Recurrence of HCV infection in patients with chronic hepatitis C virus (HCV) at the time of liver transplantation is nearly universal and reduces the likelihood of graft and patient survival.
Materials and methodsWe evaluated outcomes of 17 patients (16 with HCV genotype 1 and 1 with genotype 4) who received up to 12 or 24 weeks of ledipasvir/sofosbuvir plus ribavirin prior to or up to the time of liver transplant in the SOLAR-1 and SOLAR-2 trials. In all patients, HCV RNA was < 15 IU/mL prior to transplant. At screening, 6 patients were Child-Pugh-Turcotte (CPT) class B and 11 were CPT class C. Seven patients underwent transplant prior to completing assigned treatment, with 4 treated for < 12 weeks. The primary endpoint was posttransplant virologic response 12 weeks after transplant (pTVR12) in patients with HCV RNA < 15 IU/mL at their last measurement prior to transplant.
ResultsOverall, 94% (16/17) achieved pTVR12. All who achieved pTVR12 received at least 11 weeks of treatment. The single patient who did not achieve pTVR12 discontinued study drug on day 21 and underwent liver transplant the following day. The patient had HCV RNA < 15 IU/mL at post-transplant week 2 but died 15 days post-transplant because of multi-organ failure and septic shock.
ConclusionAmong a small population of HCV patients with decompensated cirrhosis, virologic response to ledipasvir / so-fosbuvir plus ribavirin prior to liver transplantation was maintained after transplantation, even if treatment was stopped early. Administration of ledipasvir / sofosbuvir plus ribavirin before liver transplant can prevent post-transplant HCV recurrence.
Recurrence of hepatitis C virus (HCV) after liver transplant is nearly universal in those who undergo implantation of an allograft in the setting of active viremia.1 Moreover, the immunosuppressive effects of antirejection drugs accelerate the natural history of post-transplant HCV graft hepatitis, with 10-30% of transplant recipients progressing to cirrhosis within 5 years.2 A minority develop a particularly aggressive form of liver injury known as fibrosing cholestatic hepatitis3 that, if untreated, results in graft loss within months. Retransplantation for HCV-associated graft loss is associated with poor outcomes and overall survival.4 With peginterferon and ribavirin, post-transplant treatment of HCV was suboptimal as it was poorly tolerated, and the likelihood of achieving a sustained viral response (SVR) was less than 30%.5 Treating patients with decompensated cirrhosis pretransplant with peginterferon and ribavirin was also associated with poor tolerability, increased risk of bacterial infections, and SVR in the 20% range;6 therefore, treatment in these patients was generally not recommended.
Interferon-free regimens with direct-acting antiviral agents (DAAs) have provided opportunities to treat sub-populations of HCV patients who previously had no treatment options. For the combined SOLAR-1 and SOLAR-2 data in HCV genotype 1 and 4 nontransplanted patients with decompensated cirrhosis, treatment with ledipasavir/ sofosbuvir with ribavirin resulted in rates of SVR 12 weeks following the end of treatment (SVR12) of 81% to 92% depending on Child-Pugh-Turcotte (CPT) class C or B status, and treatment duration.7,8 Sofosbuvir/velpatasvir with ribavirin demonstrated an SVR12 rate of 94% in patients with CPT B disease.9 Interferon-free DAA regimens have also had favorable clinical outcomes in liver transplant recipients, irrespective of stage of liver disease. For the pooled SOLAR-1 and -2 data of ledipasavir/sofosbuvir with ribavirin in liver transplant recipients, SVR12 rates of 95 to 99% for combined data 100% have been reported in patients without cirrhosis or with compensated cirrhosis, 90-94% in those with CPT class B disease, and 50-78% in those with CPT C disease.7,8 Similar efficacy has also been reported in the “real world” clinical setting of post-liver transplant patients.10,11 Most importantly, the sofosbuvir-based regimens are reported to be well-tolerated in these populations, with manageable drug-drug interactions.
Previously, in the peginterferon and ribavirin era, post-transplant recurrence of HCV in patients transplanted with an undetectable HCV viral load was a significant issue if the duration of pretransplant antiviral therapy was less than 16 weeks.12 With sofosbuvir plus ribavirin treatment in patients with hepatocellular carcinoma awaiting liver transplantation, duration of time with HCV RNA less than the lower limit of quantification (LLOQ) was the only factor associated with post-transplant recurrence.13 The post-transplant virologic response for patients with decompen-sated cirrhosis treated with combinations of NS5A and NS5B inhibitors pretransplant are currently not known.
The outcome of patients who have DAA treatment interrupted by a liver transplant is important because of the clinical uncertainty and pragmatic concerns surrounding those who are on the waiting list for a liver transplant. It is not clear whether patients should be suspended from the transplant waiting list until completing DAA therapy in case it is interrupted in the event of liver transplant surgery. However, given the scarcity of suitable organs for any given patient on the transplant waitlist, to do so may be placing a patient at risk of losing a life-saving opportunity. To gain insight into this issue, we conducted a post-hoc analysis of a subset of patients from the SOLAR-17 and SOLAR-28 clinical trials. SOLAR-1 and -2 had similar trial designs and randomly assigned patients with decompen-sated liver disease and post-transplant patients with graft hepatitis to ledipasavir/sofosbuvir with ribavirin for 12 or 24 weeks within each of 7 disease groups. In our analysis, we evaluated the virologic response outcomes of patients who underwent liver transplant surgery during the study, some of whom had not completed the assigned course of therapy.
Material and MethodsThe study designs of the SOLAR-1 and SOLAR-2 clinical trials have been previously described.7,8 SOLAR-1 and -2 enrolled and treated 670 patients at 63 clinical sites in 13 countries. Ethical approval for the clinical trials was obtained by each site from the site's institutional review board. Eligible patients were at least 18 years old with chronic genotype 1 or 4 HCV infection. Patients with CPT scores of 13-15 were excluded, as were patients with HIV or hepatitis B infection, history of hepatocellular carcinoma, significant renal impairment (creatinine clearance ≤ 40 mL/min), or significant thrombocytopenia (platelet count < 30,000 x 103/μL).
In both studies, patients were enrolled into 2 cohorts. Cohort A comprised patients with decompensated cirrhosis who had not undergone liver transplant: Group 1 patients had CPT B cirrhosis (CPT score 7-9), and group 2 patients had CPT C cirrhosis (CPT score 10-12). Cohort B consisted of post-liver transplant patients: group 3 patients did not have cirrhosis, group 4 patients had CPT A cirrhosis (CPT score 5-6), group 5 patients had CPT B cirrhosis, group 6 patients had CPT C cirrhosis, and group 7 patients had fibrosing cholestatic hepatitis. CPT class for assignment to disease groups was based on the screening CPT score.
Patients in each of the 7 disease groups were randomized 1:1 to receive 12 or 24 weeks of treatment with ribavirin and the fixed-dose combination tablet of ledipas-vir 90 mg and sofosbuvir 400 mg. For groups 3, 4, and 7, ribavirin dosing was weight-based, 1,000 mg/day for patients with baseline weight < 75 mg and 1,200 mg/day for patients with baseline weight ≥ 75 kg, given in 2 divided daily doses. For groups 1, 2, 5, and 6, ribavirin dosing started at 600 mg in a divided daily dose, and, if well tolerated, could be titrated up to 1,000-1,200 mg daily (weight-based dose).
Patients who underwent liver transplantation during the study before completing their assigned antiviral treatment were discontinued from treatment at the time of transplant and were followed post-transplant.
Serum HCV RNA was measured using the COBAS® AmpliPrep/COBAS® TaqMan® HCV Test, v2.0 (Roche Molecular Diagnostics, Pleasanton, CA, USA) with an LLOQ of 15 IU/mL. For this analysis, the primary end-point was the percentage of patients with post-transplant virologic response, defined as HCV RNA < LLOQ 12 weeks after liver transplantation (pTVR12) in patients with HCV RNA < LLOQ on last HCV RNA measurement prior to transplant.
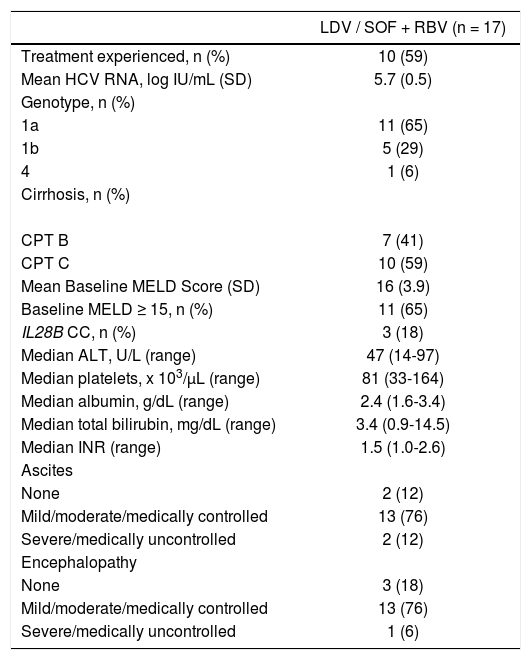
RESULTSParticipantsDuring the SOLAR-1 and SOLAR-2 studies, 17 patients underwent liver transplantation (Table 1). Eight were assigned to 12 weeks of treatment, and 9 were assigned to 24 weeks. One patient (6%) was infected with HCV genotype 4, and the remainder were infected with HCV genotype 1 (65% genotype 1a, 29% genotype 1b). The median age of patients was 57 years (range 47-69), 82% were male (n = 14), and all were Caucasian. The median body mass index (BMI) was 27.5 kg/m2 (range 20.9-40.6), and 24% (n = 4) had a BMI ≥ 30 kg/m2.
Patient Baseline Characteristics.
| LDV / SOF + RBV (n = 17) | |
|---|---|
| Treatment experienced, n (%) | 10 (59) |
| Mean HCV RNA, log IU/mL (SD) | 5.7 (0.5) |
| Genotype, n (%) | |
| 1a | 11 (65) |
| 1b | 5 (29) |
| 4 | 1 (6) |
| Cirrhosis, n (%) | |
| CPT B | 7 (41) |
| CPT C | 10 (59) |
| Mean Baseline MELD Score (SD) | 16 (3.9) |
| Baseline MELD ≥ 15, n (%) | 11 (65) |
| IL28B CC, n (%) | 3 (18) |
| Median ALT, U/L (range) | 47 (14-97) |
| Median platelets, x 103/μL (range) | 81 (33-164) |
| Median albumin, g/dL (range) | 2.4 (1.6-3.4) |
| Median total bilirubin, mg/dL (range) | 3.4 (0.9-14.5) |
| Median INR (range) | 1.5 (1.0-2.6) |
| Ascites | |
| None | 2 (12) |
| Mild/moderate/medically controlled | 13 (76) |
| Severe/medically uncontrolled | 2 (12) |
| Encephalopathy | |
| None | 3 (18) |
| Mild/moderate/medically controlled | 13 (76) |
| Severe/medically uncontrolled | 1 (6) |
ALT: Alanine aminotransferase. CPT: Child-Pugh-Turcotte. INR: International normalized ratio of prothrombin time. LDV: Ledipasvir. MELD: Model for End-Stage Liver Disease. RBV: Ribavirin. SOF: Sofosbuvir. Baseline = last value prior to first dose date of study drug.
Sixteen patients were in Cohort A, with no previous history of liver transplant. Five of these had CPT B cirrhosis at screening, and 11 had CPT C cirrhosis. The patient who had a prior transplant was in Cohort B group 5, with CPT B cirrhosis of the graft. The mean baseline model for end-stage liver disease (MELD) score of the patients was 16 (SD 3.9), and the majority of patients (88%, 15/17) had ascites.
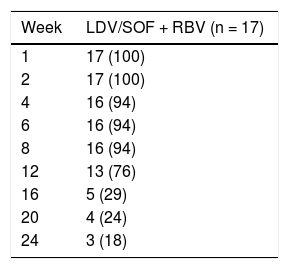
Seven patients (41%) underwent liver transplantation prior to completing the full 12 or 24 weeks of assigned treatment with ledipasvir / sofosbuvir plus ribavirin. The remainder underwent liver transplant in the post-treatment period; 6 had achieved SVR4 and 4 had achieved SVR12 prior to transplant. Overall, the mean exposure to antiviral medications was 14.7 weeks (SD 5.9), and 13 patients (76%) completed at least 12 weeks of treatment (Table 2).
Duration of Exposure to Study Regimen.
| Week | LDV/SOF + RBV (n = 17) |
|---|---|
| 1 | 17 (100) |
| 2 | 17 (100) |
| 4 | 16 (94) |
| 6 | 16 (94) |
| 8 | 16 (94) |
| 12 | 13 (76) |
| 16 | 5 (29) |
| 20 | 4 (24) |
| 24 | 3 (18) |
LDV: Ledipasvir. RBV: Ribavirin. SOF: sofosbuvir. Weeks on study regimen = (last dose date any study drug - first dose date any study drug + 1) / 7.
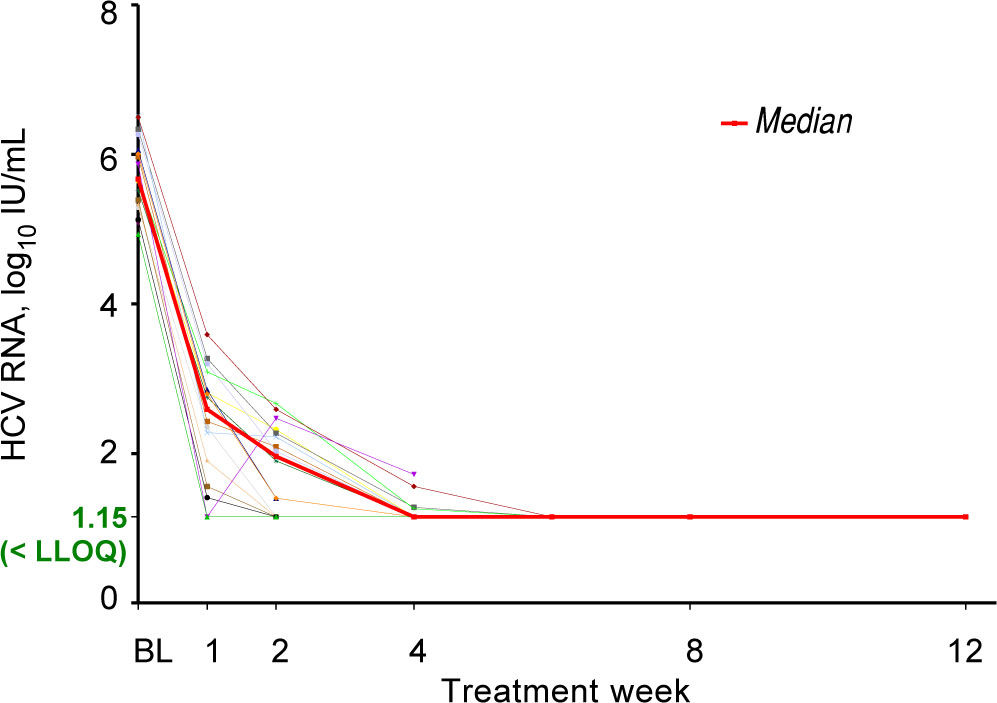
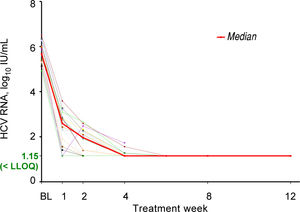
All seventeen patients had HCV RNA < LLOQ by treatment week 6 (Figure 1) and at the time of liver transplantation. Sixteen of the 17 patients (94%) achieved pTVR12. The 1 patient who did not achieve pTVR12 died 2 weeks after transplant. Further details on this patient are described in the Adverse Events section below. All patients who achieved pTVR12 received at least 11 weeks of treatment with ledipasvir / sofosbuvir plus ribavirin, and all had HCV RNA < LLOQ by Week 8 of treatment (Figure 1).
Changes in MELD ScoresMELD scores had at least a 1-point improvement for 7 patients (41%) and a 1-point worsening for 8 patients (47%) from baseline to the final MELD assessment prior to transplant. Three patients (18%) had MELD ≥ 20 at their last on-study assessment prior to undergoing liver transplantation.
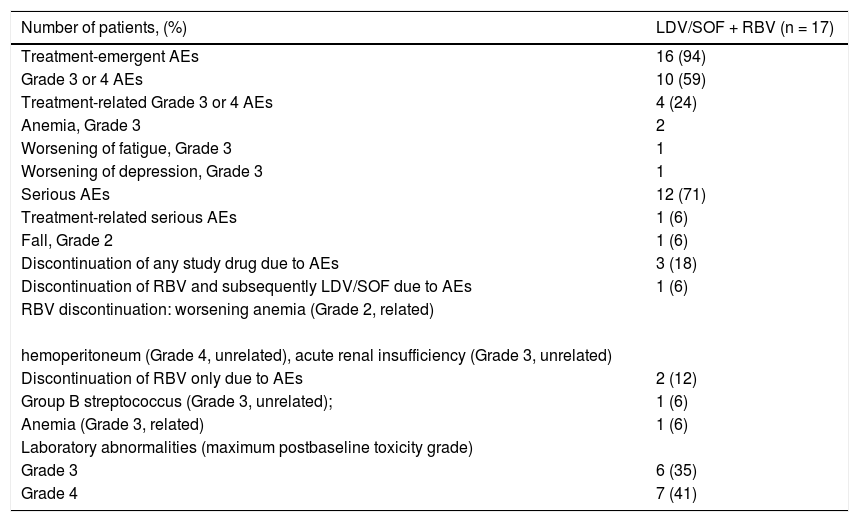
Adverse EventsMost of the adverse events were related to the primary liver disease. Of the 3 patients who discontinued any study drug (Table 3), 2 had adverse events for which only ribavirin was discontinued but treatment with ledipasvir / sofosbu-vir continued.
Safety Summary.
| Number of patients, (%) | LDV/SOF + RBV (n = 17) |
|---|---|
| Treatment-emergent AEs | 16 (94) |
| Grade 3 or 4 AEs | 10 (59) |
| Treatment-related Grade 3 or 4 AEs | 4 (24) |
| Anemia, Grade 3 | 2 |
| Worsening of fatigue, Grade 3 | 1 |
| Worsening of depression, Grade 3 | 1 |
| Serious AEs | 12 (71) |
| Treatment-related serious AEs | 1 (6) |
| Fall, Grade 2 | 1 (6) |
| Discontinuation of any study drug due to AEs | 3 (18) |
| Discontinuation of RBV and subsequently LDV/SOF due to AEs | 1 (6) |
| RBV discontinuation: worsening anemia (Grade 2, related) | |
| hemoperitoneum (Grade 4, unrelated), acute renal insufficiency (Grade 3, unrelated) | |
| Discontinuation of RBV only due to AEs | 2 (12) |
| Group B streptococcus (Grade 3, unrelated); | 1 (6) |
| Anemia (Grade 3, related) | 1 (6) |
| Laboratory abnormalities (maximum postbaseline toxicity grade) | |
| Grade 3 | 6 (35) |
| Grade 4 | 7 (41) |
Adverse events and laboratory abnormalities were assigned a Grade 1-4. Grade 1 = mild, self-limited. Grade 2 = moderate with no or minimal medical intervention required. Grade 3 = severe with medical intervention required and hospitalization possibly required. Grade 4 = potentially life-threatening, medical intervention required and hospitalization required or likely. AE: Adverse event. LDV: Ledipasvir. RBV: ribavirin. SOF: sofosbuvir.
The patient who died after undergoing liver transplantation was initially listed for liver transplantation approximately 1.5 years prior to enrolling in the SOLAR-1 study. Thereafter, for unknown reasons he went through brief periods of deactivation from the list with subsequent reactivation. At the time of screening for the study, he had a CPT score of 10 and a MELD score of 16. At Day 1 of treatment the CPT score remained 10 and the MELD score was 13. On Day 4 of treatment with ledipasvir / so-fosbuvir plus ribavirin, the patient developed worsening of his encephalopathy and was found to have streptococcal bacteremia and Clostridium difficile infection. During hospi-talization, the patient developed acute kidney injury, thought to be secondary to hepatorenal syndrome. Diuresis with assistance of intravenous albumin was begun on Day 3 and ribavirin was discontinued on Day 4. The patient failed a trial of diuretics, metolazone, and Lasix. The patient began hemodialysis on Day 11 and was dialyzed intermittently until undergoing liver transplant with no adjustment to dosing of ledipasvir / sofosbuvir. During treatment, the patient's MELD score increased to 40 at Week 1. The last on-treatment MELD score assessed was 38 at Day 22. The patient discontinued ledipasvir/sofosbu-vir on Day 21 and underwent liver transplant the next day. The subject still had HCV RNA < LLOQ at post-transplant Week 2. He died 15 days after transplant from septic shock and multi-organ failure. The death was not considered by the investigator to be related to study drugs.
DiscussionThe durability of a post-transplant virologic response is an important clinical issue, because one of the objectives of HCV treatment before transplantation is preventing HCV graft reinfection. With peginterferon and ribavirin treatment, HCV undetectability at the time of liver transplant was not a guarantee that the newly transplanted liver allograft would not become reinfected. A recent clinical trial examined the efficacy and safety of pretransplant treatment with peginterferon and ribavirin.12 Of 59 patients treated pretransplant, only 19% had undetectable HCV RNA 12 weeks post-transplant. The likelihood of pTVR increased with longer duration of pre-transplant therapy. Those who were treated for less than 16 weeks were very unlikely to achieve pTVR despite having undetectable HCV at the time of transplant surgery. In a clinical trial of sofosbuvir with ribavirin pretransplant 23% of patients had HCV recurrence in those with undetectable HCV RNA at the time of transplantation.13 Although there was overlap in pretransplant duration of HCV undetectability between patients receiving sofosbuvir and ribavirin who achieved a pTVR and those who relapsed, the mean treatment duration for post-transplant success was 30 days.13
The SOLAR studies demonstrated that 12 weeks of treatment with ledipasvir / sofosbuvir plus ribavirin leads to high SVR rates in patients with decompensated cirrhosis. Of the 17 patients who underwent liver transplant, 13 received 12 or more weeks of treatment and therefore could be considered to have received curative therapy. Data from the other 3 patients who achieved pTVR after receiving less than the approved length of treatment can address the question of the minimum duration of treatment required to prevent post-transplant recurrence. A dedicated study is needed to examine the potential to prevent HCV recurrence in this population even if 12 weeks of treatment is not administered prior to liver transplant.
One may question whether in the future there will be a need for liver transplantation in patients with chronic HCV infection. Although the majority of patients who clear HCV with DAA-based regimens subsequently experience improvement in their liver function, in SOLAR-1 and SOLAR-2, 17 patients required liver transplantation during or after treatment, suggesting the need for transplants was not eliminated for all patients. In a recent clinical trial11 of combination treatment with sofosbuvir, ribavirin, and the latest-generation NS5A inhibitor, vel-patasvir, a minority (11%) of enrolled decompensated cir-rhotic patients experienced a clinical deterioration in CPT score even though the majority had improved CPT scores. A recent retrospective analysis of real-world patients in Europe with HCV monoinfection and decom-pensated cirrhosis without hepatocellular carcinoma demonstrated that treatment with sofosbuvir-based regimens led to delisting from the liver transplant wait list for many patients.14 It is not possible to know if there was a similar delisting for the patients in the SOLAR trials because data on transplant wait list status was not collected as part of the studies. Given that HCV patients with or without decompensated cirrhosis will continue to develop hepatocellular carcinoma, which is untreatable with curative intent except by transplantation, it is likely that the need for liver transplantation in the setting of chronic HCV will continue for the foreseeable future.
As the SOLAR-1 and SOLAR-2 clinical trials did not enroll patients with the most severe hepatic impairment (CPT scores 13-15), it is unknown if the results of our study are generalizable to these patients. Patients with this degree of decompensation, however, are desperately ill, and many await transplantation as hospitalized in-patients. It may be more appropriate to make transplantation the priority for these patients and to treat their HCV post-transplant once they are clinically stable. Although our study does not have the power for statistical analysis to determine factors that may lead to liver transplantation in patients with decompensated cirrhosis treated with ledi-pasvir / sofosbuvir plus ribavirin (and the SOLAR-1 and SOLAR-2 studies were not designed for this purpose), we do note that the majority of the transplanted patients, despite achieving HCV RNA < LLOQ at the time of transplant, suffered hepatic encephalopathy and ascites. This suggests that these 2 clinical variables, which are incorporated into the CPT score but not into the MELD score calculation, may indicate need for liver transplantation.
In conclusion, there were no cases of HCV recurrence among the 17 patients treated with ledipasvir/sofosbuvir plus ribavirin prior to transplantation. Future dedicated studies are needed to determine the optimal duration of therapy and timing of therapy in the pretransplant setting.
Abbreviations- •
AE: Adverse event.
- •
ALT: Alanine transaminase.
- •
BMI: Body mass index.
- •
CPT: Child-Pugh-Turcotte.
- •
DAA: Direct-acting antiviral agent.
- •
HCV: Hepatitis C virus.
- •
INR: International normalized ratio of prothrombin time.
- •
LDV: Ledipasvir.
- •
LLOQ: Lower limit of quantification.
- •
MELD: Model for End-Stage Liver Disease.
- •
pTVR: Post-transplant virologic response.
- •
RBV: Ribavirin.
- •
SOF: Sofosbuvir.
- •
SVR: Sustained virologic response.
This analysis, as well as the SOLAR-1 and SOLAR-2 clinical trials, were funded by Gilead Sciences, Inc.
Author ContributionsJohn McHutchison and Diana Brainard conceived and designed the study. All authors were involved with data generation, collection, assembly, analysis, and/or interpretation as well as with drafting or revising the manuscript. All authors approved the final version of the manuscript.
Conflicts of InterestEric Yoshida is an investigator of clinical trials sponsored by Gilead Sciences, Inc., AbbVie, Merck & Co., Janssen Pharmaceuticals, Intercept, and Springbank and has received honoraria for lectures provided for Gilead Canada, Merck Canada, AbbVie Canada, and Celgene Canada. Kosh Agarwal has served as a consultant and speaker for AbbVie, Achillion Pharmaceuticals, Astellas Pharma, Bristol Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Intercept Pharmaceuticals, Janssen Pharmaceuticals, Merck & Co., and No-vartis and has received research grants from Bristol Myers Squibb, Gilead Sciences, and Roche. François Durand has received grants from Gilead France and Astellas France as well as honoraria for lectures provided for Gilead Sciences, Bristol Myers Squibb, and Astellas. Markus Peck-Ra-dosavljevic is an investigator for clinical trials sponsored by Gilead Sciences, Bristol Myers Squibb, Bayer, Lilly, Intercept Pharmaceuticals, and Arqle-Daichi and has received grants and honoraria from AbbVie, Bristol Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, and Merck & Co. Yves Horsmans has been a consultant for Gilead Sciences, AbbVie, Merck & Co., Janssen Pharmaceuticals, and Bristol Myers Squibb. Shampa De-Oertel, Sarah Arterburn, Hadas Dvory-Sobol, Diana M. Brainard, and John G. McHutchison are employees of Gilead Sciences and hold stock interest in the company. Beat Müll-haupt is an investigator of clinical trials sponsored by and has served as an advisor for Gilead Sciences, Inc., AbbVie, Merck & Co., Bristol Myers Squibb, and Intercept and has served as a consultant and received research grants from Gilead Sciences. Paul Kwo, Mario Rizzetto, Hugo Vargas, Norah Terrault, Robert Brown, Jr., Leslie Lilly, Bernard Willems, Christophe Duvoux, and Princy Kumar have no declared conflicts of interest.
This work was presented as a poster at the European Association for the Study of Liver, International Liver Congress, Barcelona, Spain, April 2016.
AcknowledgmentsThe investigators thank the patients who participated in the studies and their families. The investigators also thank the study coordinators, research assistants, and hepatology nurses at each site.