study visit. The discontinuance criteria for the enrolled patients were:
- •
Occurrence of viral breakthrough.
- •
Patient’s desire to terminate; and
- •
Occurrence of severe adverse events (> grade 3), as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
We enrolled patients who had chronic HCV genotype 1b infection for at least 6 months, plasma HCV RNA levels exceeding 2 log10 IU/mL (using the COBAS TaqMan HCV test; version 2.0; Roche Diagnostics, Basel, Switzerland), and wild type resistance-associated variants (RAVs) of NS5A-L31 and -Y93 detected by direct sequencing.11
No age limit was applied, and elderly patients were defined as individuals aged 65 years or older. If drug interactions with OBV/PTV/r were observed in any patient, the patient was enrolled after stopping or changing the treatment for at least one week. Patients on hemodialysis considered to have end-stage renal disease were also enrolled.
The exclusion criteria were:
- •
Decompensated liver disease, which was marked as Child-Pugh classes B or C.
- •
Current hepatocellular carcinoma by ultrasonography or CT (patients with a history of hepatocellular carcinoma were included).
- •
Another form of liver disease in addition to viral hepatitis.
- •
Infection with hepatitis B virus or human immunodeficiency virus.
- •
Use of antiviral therapy within one month before drug administration.
- •
Defined laboratory abnormalities, such as alanine aminotransferase (ALT) levels more than five times the upper limit of the normal range, platelet counts lower than 50,000/mm3, white blood cell counts < 4,000/mm, or severe anemia with hemoglobin levels < 8.5 g/dL.
The primary endpoint of this trial was the proportion of elderly patients with an SVR 12 weeks after the completion of treatment (SVR12), which was determined using intention-to-treat (ITT) analysis and modified ITT analysis. The secondary endpoint was pharmacological effects and tolerability of the combination therapy.
Statistical evaluationData were analyzed using the R Foundation for Statistical Computing, version 3.3.1. All data were expressed as the median. Separate analyses for patient age and eGFR levels were compared using the Mann-Whitney U test. All differences with a P value < 0.05 were considered significant. Univariate and multivariate analyses with logistic regression models were used to calculate the odds ratios and 95% confidence intervals to assess the correlation between treatment factors and adverse events.
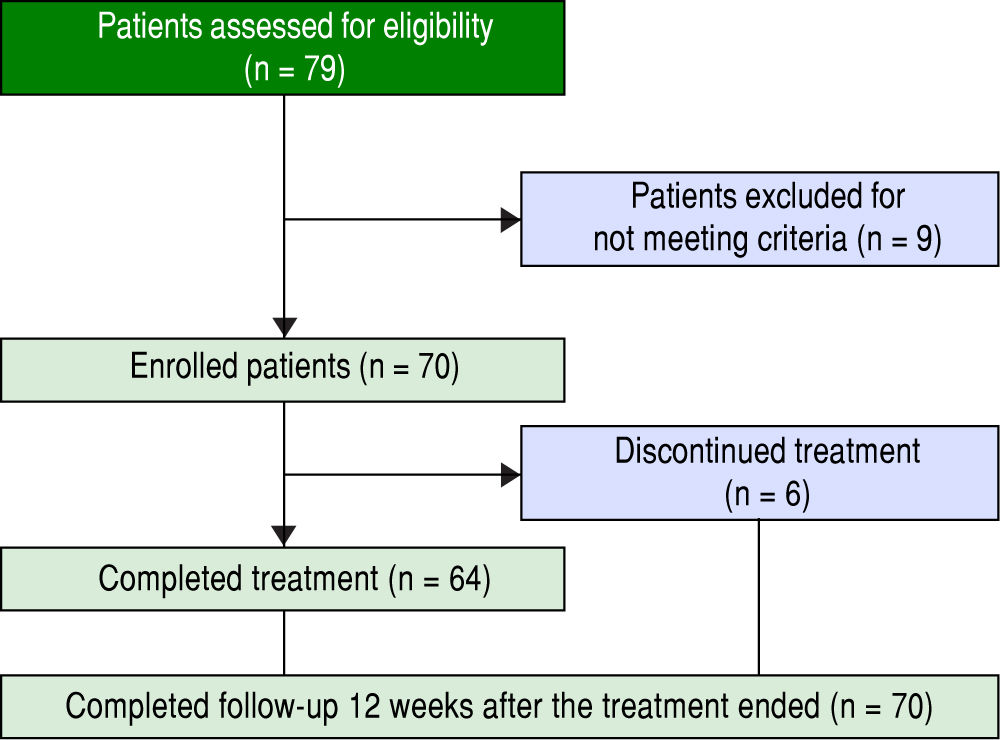
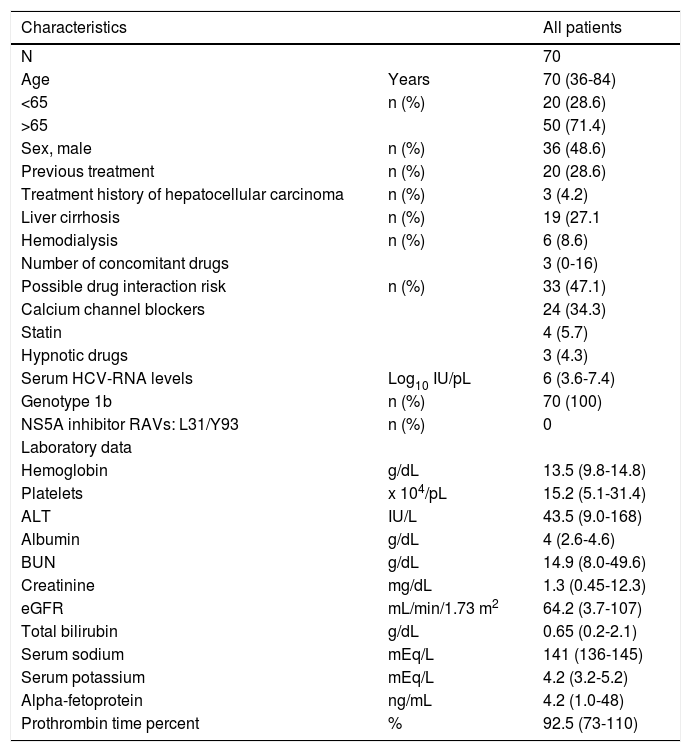
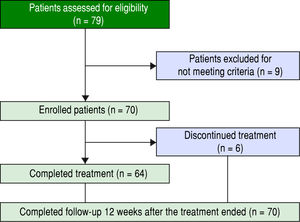
ResultsPatients and baseline characteristicsSeventy-nine patients were assessed (Figure 1). Of these, nine patients were excluded during the run-in period because of failure to meet the eligibility criteria. The remaining 70 patients received OBV/PTV/r. The baseline clinical characteristics of the enrolled patients are summarized in table 1.
Patient baseline clinical characteristics.
| Characteristics | All patients | |
|---|---|---|
| N | 70 | |
| Age | Years | 70 (36-84) |
| <65 | n (%) | 20 (28.6) |
| >65 | 50 (71.4) | |
| Sex, male | n (%) | 36 (48.6) |
| Previous treatment | n (%) | 20 (28.6) |
| Treatment history of hepatocellular carcinoma | n (%) | 3 (4.2) |
| Liver cirrhosis | n (%) | 19 (27.1 |
| Hemodialysis | n (%) | 6 (8.6) |
| Number of concomitant drugs | 3 (0-16) | |
| Possible drug interaction risk | n (%) | 33 (47.1) |
| Calcium channel blockers | 24 (34.3) | |
| Statin | 4 (5.7) | |
| Hypnotic drugs | 3 (4.3) | |
| Serum HCV-RNA levels | Log10 IU/pL | 6 (3.6-7.4) |
| Genotype 1b | n (%) | 70 (100) |
| NS5A inhibitor RAVs: L31/Y93 | n (%) | 0 |
| Laboratory data | ||
| Hemoglobin | g/dL | 13.5 (9.8-14.8) |
| Platelets | x 104/pL | 15.2 (5.1-31.4) |
| ALT | IU/L | 43.5 (9.0-168) |
| Albumin | g/dL | 4 (2.6-4.6) |
| BUN | g/dL | 14.9 (8.0-49.6) |
| Creatinine | mg/dL | 1.3 (0.45-12.3) |
| eGFR | mL/min/1.73 m2 | 64.2 (3.7-107) |
| Total bilirubin | g/dL | 0.65 (0.2-2.1) |
| Serum sodium | mEq/L | 141 (136-145) |
| Serum potassium | mEq/L | 4.2 (3.2-5.2) |
| Alpha-fetoprotein | ng/mL | 4.2 (1.0-48) |
| Prothrombin time percent | % | 92.5 (73-110) |
The median age of the patients was 70 years (range: 3684), and the number of patients over 65 years of age was 50 (71.4%). With regard to renal disease, the number of patients with an eGFR < 60 mL/min/1.73 m2 was 30 (42.8%). The median number of concomitant drugs used at baseline was 2.6 (range: 0-18). The number of patients with a treatment history of hepatocellular carcinoma was 3 (4.3%). To prevent drug interactions with this regimen, the co-administered drugs were discontinued or changed in 47.1% of the patients. Calcium channel blockers (CCB) were the most frequently reported interacting drugs, followed by statins, hypnotics, and phosphodiesterase type 5 inhibitors. RAVs of NS5A-Y93H were not detected at baseline in any patient. All patients reported taking medications according to the manufacturer’s prescribed information except in cases of adverse events.
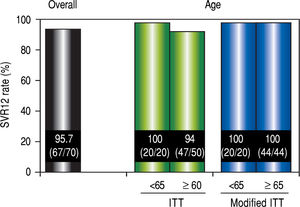
Virological responseIn ITT analysis, the overall SVR12 rate was 95.7% (67 / 70) (Figure 2). The SVR for patients over 65 years of age was 94% (47 / 50), which was lower than the SVR for younger patients (100% [20 / 20]). However, no significant differences in the SVR12 rate were observed between the two age groups (P = 0.552). In the modified ITT, SVR for both groups of patients was 100% (44 / 44 and 20 / 20, respectively).
SVR12 rates. Efficacy was compared by performing separate analyses in patients over 65 years of age and younger than 65 years of age. Separate analyses for patient age were compared using the Mann-Whitney U test The modified ITT analysis was defined as all patients in the ITT population excluding those who discontinued the study for reasons other than virologic failure. SVR12: sustained virological response 12 weeks after the completion of treatment. ITT: intention-to-treat.
Plasma HCV-RNA levels declined following combination therapy of OBV/PTV/r, and by treatment week 4, the levels decreased below the lower limit of quantification in 81.4% of the patients (57/70). The mean decrease in HCV RNA between baseline and week 4 in patients over 65 years of age and younger than 65 years of age was 4.5 and 4.2 log10 IU/mL, respectively.
Furthermore, the SVR12 for patients with an eGFR < 60 (90% [27 / 30]) was lower than the SVR12 for patients with an eGFR > 60 (100% [40 / 40]). Patients undergoing hemodialysis achieved an SVR rate of 100% (6 / 6). No significant differences in the SVR12 rate were observed in patients with and without chronic kidney disease.
Virological failureVirological failure was observed in three patients (4.3%) who had insufficient treatment duration of less than 2 weeks. All the three patients were over 65 years of age and were administered more than three concomitant drugs. In addition, their eGFR levels were less than 60. They changed to concomitant drugs owing to drug interactions.
We also assessed the effect of pretreatment RAVs NS5A-L31M/V and -Y93H and found that patients who showed virological failure did not exhibit RAVs at baseline or at the time of virological failure.
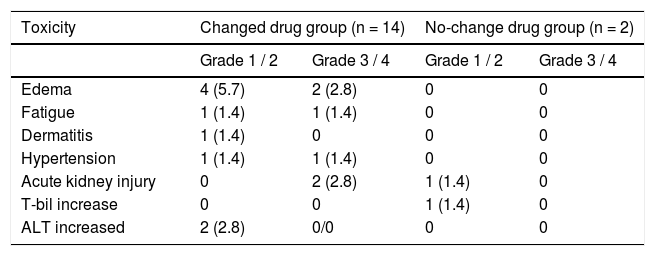
Safety assessmentThe adverse events reported are summarized in table 2. There were no treatment-related deaths. Adverse events were observed in 16 patients (22.8%) following OBV/ PTV/r administration. Of these, six cases (8.6%) required early interruption of treatment. Two patients each exhibited edema at weeks 2 and 4, two patients each experienced acute kidney injury at weeks 2 and 4, one patient reported fatigue at week 2, and one patient received diagnosis of hypertension at week 10. Edema was the most frequently reported adverse event, and incidences of fatigue, hypertension, and dermatitis were reported during the treatment. Acute kidney injury or increase in total bilirubin or ALT was observed in five patients.
Incidence of adverse events and adverse drug reactions.
| Toxicity | Changed drug group (n = 14) | No-change drug group (n = 2) | ||
|---|---|---|---|---|
| Grade 1 / 2 | Grade 3 / 4 | Grade 1 / 2 | Grade 3 / 4 | |
| Edema | 4 (5.7) | 2 (2.8) | 0 | 0 |
| Fatigue | 1 (1.4) | 1 (1.4) | 0 | 0 |
| Dermatitis | 1 (1.4) | 0 | 0 | 0 |
| Hypertension | 1 (1.4) | 1 (1.4) | 0 | 0 |
| Acute kidney injury | 0 | 2 (2.8) | 1 (1.4) | 0 |
| T-bil increase | 0 | 0 | 1 (1.4) | 0 |
| ALT increased | 2 (2.8) | 0/0 | 0 | 0 |
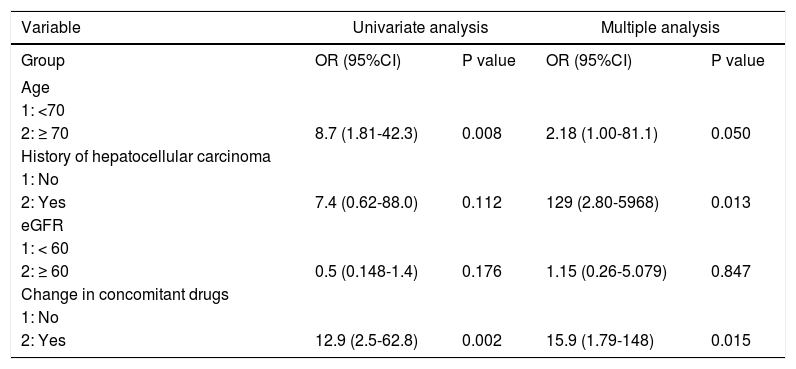
Patient age, number of concomitant drugs, hypertension, and change in concomitant drugs owing to drug interaction estimated by univariate logistic regression analysis were correlated with the occurrence of adverse events. Multivariate regression models were fitted to each of the four categories of risk factors (age, number of concomitant drugs, eGFR, and change in concomitant drugs). The analysis revealed that treatment history of hepatocellular carcinoma (HCC) and concomitant drug change due to drug interactions were independent of risk factors for adverse events associated with OBV/PTV/r combination drug therapy (history of HCC: P = 0.013; odds ratio, 129; 95% confidence interval, 2.80-5968; concomitant drug use: P = 0.015; Odds ratio, 15.9; 95% confidence interval, 1.79148) (Table 3).
Univariate and multivariate analysis of factors affecting adverse events.
| Variable | Univariate analysis | Multiple analysis | ||
|---|---|---|---|---|
| Group | OR (95%CI) | P value | OR (95%CI) | P value |
| Age | ||||
| 1: <70 | ||||
| 2: ≥ 70 | 8.7 (1.81-42.3) | 0.008 | 2.18 (1.00-81.1) | 0.050 |
| History of hepatocellular carcinoma | ||||
| 1: No | ||||
| 2: Yes | 7.4 (0.62-88.0) | 0.112 | 129 (2.80-5968) | 0.013 |
| eGFR | ||||
| 1: < 60 | ||||
| 2: ≥ 60 | 0.5 (0.148-1.4) | 0.176 | 1.15 (0.26-5.079) | 0.847 |
| Change in concomitant drugs | ||||
| 1: No | ||||
| 2: Yes | 12.9 (2.5-62.8) | 0.002 | 15.9 (1.79-148) | 0.015 |
In this study, we aimed to assess the characteristics of virological response to OBV/PTV/r combination therapy in hepatitis C virus genotype 1-infected elderly Japanese patients. We found that combination therapy with OBV/ PTV/r was highly effective for HCV genotype 1b infection in elderly patients. However, six patients (8.6%) discontinued the therapy because of serious adverse events associated with the study drugs. Among those six patients, three patients who received treatment for only 2 weeks exhibited virological failure. In a phase III study of a 12-week regimen of OBV/PTV/r, it was reported that antiviral-resistant mutations of NS5A and NS3 were correlated with response to OBV/PTV/r therapy.10 Although it is not certain whether or not HCV clearance in this treatment regimen is influenced by age and renal function, risk factors other than the presence of resistant variants should be considered when determining the duration of treatment. Therefore, virological failure may be correlated with the occurrence of adverse events after administration of OBV/PTV/r.
In subgroup analyses for age, OBV/PTV/r therapy was effective for the elderly, and there were no significant differences in SVR between the age groups. However, the prevalence of adverse events in elderly patients was slightly higher, perhaps because they exhibited altered drug absorption, distribution, metabolism, and excretion parameters. In particular, hepatic metabolism, which is primarily responsible for eliminating OBV/PTV/r, decreased markedly with age. Hepatic clearance of drugs metabolized by oxidation (in the presence of cytochrome P-450 enzymes) and hydrolysis is more likely to be prolonged in the elderly. Clearance typically decreases by 30% to 40% with age, making these patients highly susceptible to unexpected adverse events.12,13
Logistic regression analysis for adverse events revealed that a history of HCC and change or discontinuation of concomitant drugs were more important risk factors for adverse events than age. We could not conclude that a history of HCC is associated with the occurrence of adverse events because there were only three patients with a history of HCC. Patients who changed or discontinued concomitant medications need to be closely monitored. We propose three explanations for the above result. First, some patients exhibited poor blood pressure control after discontinuation of CCB. One case of treatment failure with reduced kidney function exhibited an elevation of median blood pressure by 20 mmHg. Second, it is possible that changing the drugs could have caused side effects because the patients were enrolled after discontinuation or change of treatment for at least one week. Third, patients can make mistakes regarding the precautions on coadministration of drugs despite receiving instructions to change the dosage of certain drugs or to discontinue others. Medications were discontinued or changed in approximately half of all the enrolled patients. Before administration of OBV/PTV/r, it is better for elderly Japanese patients to discontinue or change the concomitant medications. Close monitoring of HCV-infected elderly Japanese patients administered OBV/PTV/r was required.14-17
We also evaluated whether or not renal function was related to low efficacy and tolerability.18 In subgroup analyses for kidney function, the SVR rate in patients with insufficient renal function was high, and there was little or no significant difference in SVR between patients with normal and insufficient renal function. Furthermore, all patients with end-stage renal disease undergoing hemodialysis achieved SVR. According to a previous study on OBV/PTV/RTV therapy in HCV patients undergoing hemodialysis, severe adverse effects may have resulted from drug interactions and not from impaired renal function.19 Hemodialysis patients in our study did not alter their concomitant drug use, and only a small number of concomitant drugs were administered. Consequently, all hemodialysis patients achieved an SVR rate of 100% without adverse events.
The DAA regimen should be chosen by several factors, e.g., the HCV genotype, RAV and the progression of liver fibrosis. Furthermore, we should consider the potential hematologic toxic effects and concomitant drug interactions. Due to the development of new DAAs with better safety and stronger antiviral effects, OMB/PTV/r will likely be replaced by more effective regimens with no minimal potential drug interactions. However, new drugs almost completely lack data concerning efficacy and safety in fragile patient populations. Especially, elderly patients who suffer from more than one disease, e.g., hypertension and chronic kidney dysfunction, need to consider not only the treatment conditions of each disease but also the possibility of other related diseases. Therefore, it is necessary to carefully examine the effects of each drug, as in the present study, which proved the efficacy and tolerability of OBV/PTV/r for elderly patients. The efficacy and safety for new DAA regimens in elderly patients is a serious problem that warrants further investigation.
This study has four limitations. First, the sample size was small, so there was selection bias. However, large trials of DAAs showed that drug adherence affects virological response20 as did the results in the present study. Therefore, these findings may be safely relied upon. Second, evidence to support the involvement of RAVs is insufficient; therefore, further studies are warranted to elucidate other predictors in combination with RAVs. Third, this study did not have an equal number of elderly and young patients in each group. Finally, detailed pharmacokinetic studies of this combination therapy with multiple concomitant drugs should be done in an elderly population. Further studies are necessary to determine the safety of this treatment regimen.
This study demonstrated that the combination regimen of OBV/PTV/r was highly effective in an elderly population with HCV genotype 1b infection. Age was not significantly related to treatment response. However, tolerability should be monitored in elderly patients discontinuing or changing concomitant medications because of drug interactions.
Abbreviations- •
ALT: alanine aminotransferase.
- •
CCB: calcium channel blockers.
- •
CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events.
- •
DAA: direct-acting antiviral agent.
- •
eGFR: estimated glomerular filtration rate.
- •
HCC: hepatocellular carcinoma.
- •
HCV: Hepatitis C virus.
- •
IFN: interferon.
- •
ITT: intention-to-treat analysis.
- •
OBV/PTV/r: ombitasvir, paritaprevir, ritonavir.
- •
RAV: resistance-associated variant.
- •
SVR: sustained virological response.
The authors declares that there is no conflict of interest regarding the publication of this article.
AcknowledgmentsWe thank Ayumu Sugitani, of the Institute of Biomedical Research, Sapporo Higashi Tokushukai Hospital, Hokkaido, Japan, for assistance with the statistical analyses, and Robert E. Brandt, Founder, CEO, and CME of MedEd Japan, for editing and formatting the manuscript.