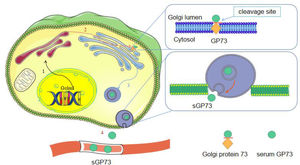
Golgi protein 73 (also known as GP73 or GOLPH2) is a transmembrane glycoprotein present in the Golgi apparatus. In diseased states, GP73 is expressed by hepatocytes rather than by bile duct epithelial cells. Many studies have reported that serum GP73 (sGP73) is a marker for hepatocellular carcinoma (HCC). For HCC diagnosis, the sensitivities of sGP73 were higher than that of other markers but the specificities were lower. Considering that the concentration of GP73 is consistent with the stage of liver fibrosis and cirrhosis, some studies have implied that GP73 may be a marker for liver fibrosis and cirrhosis. Increased sGP73 levels may result from hepatic inflammatory activity. During liver inflammation, GP73 facilitates liver tissue regeneration. By summarizing the studies on GP73 in liver diseases, we wish to focus on the mechanism of GP73 in diseases.
Golgi protein 73 (GP73) is a type II Golgi membrane protein with a molecular weight of 73 kDa that was first identified by Kladney et al. It is mainly expressed by bile duct epithelial cells in the normal liver [1]. Aberrant hepatocyte GP73 expression has been demonstrated in chronic liver diseases such as viral and non-viral hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) [1–2]. GP73 is related to the transforming growth factor-β(TGF-β)/Smad and PIK3-AKT signaling pathways in HCC [3–4] and is involved in the outcomes of patients with HCC [5]. Immune checkpoint inhibitors (ICIs) have the potential to treat biliary tract cancer and HCC [6]. However, without any other agents, ICI performs well in a very small group. This encourages the combination of immunotherapy with anti-angiogenic agents, which requires biomarker guidance. Hence, some emerging biomarkers got attention, including programmed death-1 and tumor mutation burden [7–8].
Serum GP73 (sGP73) is a component of GP73. In the diseased state, the C-terminus of GP73 is released into circulation [9]. Increased sGP73 was first found in HCC and has been regarded as a promising biomarker for HCC [10]. Considering that HCC is induced by viral infection or hepatitis, some scholars believe that GP73 alone may not be specific for diagnosing HCC [11–14]. It has been found that GP73 increases in prostate cancer [15–16], breast cancer [17] and bladder cancer [18]. Tissue GP73 is upregulated in prostate and bladder cancers, while sGP73 is upregulated in breast tumor [16–18]. sGP73 expression is significantly elevated in patients with acute hepatitis, but this elevation is reversible after disease remission [19]. Regardless of the cause of the injury, there is an increase in sGP73 [12]. Therefore, it is important to investigate the regulation of GP73.
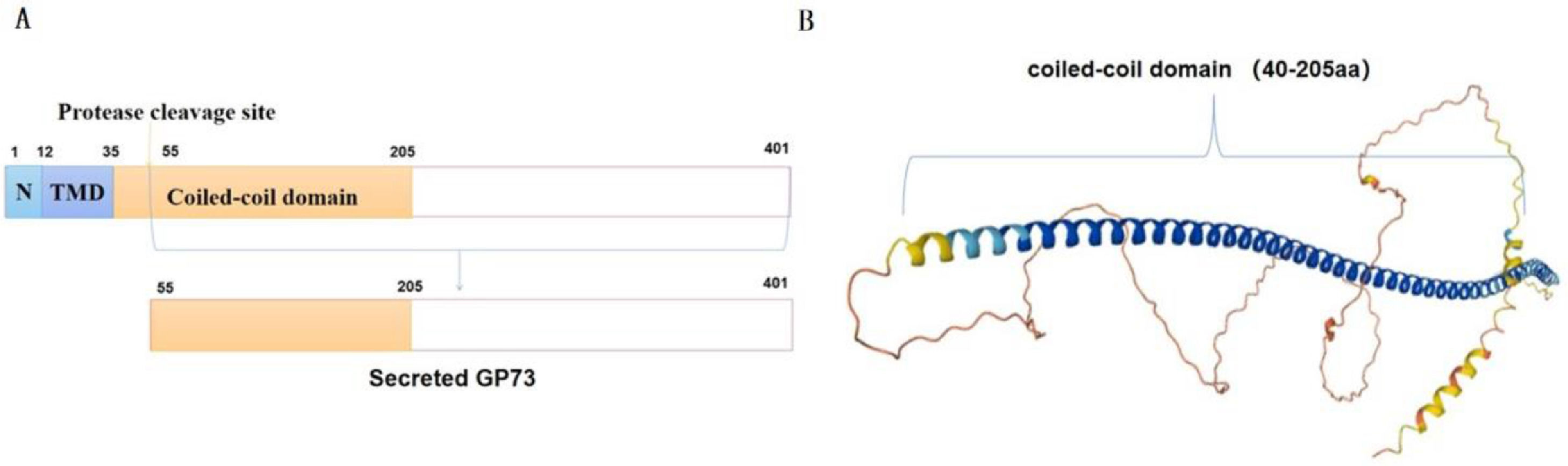
2Structure of GP73The coding gene for human GP73 is located on chromosome 9p21.33. It has a full-length sequence of 3080 bp, which includes a 1200 bp open reading frame. Human GP73 encodes 401 amino acids. It mainly contains an N-terminal domain, a transmembrane domain (TMD), and a coiled-coil domain. For GP73 transportation, the N-terminal domain and TMD are important, while the TMD also determines its localization [9]. The length of the TMD is a determinant of localization, and a 19-26 aa long TMD is sufficient to support Golgi localization, while a longer TMD facilitates transport to the plasma membrane. The shorter TMD of GP73 prevents it from entering. The coiled-coil domain not only facilitates transport to the plasma membrane but also mediates dimerization of GP73 [9]. Figure 1A shows the N-terminal, TMD, and coiled-coil domain. Based on the amino sequences of GP73, its tertiary structure was predicted and the coiled-coil domain was marked (Fig. 1B). The biochemical functions of GP73 remain unknown, and some studies have addressed the regulation of its expression. The coiled-coil structural domain is essential for binding, docking, and trafficking [20]. Therefore, focusing on the coiled-coil domain provides new insights into how GP73 enters the serum during disease.
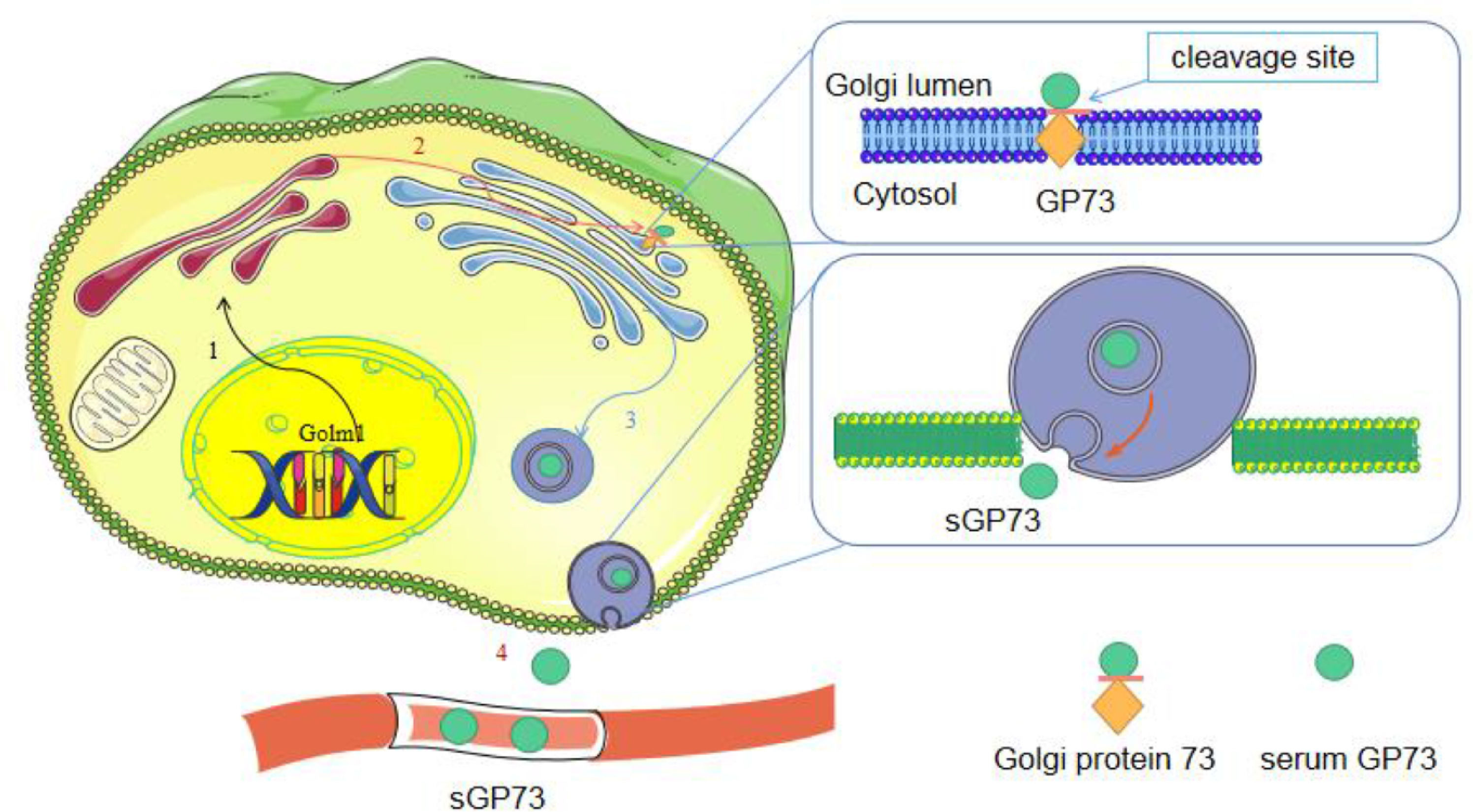
3Secretion and transportation of GP73Under normal conditions, the N-terminus of GP73 is cleaved by proteases and then transported to the plasma membrane, eventually returning to the Golgi via the late endosomal pathway [21]. The amino acid site at position R52VRR55 of GP73 contains a recognition site for preproteases, such as furin. In the presence of inflammatory factors, GP73 is cleaved by furin and transported from cis-Golgi to trans-Golgi [21]. Its C-terminal fragment is transported to the cytoplasm via vesicles and is eventually released extracellularly to produce sGP73 [9, 21] (Fig. 2). In response to endoplasmic reticulum (ER) stress, full-length GP73 and cleaved sGP73 are secreted into the extracellular space as signaling molecules, and then they interact with glucose-regulated protein 78 (GRP78) outside the cell surface of neighboring macrophages to activate ER stress signaling and induce the release of factors from macrophages [22]. This provides insights into the function of extracellular GP73 in the regulation of unfolded protein response (UPR)-triggered intercellular communication and identifies GRP78, which belongs to the UPR family, as its cellular receptor for intracellular signal transduction.
4Homology of GP73GP73 has no apparent sequence homology or structural similarity to any known nucleotides. We compared the cytoplasmic tails and transmembrane structural domains of GP73 in six model organisms, including human, mouse, rattus, Xenopus, Canis lupus familiaris, and gallus. Residues in the cytoplasmic tail were identical (Fig. 3A). The human TMD (residues 13‒35) includes 13 identical residues, nine highly conserved amino acid residues, no semi-conserved residues, and one relatively variable residue compared to the cytoplasmic tail of GP73 in the other five model organisms (Fig. 3B).
5GP73 is related to inflammation and liver regeneration5.1GP73 is related to inflammationGP73 levels increase in many diseases, including hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, non-alcoholic fatty liver disease (NAFLD), and other liver diseases. HCV is a chronic liver disease. Without treatment, the liver tends to be damaged. Upregulation of GP73 is observed in HCV [23–25]. In HCV, the coiled-coil domain plays an important role in the secretion of HCV, as it promotes HCV secretion. GP73 increases and interacts with lipoprotein E, a host factor for HCV secretion [24]. This evidence provides new insights into antiviral strategies. Zhang et al. [26–27] first demonstrated that overexpression of GP73 promotes the degradation of mitochondrial antiviral signaling protein (MAVS) and tumor necrosis factor receptor-associated factor 6 (TRAF6) through a protease-dependent pathway, resulting in the suppression of intrinsic host immunity and promotion of HCV infection. There may be some relationship between GP73 and inflammation, but there is no specific conclusion that explains the mechanism of GP73 regulation in an inflammatory environment. Interleukin 6 (IL-6) has been reported to be involved in this process.
IL-6 is an acute-phase response protein induced by acute inflammatory injury. IL-6 is not only involved in hepatocyte injury and repair but also promotes the proliferation of HSCs (hepatic stellate cells) [28]. Consistent with previous reports, IL-6 promoted GP73 expression in HepG2 cells in vitro. IL-6 promotes the transcription of GP73 and preprotein convertase furin by binding to the IL-6 receptor to activate the JAK/STAT3 signaling pathway, and then the GP73 is freed from the Golgi membrane by cleavage [29]. Clinical evidence shows that IL-6 levels progressively increase in HBV patients during disease progression [29], and the connection between GP73 and HBV has also been reported [14, 30]. Additionally, sGP73 levels are increased in patients with HBV [30]. Moreover, it has been suggested that GP73 promotes HBV infection by hindering the immune response [14]. Taken together, these results suggest that GP73 interacts with IL-6 to induce hepatitis development.
In patients with NAFLD, the expression level of GP73 was increased. GP73 weakens the export of very-low-density lipoprotein-apolipoprotein B from intrahepatic to extrahepatic tissues through its GTPase-activating protein activity, thus blocking the extrahepatic transport of triglycerides, cholesterol, and other lipids from the liver, thereby contributing to the development of NAFLD [31]. Iftikhar et al. detected GP73 expression in patients with liver diseases such as alcoholic liver disease and chronic hepatitis. It was found that GP73 levels in hepatocytes from alcoholic liver disease and chronic hepatitis were correlated with disease stages instead of grades [19]. GP73 expression in α-smooth muscle actin positive cells during liver injury suggests that GP73 originates from activated hepatic stellate cells (HSCs) [19]. It has been shown that the main triggers of GP73 expression are the inflammatory activities in acute liver diseases and hepatic fibrosis [32]. Increased sGP73 levels have been reported to be triggered under inflammatory conditions [33]. sGP73 positively correlates with liver disease progression [12-13, 34]. These findings suggested that hepatic inflammatory activity may be a major driver of increased sGP73 levels.
5.2GP73 is related to liver regenerationRegardless of the etiology, there is overwhelming evidence linking GP73 to cell proliferation [35–37]. Thus, it is reasonable to speculate that GP73 may play an active role in liver regeneration. Wang et al. [38] reported that regenerating livers from GP73 knockout mice exhibited a less pro-inflammatory environment when performing partial liver resection. In the early stages of liver regeneration after partial liver resection, GP73 deletion inhibited residual hepatocyte proliferation and liver inflammation, however, the liver mass recovered more rapidly. This difference in recovery was mainly due to compensatory hypertrophy of hepatocytes, in which the mTOR signaling pathway plays an important role. Regenerating livers from GP73−/− mice exhibited a less pro-inflammatory environment than GP73+/+ mice because of the low expression of inflammatory cytokines in GP73−/− mice [38]. This study revealed a novel function of GP73 in liver regeneration, in which GP73 facilitates the regeneration of liver tissue and inflammation during the process. After partial hepatectomy, the absence of GP73 promoted hepatocyte hypertrophy through the activation of the mTOR signaling pathway.
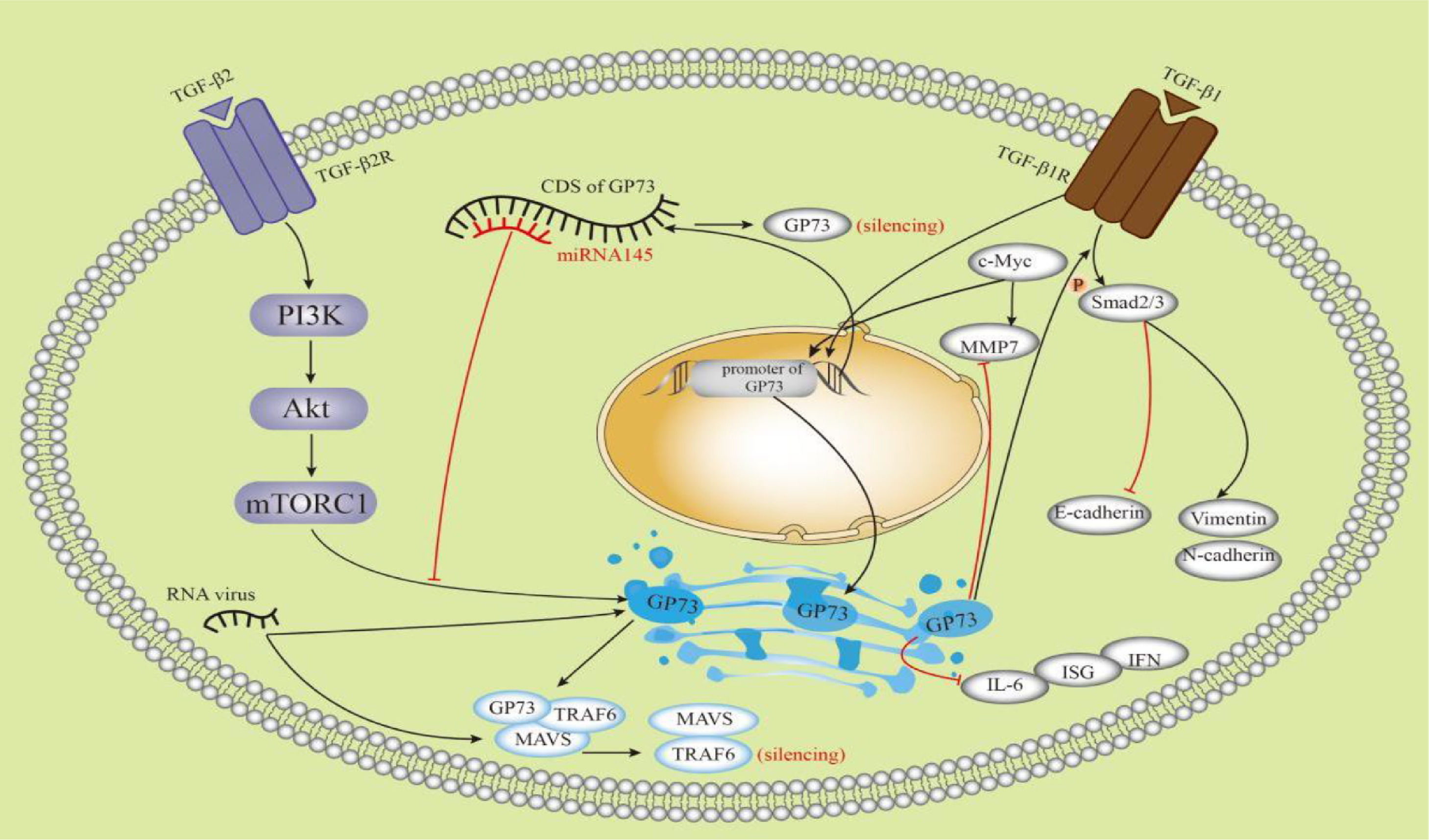
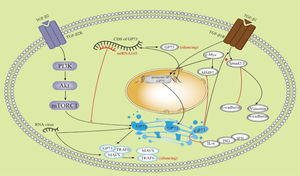
5.3GP73 is bound with HCCGP73 has been considered a promising biomarker for HCC since its upregulation was discovered in HCC patients [39–42].Before the discovery of GP73, alpha-fetoprotein (AFP) was regarded as a useful marker for HCC. Serum AFP levels and abdominal ultrasound have been widely used for the diagnosis and surveillance of HCC [43]. GP73 increases the secretion of AFP through direct binding to AFP both in vivo and in vitro, thereby promoting the proliferation and metastasis of HCC cells that express AFP and its receptor. Extracellular secretion of GP73 is required for this process [44]. GP73 is used as a screening tool for the detection of HCC, with a higher diagnostic performance than AFP [41]. The diagnostic sensitivities for HCC, as detected using time-resolved fluorescence immunological assays of GP73, AFP, and γ-glutamyl transferase isoenzyme II (GGT-II), were 73.4, 55.6 and 68.4%, respectively, and the specificities were 80.0, 86.7 and 97.1%, respectively. The combination of these markers increased the diagnostic sensitivity to 96.3% for HCC [45]. It has been shown to GP73 interacts with the TGF-β/Smad signaling pathway [4], metalloproteinases (MMPs) [35–36], CD44 [46], c-Myc [35] and other factors in HCC and plays a role in the development of HCC; for example, GP73 partially regulates epithelial mesenchymal transition(EMT) and metastasis in HCC by targeting TGF-β1/Smad2 signaling [4, 37]. In HepG2 cells, GP73 decreased the expression of the epithelial marker E-cadherin and increased the expression of mesenchymal markers, such as N-cadherin and Vimentin. Apart from TGF-β1/Smad signaling, GP73 may be involved in regulating other EMT pathways [37]. Futhermore, Chen et al. have shown that mTOR promotes HCC development by stimulating the expression of GP73, which is possibly involved in the PIK3-AKT signaling pathway [3]. GP73 enhances the invasion of HCC cells by upregulating MMP-13 [36]. GP73 promotes tumor progression through a combination of multiple factors in multiple pathways. As shown in Figure 4, some studies involved in the interactions between GP73 and other factors were illustrated. GP73 is a transcriptional target of the TGF-β1/Smad signaling pathway, which directly activates p-Smad3 and upregulates GP73 [37]. Second, mTOR complex 1 upregulates GP73 expression [3]. Third, upregulation of c-Myc promotes transactivation of GP73. However, GP73 overexpression reduces MMP7 [35]. microRNA (miRNA)-145 directly targeted the coding sequence region of GP73 and then abolished GP73 [47]. Overexpression of GP73 promotes degradation of MAVS and TRAF6 and downregulates interferon-β, interferon-λ1, IL-6, and immune serum globulin 56 [26–27]. High levels of sGP73 are associated with aggressive tumor behavior and poor disease-free survival and overall survival [48]. It has been suggested that sGP73 has important prognostic value for HCC. GP73 has been shown to promote proliferation, migration, invasion, and metastasis of HCC cell lines and xenograft tumors in mice [3].
Schematic diagram showing the multiple factors connected with GP73. TGF-β1, transforming growth factor-β1; TGF-β2, transforming growth factor-β2; mTORC1, mTOR complex 1; MMP 7, matrix metalloproteinase-7; CDS, coding sequence; MAVS, mitochondrial antiviral signaling protein; TRAF6, TNF receptor-associated factor 6; IL-6, interleukin 6; ISG, IFN-stimulated gene.
The differential expression of miRNAs in HCC suggests that miRNAs can be used as new potential targets, either as carcinogens or tumor suppressors. Gai et al. found that miR-145 inhibits HCC development and metastasis by binding to GP73 mRNA [47]. Zhao et al. [49] revealed that miR-493-5p inhibited HCC cell proliferation and induced cell cycle arrest and apoptosis by directly targeting GP73. Hou et al. [50] proposed that miR-141-3p inhibits EMT in HCC cells by promoting mesenchymal-to-epithelial transition through GP73. Overall, some miRNAs were involved in the expression of GP73. Therefore, miRNA expression may be crucial in HCC. However, the mechanism by which GP73 promotes tumor progression remains unclear. Upregulation of sGP73 expression occurs not only in patients with HCC but also in patients with prostate cancer [15], bladder cancer [18], breast cancer [17] and gastric cancer [51]. Thus, as a serum marker for diagnosing other cancers, the levels of GP73 expression and other biomarkers should be considered.
5.4GP73 is linked with cirrhosis and hepatic fibrosisAs some HCC cases are accompanied by cirrhosis, research suggests that sGP73 may be unsuitable for diagnosing HCC [11, 32, 52]. Cirrhosis is an end-stage manifestation of diffuse liver injury. Usually, it occurs by two mechanisms: (1) the formation of regenerated nodules of hepatocytes. (2) proliferation of fibrous tissue and reconstruction of liver circulation [53]. It is a long and complex pathological process with the development of hepatitis to hepatic fibrosis, cirrhosis, and HCC. In the course of chronic liver disease due to different etiologies, sGP73 levels gradually increase with the progression of liver fibrosis and development of cirrhosis. A previous study reported that the concentration of GP73 was consistent with the stage of liver fibrosis and cirrhosis [54]. Through case review analysis, it has been demonstrated that GP73 alone is an effective marker of cirrhosis. It is superior to the aspartate transaminase to platelet ratio index (APRI) assay. Its diagnostic performance was further improved when used in combination with the APRI [55]. Although the function of GP73 remains unclear, it has been reported that GP73 expression represents the degree of liver fibrosis because of its expression in activated HSCs [19].
Hepatic fibrosis is a reversible wound healing response characterized by the accumulation of extracellular matrix or “scar,” that follows chronic but not self-limited liver disease. Activation of HSCs is the central event in liver fibrosis [56–57]. Other liver-specific biomarkers, such as hyaluronic acid, laminin, and collagen type III, have also been reported to be positively correlated with GP73, indicating their potential roles in liver fibrosis [55]. During the regression of liver fibrosis, upregulation of GP73 is reversible [19], implying that sGP73 may be a promising marker for monitoring the progression of liver fibrosis.
As mentioned above, for HCC diagnosis, the sensitivity of sGP73 is higher than the sensitivities of AFP and GGT-II but the specificity is lower. Combining sGP73 and AFP with GGT-II can improve the sensitivity [45]. Therefore, sGP73 alone may be unsuitable for HCC treatment. Although sGP73 is better than APRI for diagnosing cirrhosis, its combination improves significantly [55]. Thus, altogether, sGP73 is beneficial in identifying patients at a high risk of disease progression.
6ConclusionsClinically, GP73 is widely used to aid the early diagnosis and postoperative monitoring of HCC. Therefore, most of the current studies of GP73 upregulation are devoted to HCC. The application of GP73 as a serum marker of liver disease is promising. An increasing number of studies have demonstrated its close association with liver fibrosis, cirrhosis, and HCC. However, it is not ideal if GP73 is not associated with other serum markers for diagnosing these diseases.
This review summarizes the recent studies on GP73 in HCC and alternative diseases, including hepatitis, liver fibrosis, and cirrhosis. Here, we conclude with existing reports on GP73 regulation, offering a preliminary understanding of this hot spot. We hope that this will provide some help for the foundation of GP73 function in different diseases. As it may originate from HSCs, it is speculated that GP73 could play a key role in liver fibrosis. Thus far, studies on GP73 in liver fibrosis and cirrhosis are scarce and not well elucidated.
Most of these reports focused on the correlation between upregulation of sGP73 and the occurrence or severity of diseases, but only a few studies have investigated the underlying mechanisms by which GP73 contributes to diseases. Although the related signaling pathways have not been fully discovered, studies on GP73 and the PI3K -Akt signaling and TGF-β/Smad signaling pathways may provide an entry point to investigate the mechanism of GP73 in diseases. However, the mechanisms of GP73 regulation and secretion are not well established, and those of inflammation and liver regeneration remain to be explored. Addressing these issues may provide new directions for the diagnosis and treatment of liver disease.
Authors' contributionJFW and RTZ contributed to the conception and design of the study. MYL and LH contributed to drafting the article or revising it critically for important intellectual content. WBA and HBZ contributed to final approval of the version to be submitted.
FundingThe present study was supported by the National Natural Science Foundation of China (grant no. 81670555). This work was supported by the National Natural Science Foundation of China (grant no. 81730078) and the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (2021-1-I2M-018).