Endogenous sex hormones are associated with the risk of diabetes and metabolic syndrome. Recent studies suggested the role of these hormones in nonalcoholic fatty liver disease (NAFLD). We conducted a systematic review and meta-analysis of observational studies investigating the association between sex hormones and NAFLD.
Material and MethodsA comprehensive search of the databases of the MEDLINE and EMBASE was performed from inception through April 2016. The inclusion criterion was the observational studies that assessed the association of serum total testosterone (TT) and sex-hormone binding globulin (SHBG) and NAFLD. We calculated pooled effect estimates of TT and SHBG with 95% confidence intervals (CI) comparing between subjects with and without NAFLD by using random-effects model.
ResultsSixteen trials comprising 13,721 men and 5,840 women met the inclusion criteria. TT levels were lower in men with NAFLD (MD = -2.78 nmol/l, 95%CI -3.40 to -2.15, I2 = 99%) than in those without. Men with higher TT levels had lower odds of NAFLD whereas higher TT levels increased the odds of NAFLD in women. In both sexes, SHBG levels were lower in patients with NAFLD than controls and this inverse association was stronger in women than men and higher SHBG levels were associated with reduced odds of NAFLD.
ConclusionOur meta-anal-ysis demonstrated a sex-dependent association between TT and NAFLD. Lower TT levels are associated with men with NAFLD and inversely associated with women with NAFLD, whereas higher SHBG levels are associated with lower NAFLD odds in both men and women.
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic triglyceride accumulation in the absence of excess alcohol intake. This condition ranges from simple steatosis to nonalcoholic steatohepatitis (NASH) that could progress to cirrhosis and hepatocellular carci-noma.1-3 NAFLD is one of the most common causes of chronic liver disease and its prevalence has increased substantially throughout the world affecting 10 to 24 percent of general population from several countries1,3 along with the growing epidemic of obesity and diabetes mellitus (DM). As NAFLD is known as the hepatic manifestation of metabolic syndrome, it is also associated with increased insulin resistance, visceral adipose tissue (VAT) and also the risk of cardiovascular disease.4-7
Previous studies have demonstrated the effect of endogenous total testosterone (TT) on the insulin resistance. In men, TT deficiency has been associated with increased accumulation of VAT and insulin resistance and the lower TT levels are associated with obesity8-11 whereas hyperandrogenemia has been linked with increased VAT and insulin resistance in women.9,12-15 In addition, sex hormone-binding globulin (SHBG), which is the circulating glycoprotein secreted from the liver and binds androgens to regulate their bioavailability, has been negatively associated with metabolic syndrome, insulin resistance and cardiovascular disease in both sexes.16,17
Both TT and SHBG were found to be associated with insulin resistance and adiposity, which are the contributing factors of metabolic syndrome. Several studies exploring the association between these hormones and NAFLD have demonstrated inconsistent results. Therefore, to better characterize this possible association, we conducted a systematic review and meta-analysis of all published observational studies relating TT, SHBG with NAFLD.
Material and MethodsStudy selection and inclusion criteriaThis systematic review and meta-analysis was conducted and reported according to the Meta-analysis Of Observational Studies in Epidemiology statement18 and was registered in PROSPERO (registration number: CRD42016037921). Two authors (SU and VJ) independently searched published studies indexed in MEDLINE and EMBASE databases from date of inception to April 2016. The full search strategy was detailed in figure 1. A manual search of references of selected retrieved articles was also performed. To assess the quality of all studies, review articles, case reports, abstracts, and unpublished studies were excluded.
Our inclusion criteria were:
- •
Published observational studies including cross-sectional, cohort, and case-control studies assessing the association between the level of serum SHBG and/or TT and NAFLD.
- •
Participants aged 18 years or older.
- •
Odd ratios (OR), relative risks (RR) or hazard ratios (HR) were provided or sufficient raw data to calculate those ratios were provided.
- •
Participants without NAFLD were used as a reference group.
NAFLD was diagnosed using the definition by each study including liver function, imaging study or liver biopsy.
Two authors (SU and VJ) independently reviewed titles and abstracts of all citations that were identified. After all abstracts were reviewed, data comparisons between the two investigators were conducted to ensure completeness and reliability. The inclusion criteria were independently applied to all identified studies. Differing decisions were resolved by consensus between the two authors. Full-text versions of potentially relevant papers identified in the initial screening were retrieved. If multiple articles from the same study were found, only the article with the most complete data was included.
Data extraction and quality assessmentUsing a standardized data extraction form, data concerning author, year of publication, study design, study location, participant characteristics, diagnosis ascertainment of NAFLD, effect estimates with 95%CI, and quality assessment were independently extracted by two authors. Discrepancies were resolved with group discussions. We contacted the authors of the primary reports to request any unpublished data. If the authors did not reply, we used the available data for our analyses.
A subjective assessment of methodological quality for observational studies was evaluated by two authors (SU and VJ) using the Newcastle-Ottawa Scale (NOS). The NOS is a quality assessment tool for non-randomized studies. A total score of 3 or less was considered poor, 4-6 was considered moderate, and 7-9 was deemed high quality.19 We excluded poor quality studies in the sensitivity analysis.
Statistical analysisMeasures of association were analyzed for men and women, separately. To compare TT and SHBG levels between subjects with and without NAFLD, pooled analyses were performed using unstandardized mean difference (MD). The heterogeneity of effect size estimates across these studies was quantified using the Q statistic, its p-val-ue, and I2 (P < 0.10 was considered significant). A value of I2 of 0-25% indicates insignificant heterogeneity, 26-50% low heterogeneity, 51-75% moderate heterogeneity and 76-100% high heterogeneity.20 Clinical heterogeneity was assessed by grouping trials within each sex according to age of participants, BMI, menopausal status, study design and study quality. For these analyses, studies were stratified according to mean age (< 55 vs. ≥ 55), mean BMI (< 30 vs. ≥ 30), menopausal status (premenopausal vs. postmeno-pausal), study design (case-control vs. cross-sectional) and study quality (high vs. moderate). Furthermore, We used the techniques of meta-regression to quantitatively analyze which components of NAFLD best explained variance in sex hormone levels. If studies reported only median and interquartile range, we used the formula by Hozo, et al.21 to estimate mean value and standard deviation. Adjusted point estimates were combined using a random-effects model because the heterogeneity was high. Publication bias was assessed using funnel plot, Egger's regression test and its implications with the trim and fill method.22 Insufficient numbers of sex-stratified populations and inconsistent methods precluded the meta-analysis of free testosterone in this study. Test for interaction was applied to identify the difference between MD from subgroup analysis. All analyses were conducted using Comprehensive Meta-Analysis 3.3 software from Biostat, Inc.
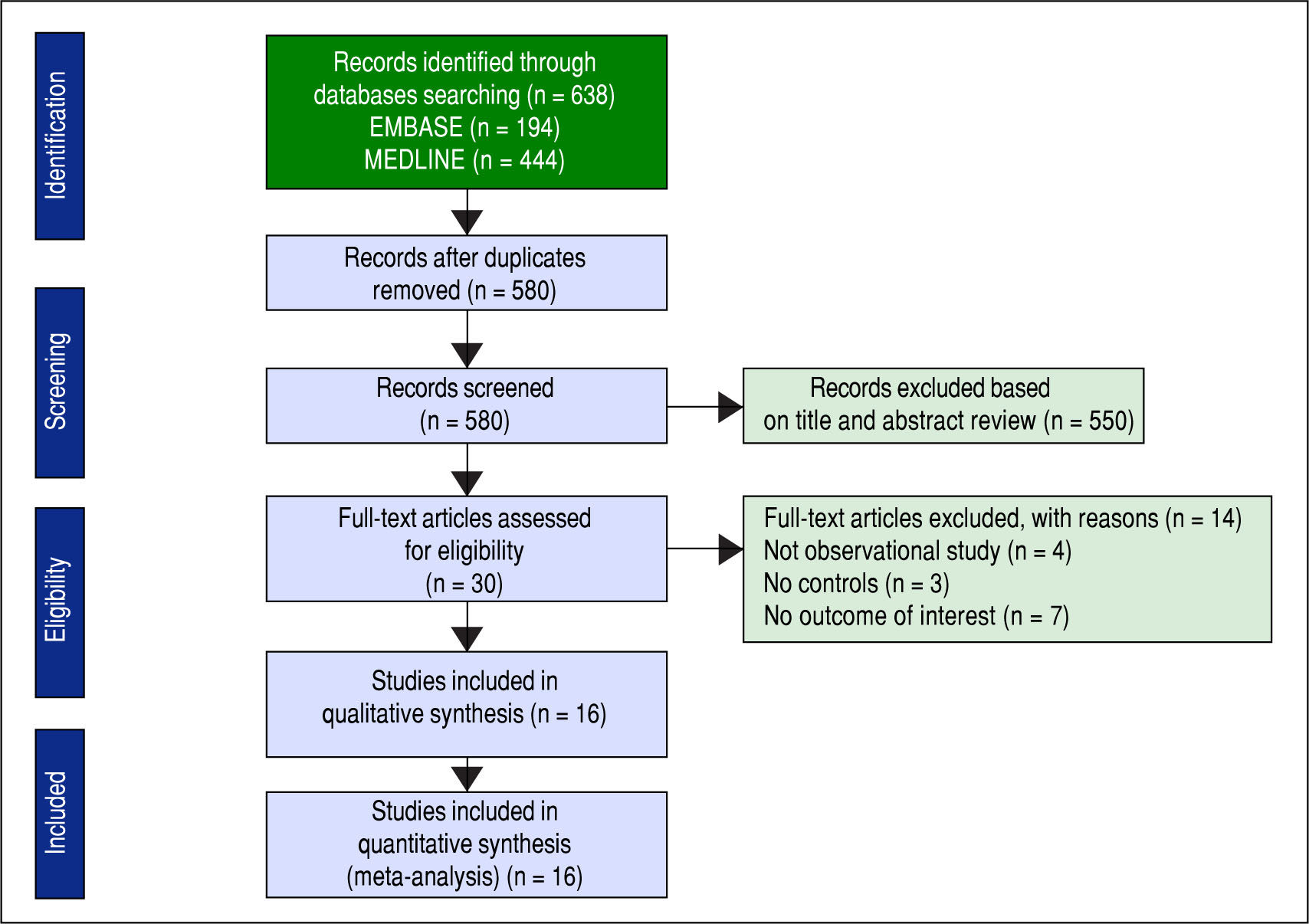
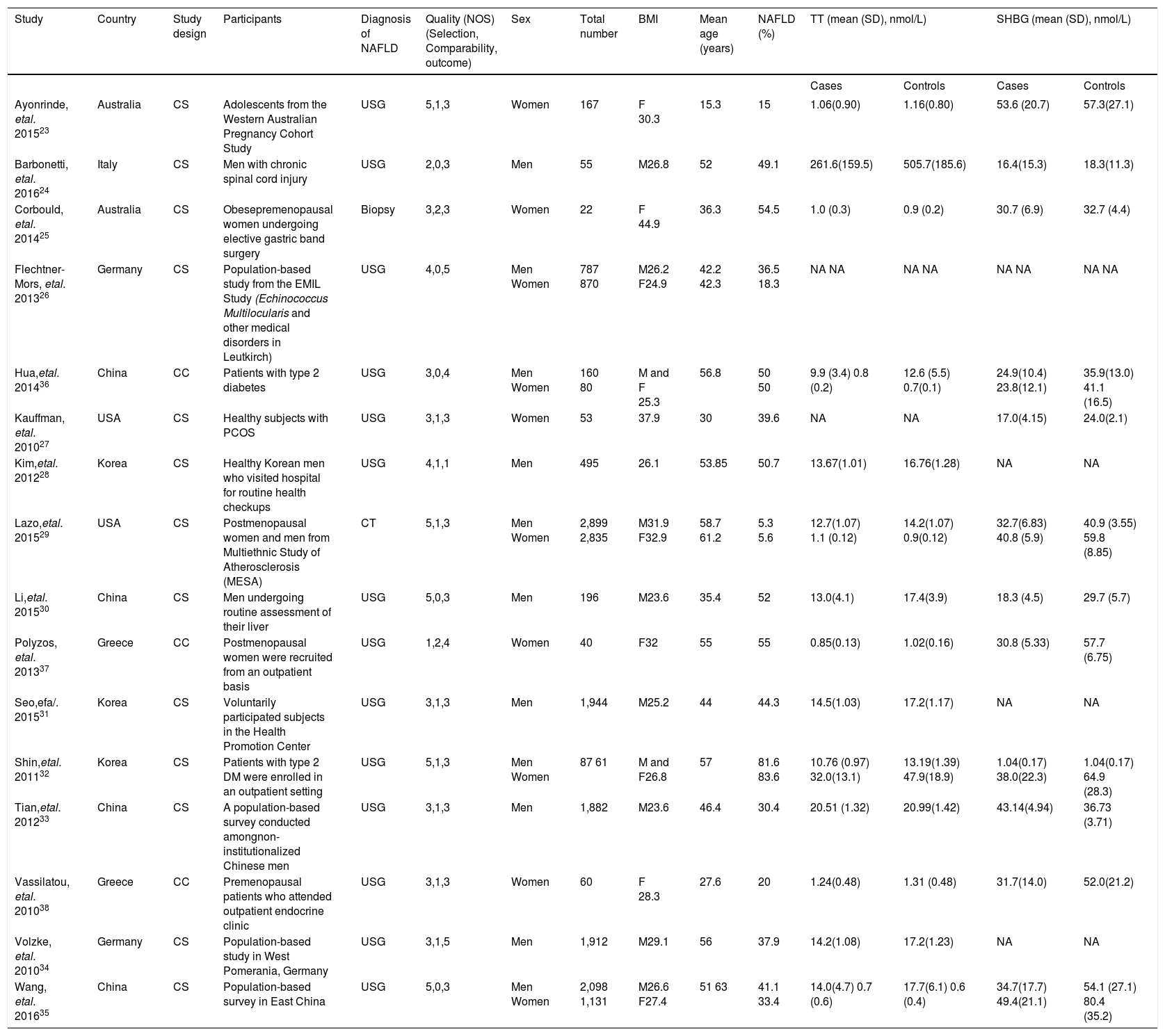
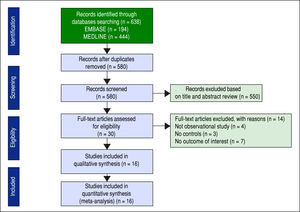
ResultsDescription of included studiesThe initial search yielded 580 articles; 550 articles were excluded based on title and abstract review. A total of 30 articles underwent full-length review. Fourteen articles were excluded (4 articles were not observational studies, 3 articles did not have control group, and 7 articles did not report outcome of interest). Data was extracted from 16 observational studies (13 cross-sectional studies23-35 and 3 case-control studies36-38). Figure 2 outlines the search methodology and selection process. Table 1 describes the detailed characteristics and quality assessment of included studies. NAFLD was ascertained by either imaging studies or biopsy by all included studies. None of the studies used only liver function to diagnose NAFLD.
Characteristics of studies included in the meta-analysis.
| Study | Country | Study design | Participants | Diagnosis of NAFLD | Quality (NOS) (Selection, Comparability, outcome) | Sex | Total number | BMI | Mean age (years) | NAFLD (%) | TT (mean (SD), nmol/L) | SHBG (mean (SD), nmol/L) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||||||||||
| Ayonrinde, etal. 201523 | Australia | CS | Adolescents from the Western Australian Pregnancy Cohort Study | USG | 5,1,3 | Women | 167 | F 30.3 | 15.3 | 15 | 1.06(0.90) | 1.16(0.80) | 53.6 (20.7) | 57.3(27.1) |
| Barbonetti, etal. 201624 | Italy | CS | Men with chronic spinal cord injury | USG | 2,0,3 | Men | 55 | M26.8 | 52 | 49.1 | 261.6(159.5) | 505.7(185.6) | 16.4(15.3) | 18.3(11.3) |
| Corbould, etal. 201425 | Australia | CS | Obesepremenopausal women undergoing elective gastric band surgery | Biopsy | 3,2,3 | Women | 22 | F 44.9 | 36.3 | 54.5 | 1.0 (0.3) | 0.9 (0.2) | 30.7 (6.9) | 32.7 (4.4) |
| Flechtner-Mors, etal. 201326 | Germany | CS | Population-based study from the EMIL Study (Echinococcus Multilocularis and other medical disorders in Leutkirch) | USG | 4,0,5 | Men Women | 787 870 | M26.2 F24.9 | 42.2 42.3 | 36.5 18.3 | NA NA | NA NA | NA NA | NA NA |
| Hua,etal. 201436 | China | CC | Patients with type 2 diabetes | USG | 3,0,4 | Men Women | 160 80 | M and F 25.3 | 56.8 | 50 50 | 9.9 (3.4) 0.8 (0.2) | 12.6 (5.5) 0.7(0.1) | 24.9(10.4) 23.8(12.1) | 35.9(13.0) 41.1 (16.5) |
| Kauffman, etal. 201027 | USA | CS | Healthy subjects with PCOS | USG | 3,1,3 | Women | 53 | 37.9 | 30 | 39.6 | NA | NA | 17.0(4.15) | 24.0(2.1) |
| Kim,etal. 201228 | Korea | CS | Healthy Korean men who visited hospital for routine health checkups | USG | 4,1,1 | Men | 495 | 26.1 | 53.85 | 50.7 | 13.67(1.01) | 16.76(1.28) | NA | NA |
| Lazo,etal. 201529 | USA | CS | Postmenopausal women and men from Multiethnic Study of Atherosclerosis (MESA) | CT | 5,1,3 | Men Women | 2,899 2,835 | M31.9 F32.9 | 58.7 61.2 | 5.3 5.6 | 12.7(1.07) 1.1 (0.12) | 14.2(1.07) 0.9(0.12) | 32.7(6.83) 40.8 (5.9) | 40.9 (3.55) 59.8 (8.85) |
| Li,etal. 201530 | China | CS | Men undergoing routine assessment of their liver | USG | 5,0,3 | Men | 196 | M23.6 | 35.4 | 52 | 13.0(4.1) | 17.4(3.9) | 18.3 (4.5) | 29.7 (5.7) |
| Polyzos, etal. 201337 | Greece | CC | Postmenopausal women were recruited from an outpatient basis | USG | 1,2,4 | Women | 40 | F32 | 55 | 55 | 0.85(0.13) | 1.02(0.16) | 30.8 (5.33) | 57.7 (6.75) |
| Seo,efa/. 201531 | Korea | CS | Voluntarily participated subjects in the Health Promotion Center | USG | 3,1,3 | Men | 1,944 | M25.2 | 44 | 44.3 | 14.5(1.03) | 17.2(1.17) | NA | NA |
| Shin,etal. 201132 | Korea | CS | Patients with type 2 DM were enrolled in an outpatient setting | USG | 5,1,3 | Men Women | 87 61 | M and F26.8 | 57 | 81.6 83.6 | 10.76 (0.97) 32.0(13.1) | 13.19(1.39) 47.9(18.9) | 1.04(0.17) 38.0(22.3) | 1.04(0.17) 64.9 (28.3) |
| Tian,etal. 201233 | China | CS | A population-based survey conducted amongnon-institutionalized Chinese men | USG | 3,1,3 | Men | 1,882 | M23.6 | 46.4 | 30.4 | 20.51 (1.32) | 20.99(1.42) | 43.14(4.94) | 36.73 (3.71) |
| Vassilatou, etal. 201038 | Greece | CC | Premenopausal patients who attended outpatient endocrine clinic | USG | 3,1,3 | Women | 60 | F 28.3 | 27.6 | 20 | 1.24(0.48) | 1.31 (0.48) | 31.7(14.0) | 52.0(21.2) |
| Volzke, etal. 201034 | Germany | CS | Population-based study in West Pomerania, Germany | USG | 3,1,5 | Men | 1,912 | M29.1 | 56 | 37.9 | 14.2(1.08) | 17.2(1.23) | NA | NA |
| Wang, etal. 201635 | China | CS | Population-based survey in East China | USG | 5,0,3 | Men Women | 2,098 1,131 | M26.6 F27.4 | 51 63 | 41.1 33.4 | 14.0(4.7) 0.7 (0.6) | 17.7(6.1) 0.6 (0.4) | 34.7(17.7) 49.4(21.1) | 54.1 (27.1) 80.4 (35.2) |
BMI: Body mass index. CC: Case control. CS: Cross-sectional. NA: Non available. NOS: Newcastle Ottawa scale. PCOS: Polycystic ovary syndrome. SHBG: Sex-hormone binding globulin. TT: Total testosterone. USG: Liver ultrasonography.
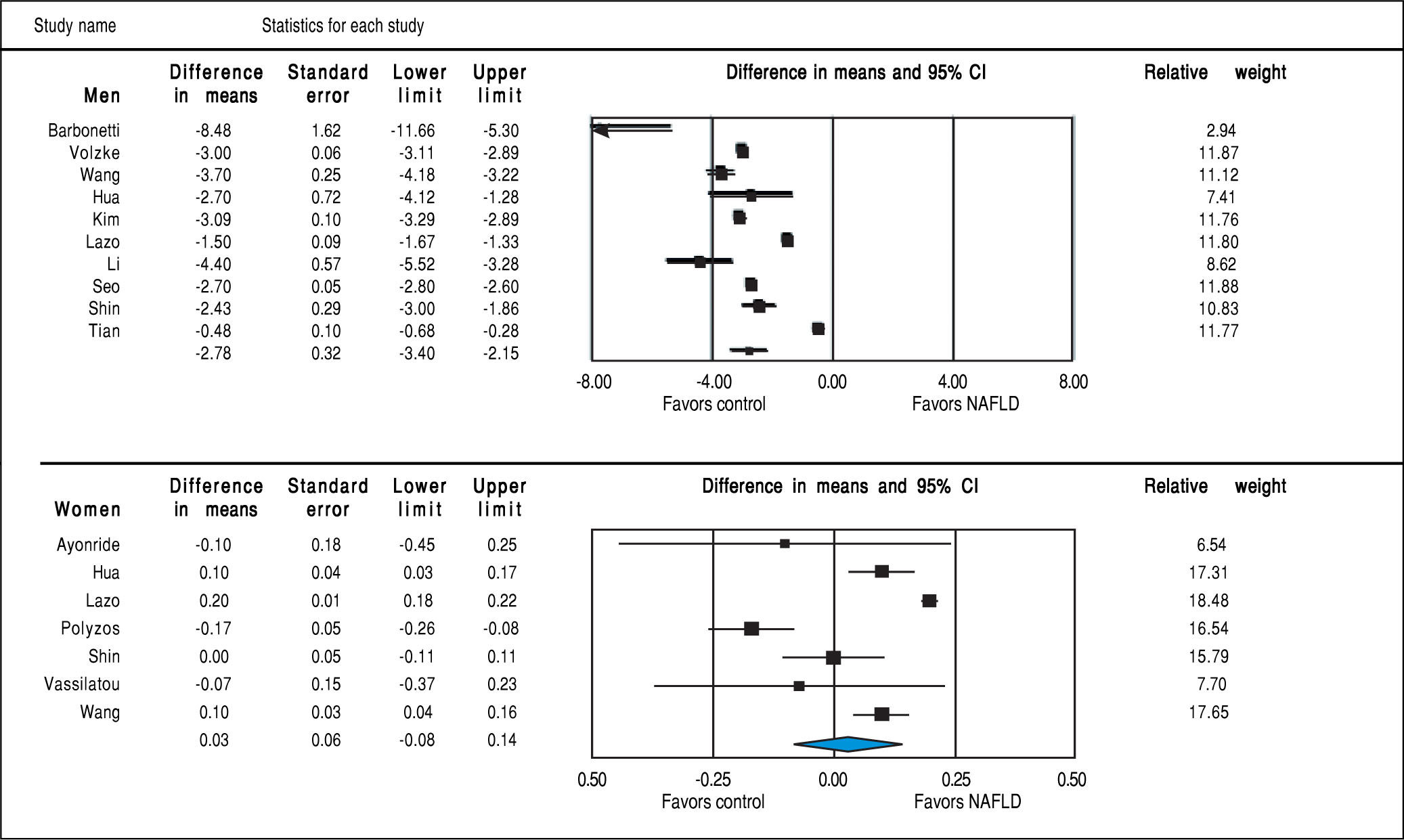
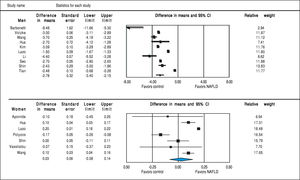
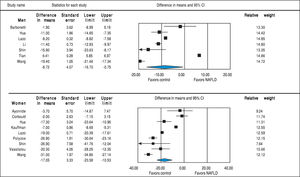
Studies assessing TT levels in subjects with and without NAFLD involving 12,388 men (10 studies24, 28-36) and 4,855 women (7 studies23,29,32,35-38) were included in the meta-analysis. Men with NAFLD had significantly lower levels of TT with MD of -2.78 nmol/l (95%CI -3.40 to -2.15, I2 = 99%, Pheterogeneity < 0.01), whereas women with NAFLD did not show significant difference of TT levels with MD of 0.03 (-0.08 to 0.14, I2 = 93%, Pheterogeneity = 0.01) compared to those without NAFLD (Figure 3). Substantial between-study heterogeneity was observed in both men (I2 = 99%) and women (I2 = 93%). Subgroup analyses were performed based age of participants, BMI, menopausal status, study design and study quality. In men, the association tended to be more pronounced in subgroup of mean age < 55 years, BMI < 30 kg/m2 and studies with moderate quality. The test for interaction showed significant results between study groups of the mean age of participants, BMI, and study design. These results indicated that the mean age of participants, BMI, and study design were partly the reason why there was high heterogeneity in the overall analysis (Table 2). However, meta-regression found no significant association between BMI and TT levels in both men (beta coefficient = 0.052, P-value = 0.72), and women (beta coefficient = -0.006, P-value = 0.80).
Subgroup analyses of included studies.
| Studies (n) | MenTT meandifference(95%CI) (nmol/l) | I 2 (%) and (P) | P-interaction | Studies (n) | WomenTT meandifference(95%CI) (nmol/l) | I2 (%) and (P) | P-interaction | |
|---|---|---|---|---|---|---|---|---|
| Overall random effects | 10 | -2.78 | 99 | |||||
| (-3.40 to -2.15) | (< 0.01) | 7 | 0.03 (-0.08 to 0.14) | 93 (0.01) | ||||
| Age (years) | ||||||||
| <55 | 6 | -2.88 | 99 | |||||
| (-3.77 to -2.00) | (< 0.01) | <0.01 | 3 | 0.06 (-0.17 to 0.29) | 65 (0.05) | 0.71 | ||
| ≥ 55 | 3 | -2.81 | 48 | |||||
| (-3.23 to -2.39) | (0.15) | 4 | 0.01 (-0.11 to 0.13) | 89 (0.03) | ||||
| BMI (kg/m2) | ||||||||
| < 30 | 9 | -2.94 | 99 | |||||
| (-3.60 to -2.28) | (< 0.01) | <0.01 | 4 | 0.08 (0.03 to 0.13) | 22 (0.28) | 0.57 | ||
| ≥ 30 | 1 | -1.50 | ||||||
| (-1.67 to 1.33) | NA | 3 | -0.01 (-0.32 to 0.29) | 97 (0.08) | ||||
| Menopausal status | ||||||||
| Premenopause | NA | NA | NA | NA | 1 | -0.07 (-0.37 to 0.23) | NA | 0.09 |
| Postmenopause | NA | NA | NA | 2 | 0.15 (0.06 to 0.25) | 90 (< 0.01) | ||
| Study design | ||||||||
| CS | 9 | -2.78 | 99 | |||||
| (-3.44 to -2.13) | (< 0.01) | 0.79 | 4 | 0.09 (-0.01 to 0.20) | 88 (0.02) | < 0.01 | ||
| CC | 1 | -2.70 | ||||||
| (-4.12 to 1.28) | NA | 3 | -0.04 (-0.26 to 0.18) | 91 (0.01) | ||||
| Study quality | ||||||||
| High | 8 | -2.54 | 99 | |||||
| (-3.25 to -1.84) | (< 0.01) | <0.01 | 7 | 0.03 (-0.08 to 0.14) | 93 (0.01) | < 0.01 | ||
| Moderate | 2 | -5.54 | 91 | |||||
| (-10.80 to -0.28) | (< 0.01) | NA | NA | NA | ||||
| Overall | 7 | -8.72 | 99 | -17.05 | ||||
| random effects | (-16.70 to -0.75) | (< 0.01) | 9 | (-23.58 to -10.53) | 97 (< 0.01) | |||
| Age (years) | ||||||||
| < 55 | 4 | -6.63 | 99 | -7.99 | ||||
| (-20.65 to 7.40) | (< 0.01) | <0.01 | 4 | (-14.09 to -1.88) | 80 (< 0.01) | < 0.01 | ||
| ≥ 55 | 3 | -10.23 | 66 | -23.99 | ||||
| (-13.65 to -6.81) | (0.05) | 5 | (-30.07 to -17.92) | 91 (< 0.01) | ||||
| BMI (kg/m2) | ||||||||
| < 30 | 6 | -8.83 | 99.5 | -23.84 | ||||
| (-20.11 to 2.44) | (< 0.01) | <0.01 | 4 | (-31.94 to -15.73) | 81 (< 0.01) | < 0.01 | ||
| ≥ 30 | 1 | -8.20 | -12.25 | |||||
| (-8.82 to -7.58) | NA | 5 | (-20.48 to -4.02) | 98 (< 0.01) | ||||
| Menopausal status | ||||||||
| Premenopause | NA | NA | NA | NA | 4 | -7.99 (-14.09 to 1.88) | 80 (0.02) | < 0.01 |
| Postmenopause | NA | NA | NA | 3 | -25.48 (-33.38 to -17.58) | 95 (0.04) | ||
| Study design | ||||||||
| CS | 6 | -8.34 | 99 | -15.45 | ||||
| (-17.01 to 0.33) | (< 0.01) | < 0.01 | 7 | (-23.03 to -7.88) | 97 (< 0.01) | < 0.01 | ||
| CC | 1 | -11.00 | -22.46 | |||||
| (-14.65 to -7.35) | NA | 2 | (-31.84 to -13.07) | 85 (0.01) | ||||
| Study quality | ||||||||
| High | 6 | -9.77 | 99 | -17.05 | 97 | |||
| (-18.37 to -1.17) | (< 0.01) | 0.98 | 9 | (-23.58 to -10.53) | (< 0.01) | |||
| Moderate | 1 | -1.90 | ||||||
| (-8.99 to 5.19) | NA | NA | NA | NA |
BMI: Body mass index. CC: Case-control. CS: Cross-sectional. NA: Not applicable. NAFLD: Non-alcoholic fatty liver disease. SHBG: Sex-hormone binding globulin. TT: Total testosterone.
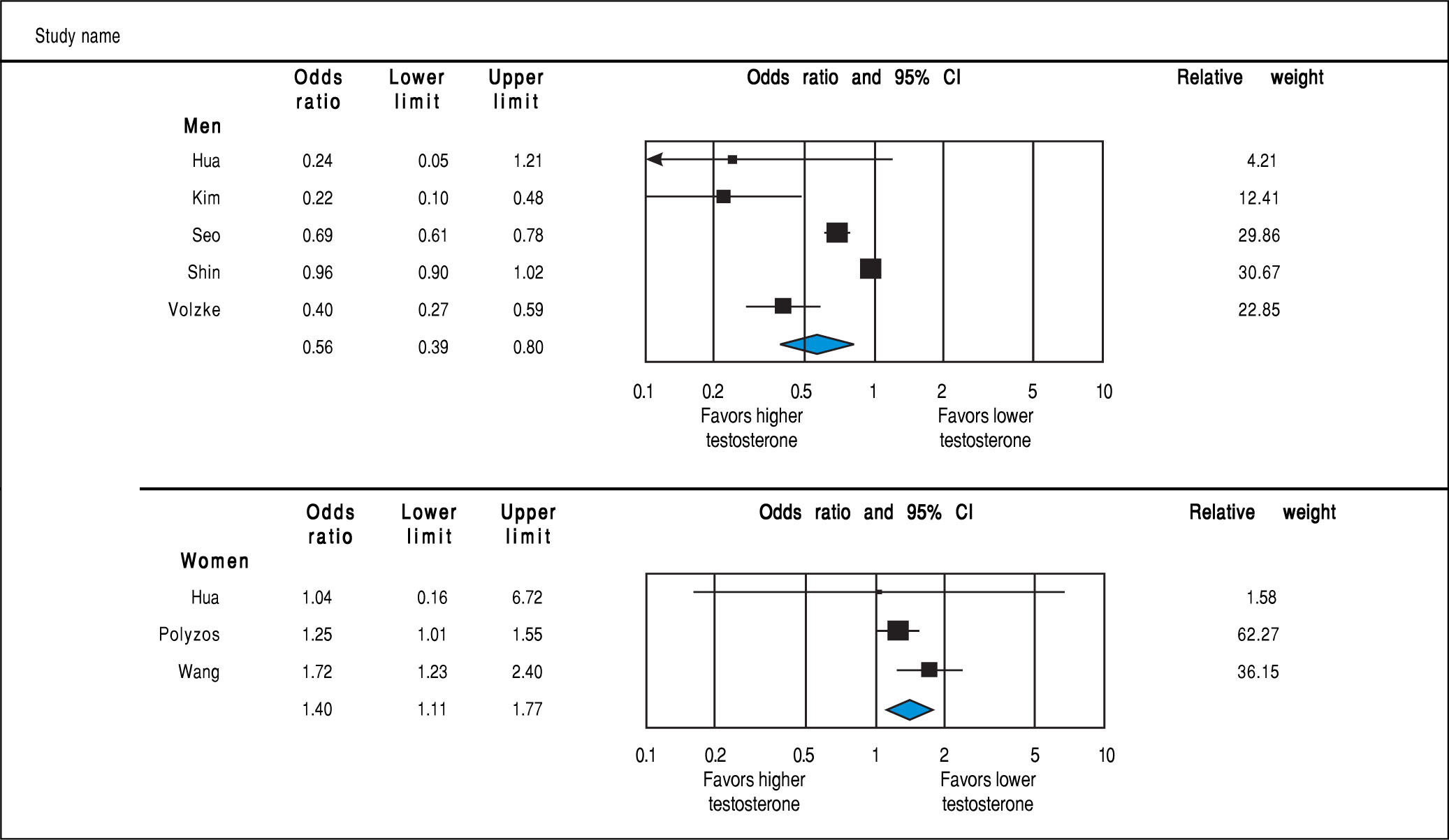
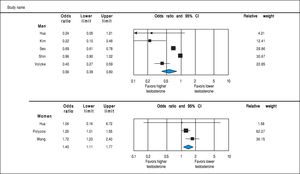
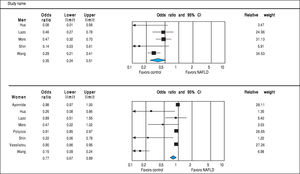
Analysis of OR estimates for TT levels in NAFLD comprised 4,715 men (5 studies28,31,32,34,36) and 1,581 women (3 studies35-37). Pooled analysis showed a reduced odds of NAFLD in men with higher TT levels with pooled OR of 0.56 (95%CI 0.39 to 0.80, I2 = 92%, Pheterogeneity = 0.07) whereas an opposite association was observed in women with pooled OR of 1.40 (95%CI 1.11 to 1.77, I2 = 21%, Pheterogeneity < 0.01) (Figure 4).
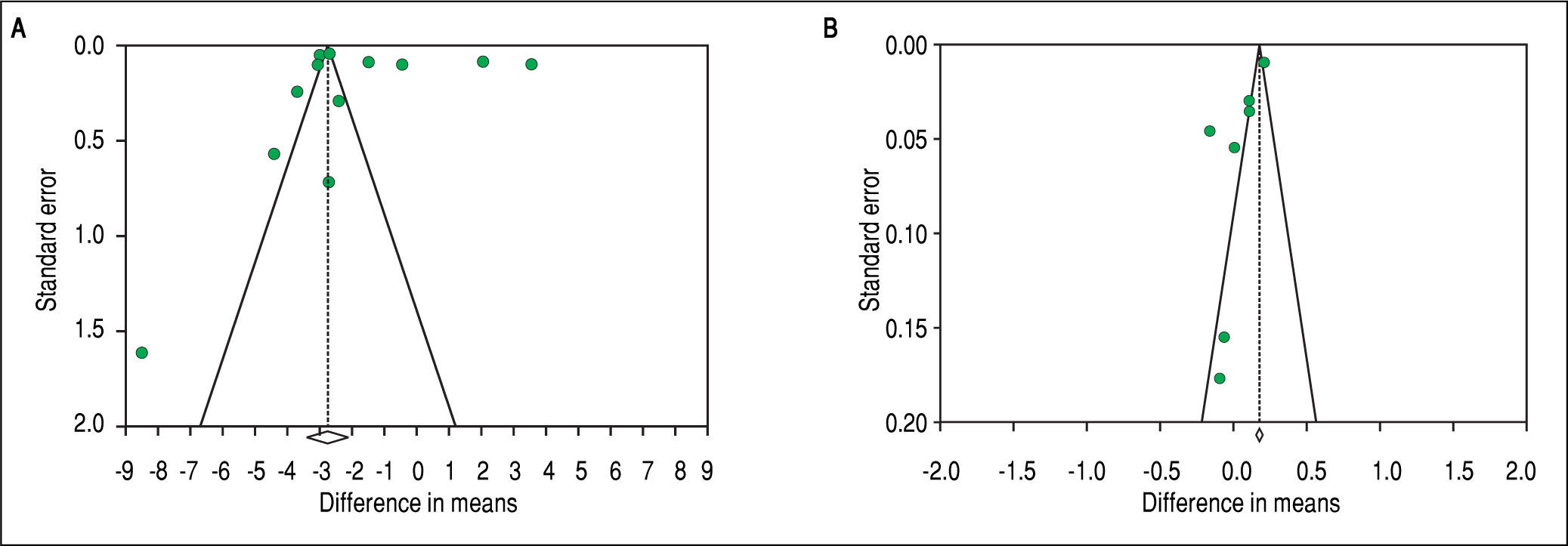
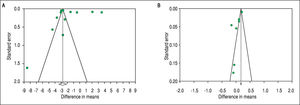
To investigate potential publication bias, we examined the contour-enhanced funnel plot of the included studies that assessed mean difference of TT in both men and women (Figure 5). For both men and women, the plots exclude bias since there are symmetrical distributions of studies on both sides of the mean. Furthermore, the Egg-er's test was non-significant (P = 0.97 and 0.039, respectively). Using the trim and fill methods in the random-effects model, there was no difference of the imputed mean difference.
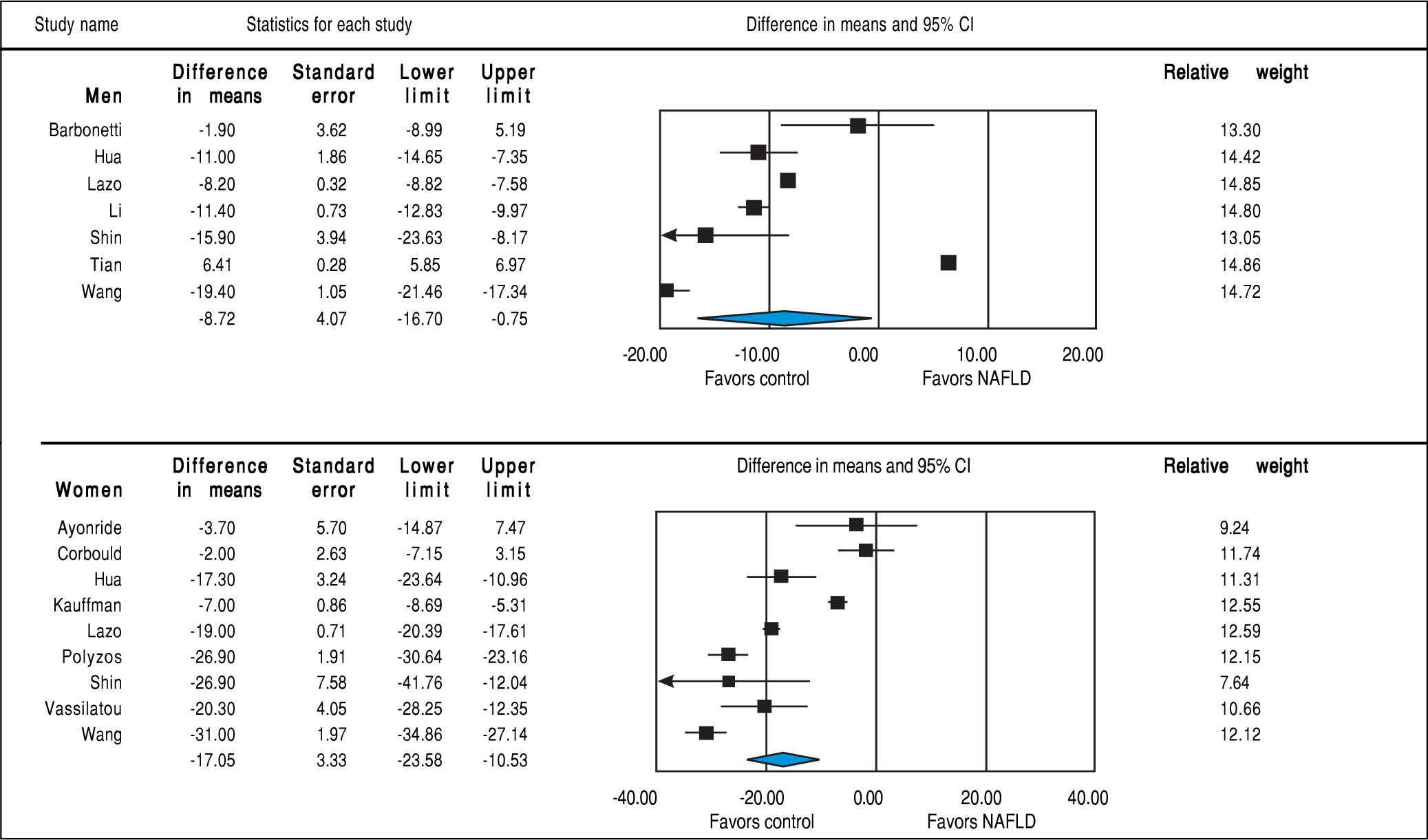
SHBGStudies assessing SHBG levels in subjects with and without NAFLD involving 7,841 men (7 studies24,29,30,32,33,35,36) and 4,930 women (9 studies23,25,27,29,32,35-38) were included in the meta-analysis. In both sexes, SHBG was lower in subjects with NAFLD (men: MD = -8.72 nmol/l, 95%CI -16.70 to -0.75, I2 = 99%, Pheterogeneity < 0.01; women: MD = -17.05, -23.58 to -10.53, I2 = 97%, Pheterogeneity < 0.01) compared to those without NAFLD (Figure 6). This inverse association between SHBG levels and NAFLD was stronger in women than men (Psexdifference < 0.01). Substantial be-tween-study heterogeneity was observed in both men (I2 = 99%) and women (I2 = 97%). In men, this significant heterogeneity could be partly explained by differences in age, BMI and study design (all P-interaction < 0.01). In women, the association between SHBG levels and NAFLD was stronger in those with age ≥ 55 years, BMI < 30, post-menopause, and case-control studies. So the differences in age, BMI, menopausal status and study design could partly contribute to the high between-study heterogeneity (all P-in-teraction < 0.01) (Table 2). By meta-regression, we show a significant association between BMI and SHBG in women such that patients with increased BMI will have higher SHBG levels (beta coefficient = 1.20, 95% CI 0.56-1.85, P-value < 0.001) whereas BMI did not significantly impact on the change in SHBG in men (beta coefficient = -0.67, P-value = 0.72).
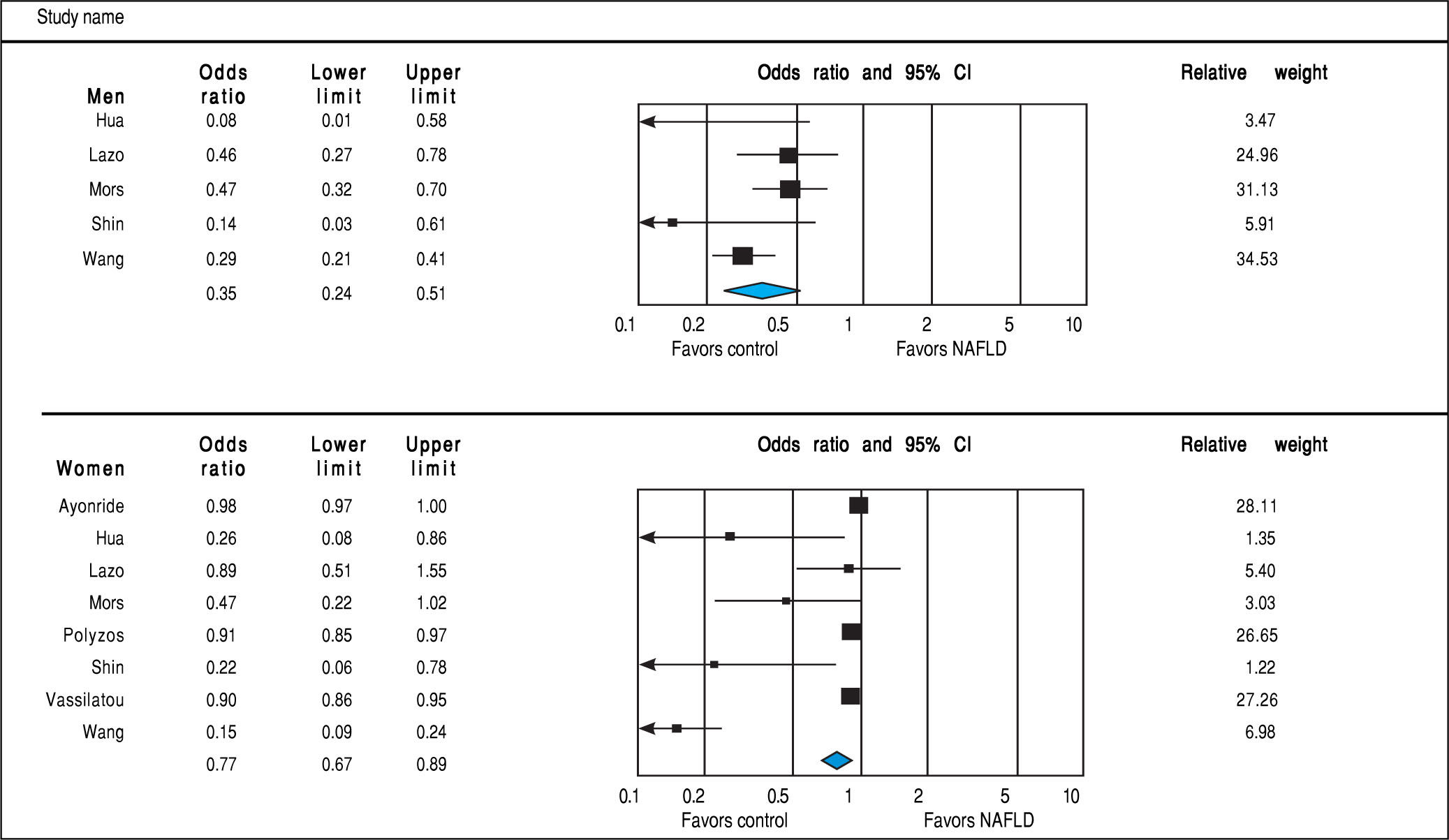
Analysis of OR estimates for SHBG levels in NAFLD comprised 6,691 men (5 studies26,29,32,35,36) and 5,725 women (8 studies23,26,29,32,35-38). Pooled analysis showed a reduced risk of NAFLD with higher SHBG levels in both men (pooled OR of 0.35, 95%CI 0.25 to 0.45, I2 = 50%, Pheterogeneity = 0.09) and women (pooled OR of 0.77, 95%CI 0.67 to 0.89, I2 = 92%, Pheterogeneity = 0.02) (Figure 7).
To investigate potential publication bias, we examined the contour-enhanced funnel plot of the included studies that assessed mean difference of SHBG in both men and women (Figure 5). For both men and women, the plots exclude bias since there are symmetrical distributions of studies on both sides of the mean. Furthermore, the Egger's test was non-significant (P = 0.36 and 0.74, respectively). Using the trim and fill methods in the random-effects model, there was no difference of the imputed mean difference.
DiscussionThe association between TT and SHBG and NAFLD has been investigated in several studies. This meta-analysis aims to comprehensively review and combine all available evidence with an attempt to provide the definitive conclusion of this potential association. Our meta-analysis demonstrated the sex differences for the association between endogenous TT and NAFLD. TT was lower in men with NAFLD compared with non-NAFLD controls but did not show any associations in women. However, Higher TT levels were found to decrease odds of NAFLD in men and increase odds of NAFLD in women. There was no sex-specific association between SHBG level and NAFLD. In both men and women, lower SHBG levels were associated with NAFLD. By meta-regression, BMI appeared to be the effect modifier of the association between NAFLD and SHBG in women.
Our results were in line with the previous meta-analy-sis from Brand, et al.,39 which demonstrated the presence of sex-dependent association between testosterone and metabolic syndrome: TT and FT were lower in men with metabolic syndrome but they were higher in women with metabolic syndrome and no sex difference for the association between SHBG and metabolic syndrome, which showed higher risk of metabolic syndrome in subjects with lower SHBG levels. Another meta-analysis from Ding, et al.40 also indicated that TT differently modulated the risk of diabetes in men and women. High testosterone levels are associated with higher risk of diabetes in women but with lower risk in men; the inverse association of SHBG and NAFLD was observed in both sexes with the stronger relationship in women than in men.
The exact mechanisms underlying the sex-specific associations between testosterone and NAFLD remain poorly understood. The association between low testosterone and NAFLD in men could be explained by hypog-onadal-obesity-adipocytokine hypothesis.41,42 Visceral adiposity positively correlates with insulin resistance and NAFLD.43 Increasing visceral adipose tissue leads to increased aromatase enzyme activity that functions to convert testosterone to estrogen leading to decrease testosterone level. The low testosterone level further increases lipoprotein lipase activity, which in turn causes increased triglyceride uptake into the adipocytes resulting in increasing visceral adiposity. This exacerbates insulin resistance and causes vicious cycle by further decreasing testosterone. Moreover, the pro-inflammatory adipocy-tokines including tumor necrosis factor-alpha, inter-leukin-1 and interleukin-6 releasing from the adipose tissue could potentially inhibit pituitary axis resulting in lower testosterone levels.44,45 In women, the opposite association between TT and NAFLD was clearly observed but the mechanism still remains uncertain. Previous study showed that higher TT levels were associated with increased visceral adiposity and insulin resistance in wom-en.46 These differences could be driven by several factors. First, it could be from relative androgen excess to estrogen level, which is known to have protective effect against fatty liver, during the menopausal transition period.47 Second, the liver showed considerable sexual dimorphism in gene expression likely due to the differences in metabolic needs for reproduction including androgen receptors. The liver also displays marked changes in androgen sensitivity.48 Genetic studies in animal models compared global gene expression on testosterone between sexes showed substantial sex differences in transcription response to testosterone, which suggests that males and females may employ different pathways when responding to elevated testosterone.49 However, it should be noted that liver fat plays a key role in development of metabolic syndrome. Changes in hormone levels might be merely a consequence of fatty liver leading to visceral adiposity.
The lack of a sex-specific association between SHBG and NAFLD is not fully understood. Recent evidence suggests the correlation between plasma level of several cy-tokines and plasma SHBG levels. SHBG was found to be upregulated by proinflammatory cytokines (tumor necrosis factor alpha and interleukin 1 beta) and downregulated by anti-inflammatory cytokines (adiponectin).17 The imbalance of those cytokines seems to have a major role in the development of NAFLD as they are involved in various aspects of its pathogenesis.50
In consideration of the increasing trend of NAFLD incidence and being one of the most common indications of liver transplantation, effective prevention and treatment strategies are urgently needed. The currently mainstay treatments are lifestyle modifications including physical activity and healthy diet, which are difficult to maintain. It is of great significance to find novel treatment strategies. TT and SHBG levels have shown to be related to the risk of NAFLD and insulin resistance. Previous meta-analysis summarizing the effects of testosterone replacement therapy from RCTs have shown the significant improvements of insulin resistance, glucose control, body composition and lipid metabolism.51 Few recent randomized controlled trials have addressed this issue relating to hepatic stea-tosis, but reported conflicting results. Hoyos, et al.52 conducted 18-week testosterone treatment in obese men demonstrating significantly reduced liver fat, whereas Huang, et al.53 reported that 6-month testosterone administration in older men with mobility limitation was not associated with a reduction in hepatic fat. Determining whether improving SHBG level or testosterone administration prevents or improves NAFLD requires further research. However, caution must be taken for the role of testosterone therapy, since it was found to increase risks of cardiovascular-related events and hepatocellular carcinoma.54-58
As with any meta-analysis, we acknowledge that there are some limitations. First, a significant heterogeneity was observed in this study. The possible sources of these heterogeneities include the differences in the study design, methodology and population as demonstrated by subgroup analyses. Second, this is a meta-analysis of observational studies, which can only demonstrate an association, not causality. Therefore, we cannot make a conclusion those endogenous sex hormones themselves versus other potential confounders cause the increased NAFLD risk. Third, most of the included studies used ultrasonography to detect hepatic steatosis. Although this imaging technique is widely accepted as the diagnostic tool of choice for screening NAFLD due to its low cost, safety and accessibility, it has limited accuracy in detecting mild stea-tosis and operator dependency.59,60 Liver biopsy is regarded as a gold standard for detecting hepatic steatosis.1
In summary, our meta-analysis demonstrated a sex-dependent association between TT and NAFLD. Higher TT levels are associated with men with NAFLD and inversely associated with women with NAFLD, whereas no sex-specific association was identified between SHBG and NAFLD. Higher SHBG levels are associated with lower NAFLD odds in both men and women.
Potential Competing InterestsNone.
Ethical ApprovalThis article does not contain any studies with human participants or animals performed by any of the authors.
Informed ConsentNo informed consent.
Disclosure StatementThe authors have nothing to disclose.
Author ContributionsVJ conceived of the study, assessed the quality of the studies, and drafted the manuscript. SU searched the literature, assessed the quality of the studies, performed the statistical analysis, and drafted the manuscript. AS and TR participated in the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
AcknowledgementWe thank Mathew Roslund for validation of the search.