SERPINB3 (formerly known as squamous cell carcinoma antigen-1 or SCCA1) is a member of the family of serine-protease inhibitors. SERPINB3 protects cells from oxidative stress conditions, but in chronic liver damage this serpin may lead to hepatocellular carcinoma through different strategies, including inhibition of apoptosis, induction of epithelial to mesenchymal transition and decrease of desmosomal junctions, cell proliferation and invasiveness. SERPINB3 may also contribute to tumor cell resistance to anti-neoplastic drugs through its binding to the respiratory Complex I, protecting cells from the pro-oxidant action of chemotherapeutic agents. Mechanisms of tumor growth promotion induced by SERPINB3 include the inhibition of intratumor infiltration of natural killer cells, up-regulation of Myc oncogene and the recent identification of this serpin as a Ras-responsive factor. In the liver SERPINB3 and SERPINBB4 isoforms (known as squamous cell carcinoma antigen or SCCA) are undetectable in normal hepatocytes, but their expression progressively increases in chronic liver diseases, dysplastic nodules and hepatocellular carcinoma. High SERPINB3 levels have been recently detected in HCC tissue of patients with early tumor recurrence after surgical resection. In serum SERPINB3/4 isoforms (or SCCA) are detectable bound to IgMs (SCCA-IgM) in the majority of HCV infected patients with HCC and in patients with cirrhosis their levels and/or the progressive increase have been found correlated to the risk of HCC development. Preliminary findings in patients with HCC revealed that SCCA-IgM was predictive of HCC prognosis, since low levels of this biomarker were able to identify HCC patients with long overall and progression-free survival.
Hepatocellular carcinoma (HCC) is one of the most common forms of cancer and of cancer-related death in the world.1,2 HCC nearly always develops in the setting of liver cirrhosis and hepatitis B and C viral infections, alcohol abuse and metabolic syndrome are the main risk factors.3,4 The annual incidence of tumor development in cirrhotic patients varies from 1 to 6% and this wide range reflects differences in age, gender, etiology and duration of cirrhosis in the different studied groups.1 Advanced age and male sex have been found indeed independent risk factors for hepatocellular carcinoma development in patients with cirrhosis.5 HCC mortality index is very high, since most of the patients die within few years after diagnosis and less than 5% survive after five years.6 In addition, this tumor is extremely heterogeneous, due to the complex interplay between the biological characteristics of the tumor and the frequent presence of an underlying chronic liver disease. Despite intensive surveillance programs, considerable recent therapeutic advances and use of potentially radical treatments, prognosis and life expectancy remain still poor in this setting. Curative treatments are applicable for early stage tumors only and include resection, liver transplantation and percutaneous ablation, while transarterial chemoembolization (TACE) and sorafenib are regarded as non-curative treatments, able to improve survival in intermediate and advanced stages, respectively.7
Molecular mechanisms of liver carcinogenesisHepatocarcinogenesis is a multistep phenomenon and during the progression phase activation of cellular oncogenes, over-expression of growth factors, inactivation of tumor suppressor genes, miRNA deregulation and possibly telomerase activation, may contribute to the development of the neoplastic phenotype. In the last years data about molecular mechanisms of liver carcinogenesis, signal transduction pathways and potential therapeutic targets have been accumulated, providing new encouraging treatment options.8 At molecular level major classes of HCC, according to gene sets profiles responsible for cell proliferation and survival, have been recognized. Aberrant activation of several signaling cascades such as epidermal growth factor receptor (EGFR), Ras/ERK, PI3-K/mTOR, HGF, Wnt, Hedgehog and apoptotic signaling have been defined.9 Recent human studies have identified a molecular subclass (S1) of HCC associated with poor prognosis that is characterized by aberrant activation of Wnt signaling and TGF-beta activation. This peculiar S1 signature is characterized by overexpression of genes associated to epithelial-to-mesenchymal transition (EMT), a process originally described for embryo development and now believed to be involved in tumor invasion and metastasis and known to be regulated by TGF-beta in HCC.10
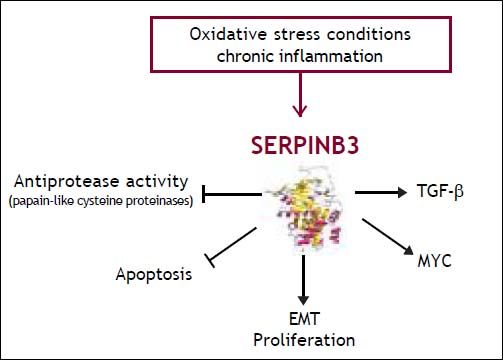
Serpinb3 and Liver CancerCarcinogenic potential of SERPINB3SERPINB3 (formerly known as squamous cell carcinoma antigen-1 or SCCA1) is a member of the family of serine-protease inhibitors (SERPINS).11 Available data suggest that this serpin may lead to hepatocellular carcinoma through different strategies (Figure 1). Initial studies indicate that SERPINB3 has an anti-apoptotic effect, since in cancer cells it was found to confer resistance to drug-induced apoptosis by inhibiting lysosomal cathepsin proteases12 and consequent inhibition of the release of mitochondrial cytochrome c. Under a variety of stress conditions this serpin also displays a protective role, with an anti-apoptotic function unrelated to its proteinase inhibition activity.13 Indeed, SERPINB3 protects cells from exposure to radiation through an inhibitory effect either on the MAP family kinase JNK14 or p38.15 More recent findings have demonstrated a novel mechanism of action of SER-PINB3, which could contribute to tumor cell resistance to anti-neoplastic drugs. This molecule was found indeed located in the inner mitochondrial compartments, where its binding to the respiratory Complex I protected cells from the toxicity of chemotherapeutic agents with a pro-oxidant action such as doxorubicin and cisplatin.16 The serpin reduced ROS generation induced by these compounds, a crucial step responsible for the opening of the mitochondrial permeability transition pore (PTP), irreversibly committing cells to apoptotic death.
In addition, SERPINB3 induces EMT and decrease of desmosomal junctions, leading to cell proliferation, increased number of colony formation in soft agar and cell invasiveness.17 In mice transgenic for SEPRINB3 lower expression of the p66shc gene, known as a signaling protein implicated in receptor tyrosine kinase signal transduction,18 has been described.19 Experimental studies have also reported that these transgenic mice showed higher liver regenerative potential compared to wild-type mice, supporting a role of this protein in promoting cell growth and proliferation.20 Further mechanisms of tumor growth promotion induced by SERPINB3 include the inhibition of intratumor infiltration of natural killer cells21 and the up-regulation of Myc oncogene transcription.22 Recent findings indicate that SERPINB3/SERPINB4 isoforms are a Ras-responsive factor that plays an important role in Rasassociated cytokine production and tumorigenesis.23
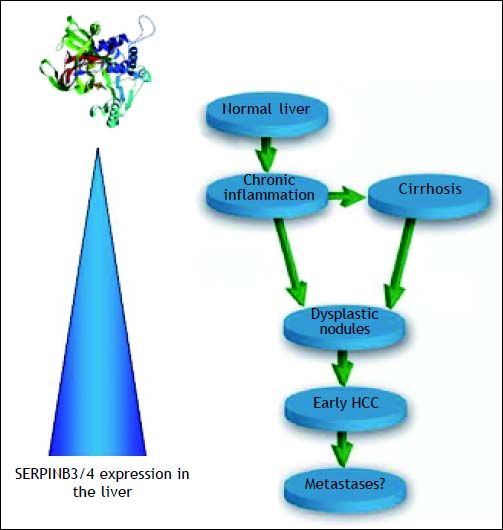
Expression of SERPINB3 in liver cancer tissueIn the liver SERPINB3 and SERPINBB4 isoforms (known as squamous cell carcinoma antigen or SCCA), are undetectable in normal hepatocytes, but their expression progressively increases in chronic liver diseases24 as a cellular response to chronic liver damage. Higher levels are detectable in dysplastic nodules25 and in hepatocellular carcinoma,26,27 suggesting that they may be also involved in relatively early events of hepatocarcinogenesis (Figure 2). Recent studies indicate that SERPINB3 is highly expressed in hepatic stem/progenitor cells of both foetal and adult livers.28 This compartment is composed by quiescent cells that proliferate under oxidative stress conditions. The occurrence of progenitor cell proliferation in humans has been described in the late stages of cirrhosis and tumors showing hepatic progenitor cell features have a worse prognosis and a higher recurrence rate compared to tumors lacking these characteristics.29,30 In agreement with these findings, high SERPINB3 levels have been detected in HCC of patients with early tumor recurrence after surgical resection.31 In this subset, a significant correlation of the serpin with over-expression of TGF-β and of β-catenin was typically found. Transcriptome data-metanalysis further supported these findings, showing accumulation of SERPINB3 in the S1 poor prognosis subclass.31 SERPINB3 has been detected recently also in hepatoblastoma, the embryonal tumor of the liver, where a direct correlation was observed between its gene expression, the up-regulation of Myc oncogene and tumor extension.22
Schematic diagram of the extent of SERPINB3 and SERPINB4 (SERPINB3/4) isoforms expression in the liver. Normal hepatocytes do not express this serpin and its expression progressively increases in relation to the extent of liver damage. The highest levels are detectable in dysplastic nodules and early HCC. No data are available to date on HCC metastases.
- •
HCC-diagnosis. On the basis of the oncogenic potential of SERPINB3 and of the reported findings of the presence of SERPINB3/4 isoforms (or SCCA) in the vast majority of HCCs specimens, in the last years ELISA assays have been developed to assess the presence of SCCA as free protein and/or as circulating immune complexes in serum.24,32 Free SCCA was not detected at significant levels in HCV infected patients with HCC, but this molecule was found coupled to IgMs (SCCA-IgM) to form circulating immunecomplexes in the majority of patients with HCC, whereas in the healthy control population their levels were below the limit of detection.24 The concentration of circulating SCCA-IgM, detected by a commercially available ELISA assay (SCCA-IC, Xeptagen), increased progressively at different stages of liver disease, from chronic hepatitis to cirrhosis and HCC, reflecting the extent of SCCA overexpression detected by immunohistochemistry in liver specimens. SCCA-IgM did not overlap with AFP, offering the possibility to increase the sensitivity for HCC detection without loosing specificity. The occurrence of this biomarker-IgM immune complex seems to be supported by the recent immunoediting model that considers natural IgMs as one of the most important players of the innate immune system.33 This pathway likely reflects host immune protective mechanisms trying to apply selective pressure on newly developed transformed cells in order to control tumor growth. The neo-epitopes, present on the tumor cells surface, recognized by commonly circulating natural IgM, are able to enhance phagocytic clearance of transformed cell by macrophages and dendritic cells.34 The possible interfering effect of rheumatoid factor in SCCA-IgM reactivity, found more frequently in HCV infected patients, has been excluded in artificially created samples, where the same results in terms of reactivity for SCCA-IgM were obtained, regardless of the presence of rheumatoid factor.35
- •
Fibrosis progression in chronic hepatitis. The behaviour of SCCA-IgM in serum over time has been also analysed in untreated patients with chronic hepatitis in relation to histological progression of the fibrosis stage.36 After a median period of 6 years a significant increase of SCCAIgM levels was observed in patients with histological fibrosis score increase > 2, but not in those without histologic deterioration. These findings suggest that monitoring SCCA-IgM levels over time might become a useful approach to identify patients with chronic hepatitis at higher risk for cirrhosis development.
- •
Antiviral therapy. In HCV infected patients treated with pegylated interferon and ribavirin a significant decrease in median SCCA-IgM levels at the end of treatment, persisting up to a year of follow-up, has been described in patients with sustained virologic response, both in patients with chronic hepatitis37 and with cirrhosis.38 No significant modifications were observed in non responder patients, indicating that SCCA-IgM monitoring in serum may be a reliable independent prognostic marker of therapeutic effectiveness in anti-HCV positive patients undergoing antiviral therapy.
- •
Risk of HCC development in patients with cirrhosis. The behaviour of SCCA-IgM in relation to HCC development has been evaluated in a cohort of HCV infected patients with cirrhosis prospectively followed up for a median period 4 years.39 The increase over time of SCCA-IgM, assessed within at least one year before clinical diagnosis of HCC, was remarkably higher in the group of patients who developed HCC than in patients who did not develop HCC during the same period of follow-up, while AFP increase was not significantly different. This initial study indicates that the assessment of SCCA-IgM behaviour over time might be useful to identify cirrhotic patients at higher risk of HCC development. These data have been confirmed in another retrospective study, addressed to evaluate whether the levels of SCCA-IgM in serum could identify HCV positive cirrhotic patients at risk of HCC development.40 The SCCA-IgM value ≤ 200 AU/ mL accurately identified patients at low risk of liver cancer in the subsequent year, with a negative predictive value of 97%. Considering an annual HCC incidence ≤ 3%, patients with SCCA-IgM ≤ 200 AU/mL had an HCC risk below the accepted threshold of a cost-effective surveillance (1.5%).41 If the results of this pilot study will be confirmed in larger studies, the authors propose that SCCA-IgM serum measurement might implement the current protocol of surveillance of cirrhotic patients,7 introducing a two step (with different costs) surveillance, consisting in an initial serological surveillance, based on the annual monitoring of this biomarker, and the conventional surveillance by semiannual ultrasound when SCCA-IgM becomes > 200 AU/mL. This proposal could improve the cost/effectiveness of surveillance of HCV infected patients at risk of HCC with an acceptable number of missed early diagnoses.
- •
HCC prognosis. In a recent study SCCA-IgM proved efficient in the prediction of HCC prognosis, identifying HCC patients with long overall and progression-free survival.42 Median survival was indeed 48 months (C.I. 29-66) for patients with low (≤ 130 AU/mL) SCCA-IgM and 26 months (C.I. 22-30) for those with high SCCAIgM (> 130 AU/mL). At multivariate analysis tumour size and SCCA-IgM levels were identified as the only independent predictors of survival. In addition, SCCA-IgM levels correlated with overall response to treatment (including surgery, TACE, percutaneous ablation), with a median time to progression of 14 months in patients with low SCCA-IgM, vs. 6 months in those with high SCCA-IgM levels. Additional studies however are required to confirm these preliminary data and to better assess the behaviour of this oncomarker in relation to different methodologies of HCC treatment.
I wish to thank my collaborators of the Molecular Hepatology Group (Alessandra Biasiolo, Santina Quarta, Mariagrazia Ruvoletto, Cristian Turato, Gianmarco Villano, Liliana Terrin, Natascia Tono, Andrea Martini, Davide Simionato), the colleagues of the Medical Clinic 5 and our Director Prof. Angelo Gatta, Giorgio Fassina and Andrea Gallotta (Xeptagen, Venice) for their important contributions to the knowledge of SERPINB3 in the biological and clinical fields of hepatology.
Abbreviations- •
EMT: epithelial-to-mesenchymal transition.
- •
HCC: hepatocellular carcinoma.
- •
SCCA: squamous cell carcinoma antigen.
- •
TACE: transarterial chemoembolization.