Cholangiocarcinoma (CCA) is a heterogeneous group of neoplasms of the bile ducts and represents the second most common hepatic cancer after hepatocellular carcinoma; it is sub-classified as intrahepatic cholangiocarcinoma (iCCA) and extrahepatic cholangiocarcinoma (eCCA), the latter comprising both perihilar cholangiocarcinoma (pCCA or Klatskin tumor), and distal cholangiocarcinoma (dCCA).
The global incidence of CCA has increased worldwide in recent decades. Chronic inflammation of biliary epithelium and bile stasis represent the main risk factors shared by all CCA sub-types.
When feasible, liver resection is the treatment of choice for CCA, followed by systemic chemotherapy with capecitabine. Liver transplants represent a treatment option in patients with very early iCCA, in referral centers only. CCA diagnosis is often performed at an advanced stage when CCA is unresectable. In this setting, systemic chemotherapy with gemcitabine and cisplatin represents the first treatment option, but the prognosis remains poor.
In order to ameliorate patients’ survival, new drugs have been studied in the last few years. Target therapies are directed against different molecules, which are altered in CCA cells. These therapies have been studied as second-line therapy, alone or in combination with chemotherapy. In the same setting, the immune checkpoints inhibitors targeting programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic T-lymphocyte antigen-4 (CTLA-4), have been proposed, as well as cancer vaccines and adoptive cell therapy (ACT). These experimental treatments showed promising results and have been proposed as second- or third-line treatment, alone or in combination with chemotherapy or target therapies.
Cholangiocarcinoma (CCA) is a heterogeneous group of neoplasms of the bile ducts [1] and represents the second most common hepatic cancer after hepatocellular carcinoma (HCC). It has been reported as a rare disease in Western countries, accounting for less than 1% of all human cancers, and around 10–15% of all primary liver cancers, and it is mostly diagnosed in the seventh decade with a small male predominance (male:female ratio of 1.2—1.5:1.0) [2]; however, over the past few decades, its overall incidence has increased worldwide and in the last 10 years, the incidence rate of intrahepatic CCA increased rapidly by 109%, from 0.67 per 100 000 in 2007 to 1.40 per 100 000 in 2016 [3].
CCA is classified as intrahepatic cholangiocarcinoma (iCCA), originating from the intra-hepatic biliary tree and accounting for 10%–20% of cases, and extrahepatic cholangiocarcinoma (eCCA), outside the liver parenchyma, the latter comprising both perihilar cholangiocarcinoma (pCCA or Klatskin tumor), the most frequent type, accounting for 50% of cases, and distal cholangiocarcinoma (dCCA) observed in 30%–40% [4].
This neoplasm still shows a high mortality rate due to its aggressiveness, late diagnosis, and immunoregulation capacity [5]. It is rarely diagnosed at an early stage owing to its silent clinical course, lack of biomarkers, difficult-to-access anatomical location, and highly desmoplastic and paucicellular nature. Therefore, in only about a third of cases, the tumor can be completely removed by surgery, while in other cases, systemic chemotherapy is usually the first-line treatment option. However, new emerging therapies are under evaluation for unresectable advanced disease. In fact, the molecular characterization and immunological analysis of these neoplasms, as well as the identification of the possible crucial role of intestinal microbiota, may open other horizons for novel therapeutic strategies, such as immunotherapy, to enhance the overall survival of these patients, for whom the late diagnosis often results in limited therapeutical options and poor clinical outcomes and survival rates.
2EpidemiologyDisease incidence and prevalence, as described by epidemiology, reflect population risk factors in different geographical areas. As for other diseases, the epidemiology of CCA is closely linked to natural and human environment changes, thus helping understand a disease about which little is known.
CCA is an emerging cancer worldwide, presenting some challenging issues and potential biases in defining its epidemiology and risk factors, with consequences on understanding etiopathogenesis and public health policy [6].
Defining CCA epidemiology is even more complex when considering its three subtypes , each exhibiting different risk factors and prevalence rates [7]. It has been observed that in recent years iCCA has shown a stable rate in Countries where a reduction in alcohol-related chronic liver disease and cirrhosis is observed. In contrast, the same form of CCA showed an increasing rate in European and American countries that experienced an increase in alcohol consumption, Hepatitis C Virus (HCV) infections, obesity, and non-alcoholic fatty liver disease (NAFLD) [8].
The latest age-standardized incidence shows an increase in iCCA and a decrease in eCCA [7], not discriminating in the last group between pCCA and dCCA forms. Indeed, the International Classification of Diseases (ICD) has long lacked a separate code for pCCA, and the previous version of the ICD for Oncology (ICD-O) identified pCCA as an intrahepatic subtype. Only classifications that came into effect in 2021 for ICD-11 and ICDO-4 provide separate codes for iCCA, pCCA and dCCA [9]. A recent retrospective review of 625 hepatobiliary cancers from three regional centers in the United Kingdom shows that only 43% of CCAs coded as intrahepatic, according to ICD-10, were true iCCAs, while 92% of those that were truly pCCAs were coded as iCCAs [10]. This miscoding of the perihepatic form might make the rising rate of iCCA merely apparent.
Regardless of methodological issues in retrieving and elaborating data, the global incidence of CCA has increased worldwide in recent decades (0.3-6 per 100,000 population per year), according to statistics available through 2020 [7].
This trend may also be explained by improved diagnostic techniques (radiological, endoscopic, and histological), increased disease awareness among physicians of this malignancy, and wider acceptance by the patients of liver biopsy, all of which contribute to achieving a diagnostic confirmation of primary liver lesions [7].
However, epidemiologic data should not be based solely on biopsy-proven cases, as there is substantial variation in the histologic or cytologic diagnosis rates of CCA cases reported in international registries. Of note, Thailand has one of the lowest rates of microscopic diagnosis for CCA and liver disease in general, despite its very high incidence of CCA. Finally, it should be noted that even in countries with larger histology-driven diagnoses, sampling issues occurring for many cases and the lack of specific radiological diagnostic criteria are possible explanations for underestimating the incidence of CCA.
CCA mortality has also increased worldwide, according to 2020 data: 1-6 per 100,00 inhabitants per year, without taking into account the Asian regions with the highest incidence (> 6 per 100,000 inhabitants per year) [7].
Mortality data also differ among subtypes: a 2019 study (limited by using only the classification into eCCA and iCCA) showed an increase in mortality for iCCA and a decrease for eCCA, considering countries with acceptable data. Regarding mortality, confounding factors, such as misdiagnosis between iCCA and HCC in cirrhotic patients who do not always undergo a biopsy, should be considered [8]. In addition, the reduction in eCCA mortality could be partly explained by the increase in the number of cholecystectomies that are now performed laparoscopically, assuming that gallstones represent an important risk factor for eCCA [11]. Therefore, in the coming years, new ICD classifications and an increasing understanding of risk factors will enable us to obtain more accurate epidemiological data for this cancer, which will be extremely useful in better understanding its etiopathogenesis and improving diagnostic and therapeutic strategies.
3Established and emerging risk factors for CCAThe considerable geographical and overtime variation in the epidemiology of CCA reflects a complex landscape of multiple and evolving risk factors driving cholangiocarcinogenesis.
Although some risk factors are shared by all forms of CCA, others seem to be more specific to distinct subtypes and more important in certain regions. A commonly shared characteristic among risk factors is the chronic inflammation of the biliary epithelium and bile stasis [7]. The trigger of chronic biliary inflammation varies across different geographic areas in which viral and parasite infections and predisposing environmental conditions show different prevalence rates. Despite this, most cases are sporadic and occur without any accepted or known risk factors. Thus, the adoption of a surveillance protocol is limited, and early diagnosis remains a challenge [12].
3.1Chronic biliary diseasesIn Western countries, Primary Sclerosing Cholangitis (PSC) is one of the most well-known risk factors for CCA [13]. PSC is a rare immune-mediated disease of the biliary tree leading to a fibroinflammatory obstruction of bile ducts, chronic cholestasis, and progressive liver failure. Previous studies have reported an up to 400‐fold higher risk for CCA in PSC patients compared to the general population with the co-existence of inflammatory bowel disease (IBD) further increasing the risk of malignancies with a significant impact on the patient's prognosis [14,15]. While several recommendations have been published for surveillance of CCA in PSC, there is no common consensus on the best prevention strategy, even if performing scheduled imaging techniques is associated with better survival [16–18].
Choledochal cysts are a rare congenital disorder characterized by the development of cystic dilatation of the biliary tree. The correlation between bile duct cysts and CCA is well established with an overall increased risk for both iCCA and eCCA with an estimated Odds Ratio (OR) of 26.71 (15.80–45.16) and 34.94 (24.36–50.12), respectively [12]. Caroli's disease is the choledochal cyst phenotype more frequently complicated by CCA, with a 38‐fold higher risk for the iCCA and a 97‐fold higher risk for the eCCA subtype [19]. In addition, when the risk factor is congenital, the development of CCA can occur even in the first decades of life, with its onset at a mean age of 32 years [20].
The most prevalent risk factor for the development of CCA in East Asia involves parasitic infection, specifically with Opisthorchis viverrini or Clonorchis sinensis (also called liver flukes) [21], which have been listed as group 1 biological carcinogens by the International Agency Research on Cancer (IARC) [22].
Liver fluke infections are associated with an up to the 5-fold risk of CCA development in endemic areas [23]. Despite anti‐helminthic effective treatment, chronic infections, and possible re-infections after consumption of undercooked freshwater fish carrying the larval parasite lead to the development of CCA in up to 10% of people [24].
While the association of intrahepatic biliary lithiasis (or hepatolithiasis) with a higher risk of iCCA has been well documented, especially in East Asia cohorts [25], the risk of CCA related to cholelithiasis and choledocholithiasis is more controversial. However, a recent meta-analysis confirms a significant association of these conditions with eCCA with a pooled OR of 18.58 (95%CI 11.07-31.18) and 2.11 (95%CI 1.64-2.73) for choledocholithiasis and cholelithiasis respectively [12].
3.2Chronic liver diseasesiCCA is a primary liver cancer and shares several similar underlying risk factors with HCC. Cirrhosis is a well‐established risk factor for HCC, with >90% of HCCs developing in cirrhotic patients [26]. In a meta‐analysis of fourteen case‐control studies, cirrhosis was also identified as a strong risk factor for iCCA (OR 15.32 (95%CI 9.33-25.15) [12]. Also, chronic viral infections such as chronic hepatitis B virus (HBV) and HCV infection may also represent a risk factor for CCA development, with a stronger association for iCCA (OR 4.57 (95% CI 3.43–6.09) and OR 4.28 (95% CI 2.98–6.16) respectively) [12]. Notably, the increased risk of CCA among HBV and HCV patients likely relies not only on the presence of cirrhosis but also on a direct carcinogenic effect of these viruses on target cells [27]; moreover, chronic liver inflammation resulting from virus infection triggers cellular proliferation, thus increasing the risk of malignant transformation [27].
NAFLD encompasses a spectrum of liver diseases ranging from steatosis to non-alcoholic steatohepatitis (NASH) and cirrhosis. While NAFLD/NASH has been identified as a risk factor for HCC, a positive association between NAFLD and CCA has also been suggested, especially for iCCA [28]. Indeed, in the last years, epidemiological studies have provided evidence that some metabolic disorders may predispose to primary liver cancers [29].
A positive association between type II diabetes and both CCA cancer types, especially iCCA, has also been reported (OR of 1.73 (95% CI 1.47–2.04) [12]. Moreover, a potential protective role of metformin for the development of CCA has been found with an OR of 0.4 (95% CI 0.2‐0.9) in patients under treatment [30].
3.3Lifestyle and professional exposureAlcohol consumption is a well-known risk factor for HCC [31]; conversely, its association with CCA has been less investigated. A recent large metanalysis using data from 15 and 11 case-control studies comprising 13,986 and 8,293 cases, respectively for iCCA and eCCA showed an increased risk of development in alcohol consumers that was higher for iCCA with respect to eCCA (OR 3.15 (95% CI 2.24–4.41) and 1.75 (95% CI 1.20–2.55), respectively) [12]. Similarly, smoking habits have been associated both with iCCA and eCCA (OR 1.25 (1.05–1.49) and 1.69 (1.28–2.22), respectively) [12]. The carcinogenic effect may be secondary to the excretion of the metabolized carcinogenic compounds (e.g., benzopyrene, formaldehyde, benzene and chromium) by hepatic microsomes to the bile.
Given the increasing prevalence of obesity and cardiovascular disease in Western countries, their putative role as a predisposing factor of CCA has been hypothesized. However, neither hypertension nor obesity has shown a statistically significant association with iCCA (OR 1.1, 95% CI 0.89–13.7 and OR 1.14, 95% CI 0.93–1.39, respectively) or eCCA, (OR 1.21, 95% CI 0.77–1.90 and OR 1.20, 95% CI 0.84– 1.70, respectively) [12].
Based on the evidence of the female predominance of gall bladder cancer (GBC) compared to other biliary tract cancers (BTCs) with a male predominance, the role of sex hormones has been suggested to be involved in cholangiocarcinogenesis. Indeed a recent case-control study reported an increased risk of gall bladder disease (GBD) associated with the use of orally-administered combined menopausal hormone therapy particularly [32].
Recently, a link between asbestos exposure and CCA has been provided in two different case-control studies. These findings have been confirmed in a population‐based case-control study on the Nordic Occupational Cancer cohort, where an increased risk of iCCA, but not of eCCA, was observed by cumulative exposure to asbestos [33,34].
3.4Genetic factorsInformation on the predisposing genetic risk factors causing CCA is currently scarce [7]. The available data are mostly available from the Genome-wide Association Study (GWAS) of patient cohorts diagnosed with PSC, which have an increased risk of CCA [35,36]. A detailed genetic signature investigated by genome sequencing has been found in CCAs caused by liver fluke infection. These tumors showed an overall higher mutational rate (median 4,700 versus 3,143 somatic mutations per tumor) with prevalent mutations in SMAD4 and TP53 as well as ERBB2 amplifications [37]. Additionally, the fact that all CCA cases cannot be explained by the currently identifiable and established risk factors may mean a significant genetic component to CCA pathogenesis, which will require identification via whole-genome sequencing [38].
4Surgical treatmentThe standard treatment for CCA is liver resection. Hepatobiliary resection is, in fact, indicated whenever it is feasible and is reserved for iCCA and pCCA [39], while the diagnostic work-up and treatment of dCCA are more similar to cancer of the head of the pancreas. The goal of the surgery is to achieve a radical (R0) resection while preserving an adequate function of the future liver remnant (FLR). Surgical resection is the only potentially curative option for CCA. Survival after resection ranges between 25%–40% at 5 years [40]. An important aspect to keep in mind is that it might be difficult to accurately assess resectability preoperatively as conventional radiology can underestimate the actual extent of the disease, and the definitive decision to resect or not is generally made at surgical exploration. A staging laparoscopy to rule out undetected liver or peritoneal metastases is clearly recommended with a reported yield above 20% [41].
Resectability depends on two main variables: the location of the tumor lesion, including its relationship with intrahepatic vascular and biliary structures, and the amount and quality of the liver parenchyma remaining after tumor resection. In terms of the type of surgical intervention, an R0 surgery for pCCA consists of an extended hemi‐hepatectomy with resection of the extrahepatic bile duct, which is considered a major surgery and requires a good performance status of the patient together with a proper pre-operative evaluation of the volume and function of the FLR. The majority of patients with iCCA have a single large tumor that requires, similarly to pCCA, an extended hemi-hepatectomy. Conversely, the results of a minimal‐invasive resection for CCA are generally disappointing [42].
In the presence of nodal involvement beyond the hepatoduodenal and gastrohepatic ligament, the benefit of surgery decreases, due to a reported high recurrence rate [43], even though controlling disease in the liver also with a no curative surgery can improve survival, as many patients risk to die of liver insufficiency [39].
In patients with normal liver parenchyma, a “safe” liver resection should leave an FLR of at least 25%, while a FLR of at least 30% to 40% needs to be considered in livers affected by steatosis, chronic cholestasis, cirrhosis or treated with chemotherapy [44]. FLR function can be assessed with the indocyanine green test together with the hepatobiliary scintigraphy. In those cases where the FLR is insufficient, strategies to increase the FLR should be considered in order to avoid post‐hepatectomy liver failure and these include portal vein embolization, generally indicated when the FLR is below 30%‐40% [45] and associating liver partition and portal vein ligation (ALPPS) for staged hepatectomy; in detail, during the first procedure of ALPPS, the liver parenchyma is transected with portal vein ligation of the liver with the tumor and in the second stage, after the FLR has become hypertrophic, involves the resection [46,47].
It is worth mentioning that lymphadenectomy of locoregional lymph nodes in the hepatoduodenal ligament is generally recommended and at least six locoregional lymph nodes should be sampled [48]. Lymph node metastases can be found at clinical diagnosis in up to 45%-65% of patients with iCCA, significantly impacting survival: 5-year overall survival (OS) is approximately 0%-20% in pN1 patients versus 35-50% in pN0 patients [49]. However, no solid evidence exists on the survival benefit provided by a systematic lymphadenectomy, and available studies in this regard are retrospective and mostly unmatched [50]. According to the available series, an aggressive surgical treatment including extended lymphadenectomy might improve survival despite the presence of lymph node metastases. Conversely, whether adequate lymphadenectomy might be beneficial in the absence of lymph node metastases is still a matter of debate; as no clear-cut data suggests that lymphadenectomy could prevent local nodal recurrences and considering the related morbidity, each case should be discussed by a hepato-biliary multidisciplinary team with specific attention to more fragile patients (i.e., cirrhotic patients).
Some procedures might be necessary before surgery. First, obstructive cholangitis is an absolute indication of preoperative biliary drainage (PBD) with approximately 15% of iCCA showing biliary obstruction requiring PBD. As liver resection in jaundiced patients is considered to be at higher surgical risk, PBD in jaundiced CCA patients is generally performed [51]. However, PBD is often associated with infectious complications, and cholangitis is an independent prognostic factor for post-operative mortality [51]; thus, the decision on making PBD should be carefully balanced. As in patients with an insufficient FLR (i.e., below 40%), biliary obstruction impairs liver regeneration, PBD of the FLR is generally suggested before portal vein embolization. Percutaneous transhepatic biliary drainage (PTBD) and endoscopic biliary drainage (EBD) are the two available procedures to drain the bile ducts, but in the absence of large randomized controlled trials, there is no definitive evidence to recommend one procedure over the other [39]. Furthermore, in patients with biliary obstruction, resection of the biliary confluence is indicated, followed by a roux‐Y hepaticojejunostomy.
Some patients might require neoadjuvant chemotherapy, although the actual benefit of preoperative chemotherapy is still unclear. According to the preliminary findings of the phase II trial of the NACRAC study [52], in 24 patients with borderline or unresectable pCCA, pre-operative chemotherapy might enhance R0 resection, while it is still to be clarified whether it also improves survival. On the other hand, no randomized controlled trials are available regarding the role of preoperative systemic chemotherapy for patients with resectable or unresectable iCCA and, according to some studies, preoperative chemotherapy does not affect OS when compared with patients undergoing upfront surgery [53]. However, the administration of chemotherapy in the pre-surgical setting might allow the downstaging from unresectable to resectable forms in selected cases.
Regarding the role of systemic chemotherapy with gemcitabine and cisplatin in the adjuvant setting, there are inconsistent findings so far. According to a large Japanese randomized controlled trial [54] including a total of 508 patients with pancreaticobiliary tumors of whom 118 with CCA, adjuvant chemotherapy did not improve OS. The BILCAP trial [55] included 447 patients with biliary cancer, of whom 84 with iCCA, compared adjuvant capecitabine with observation and found a median OS of 51 months with capecitabine versus 36 months with observation (P = 0.097). Conversely, the Prodige-11 trial [56], which compared adjuvant gemcitabine plus oxaliplatin versus observation after resection of biliary cancer, found no survival benefit. A benefit in survival for patients treated with adjuvant chemotherapy has been reported in the presence of positive lymph nodes and/or R1 resection [57–59]. However, based on available studies, it is still not possible to make a clear-cut, evidence-based recommendation, also considering that the majority of trials include all patients with biliary tumors [39].
4.1Liver transplantA specific mention needs to be made regarding the role of liver transplantation (LT) for pCCA and iCCA, which should be reserved for unresectable CCA with no evidence of extrahepatic disease. The rationale of LT in this setting is, indeed, to avoid an R1 resection and an inadequate FLR. In addition, LT allows removing of underlying liver disease including liver cirrhosis and PSC. iCCA has been a recognized contraindication for LT due to very poor initial results with a 2-year survival of around 30% [60–62].
The concept of LT for CCA has started to change since specific selection criteria, as well as neoadjuvant chemo-radiation protocols, have been introduced with promising survival outcomes [63].
According to retrospective studies, LT may offer satisfying outcomes for patients with unresectable very-early iCCA (i.e., ≤ 2 cm) [40]. Sapisochin et al. [64], in their cohort of 2301 patients transplanted for end-stage liver disease or HCC, had twenty-three patients diagnosed with an iCCA on pathology examination; they reported a 5-year OS of 45% with far better results for very early iCCA (namely single tumor ≤ 2 cm) than multifocal and larger tumors in terms of 5-year risk of recurrence (18% versus 65%, P = 0.01) and 5-year OS (65% versus 45%, P = 0.02). These promising results for early stages tumors were then confirmed in an international collaborative study containing 48 patients [64]. In order to obtain more solid evidence, a multicentric single-arm clinical trial (NCT02878473) is ongoing to confirm the effectiveness of LT for very early iCCA.
On the other hand, a recent study [63] enrolled patients with iCCA >2 cm but with favorable tumor biology (i.e., no evidence of extrahepatic disease, vascular invasion, and lymph node spread). All the patients underwent at least 6 months of chemotherapy with gemcitabine and cisplatin in order to get a sustained response. Out of 21 patients, 6 underwent LT and 1 liver resection, followed by adjuvant chemotherapy. The authors reported promising outcomes in terms of 5-year OS rate (i.e., 83%) with a recurrence rate of 50% despite the relevant tumor burden.
Given high recurrence and unacceptably low survival, LT was initially contraindicated also in patients with pCCA [65–67]. However, according to a large multicenter retrospective study including 216 patients with early-stage [68] unresectable pCCA treated with neoadjuvant chemoradiotherapy followed by LT, 5-year disease-free survival (DFS) rates of 65% have been reported. In a direct comparison between LT and resection, patients resected for pCCA and meeting transplantation criteria had a significantly worse 5-year survival compared to transplanted patients (18% vs. 64%), and the survival benefit of LT versus resection was also maintained when considering only CCA < than 3 cm with no lymph nodal involvement. [69].
In summary, also considering the well-known organ shortage and the still limited evidence on the actual benefit of LT for CCA, LT for CCA should be considered only in referral centers; in patients with very early iCCA (single tumor ≤2 cm) upfront LT might be of benefit, whereas those with advanced unresectable iCCA or pCCA neoadjuvant chemoradiation needs to be administered within specific protocols [64].
5Chemotherapy5.1Adjuvant chemotherapyAfter the radical surgical approach, the available treatments for BTC are chemotherapy and radiotherapy, or a combination of the two. In particular, the role of post-operative chemotherapy was quite recently defined due to the conflicting or few specific data on the topic.
The first study, published in 2002, demonstrated the benefit of chemotherapy over observation in the post-operative setting for GBC [54].
The ESPAC-3 trial explored the efficacy of 5-Fluoruracile or gemcitabine compared to observation in periampullary carcinoma [70].
A significant point was reached by the meta-analysis by Horgan et al, which evaluated data from more than 6000 patients who underwent different types of post-surgical treatments: the analysis confirmed the benefit of adjuvant chemotherapy and chemoradiotherapy, in particular in the group with node-positive and surgical positive margin [58].
These data were confirmed by another meta-analysis of 30 studies confirming a definitive benefit in terms of reduction of the risk of death with post-surgery chemotherapy [71].
Three randomized phase-III studies were published focusing on the adjuvant setting. In the PRODIGE12-ACCORD18 study, 193 patients were randomized to the observation or GEMOX scheme (gemcitabine plus oxaliplatin) after surgery, with no significant differences in terms of relapse-free survival [72]. Another negative phase III study in which gemcitabine was used was the BCAT study [73].
The first positive, large, randomized phase III trial was the BILCAP-study, which compared 8 cycles of capecitabine to surveillance: the median OS was 36.4 months for the control group and 51.1 months in the experimental arm (Hazard Ration (HR) 0.81 95% CI 0.63-1.04 p=0.097), reaching the statistical significance after the correction for prognostic factors [55].
Based on these data, capecitabine at the moment is considered the standard of care after curative resection of BTC.
To improve the benefit of capecitabine as an adjuvant treatment is currently recruiting the phase III trial ACTICCA-1, which compares capecitabine (control arm) with cisplatin/gemcitabine as the experimental arm. In addition, patients with R1 resection are randomized between chemotherapy (capecitabine or cisplatin plus gemcitabine) and the same regimens followed by chemoradiation with capecitabine (NCT02170090) [74].
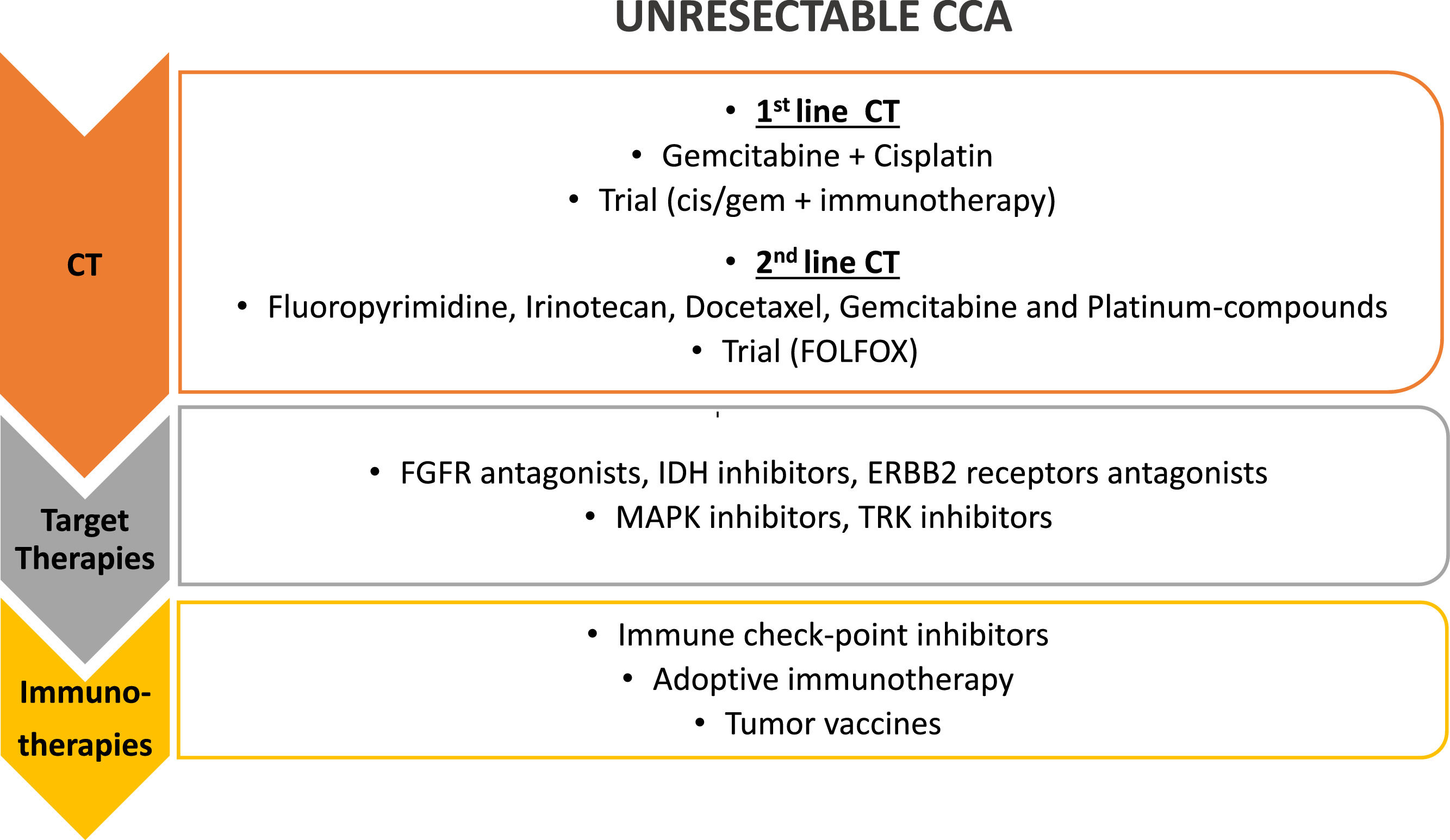
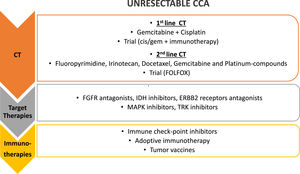
5.2Chemotherapy for metastatic disease: first and later linesSeveral pivotal trials for metastatic setting in biliary disease are ongoing Figure 1. The AC-02 trial, in which the combination of gemcitabine and cisplatin is compared with gemcitabine alone, demonstrated a higher median OS (11.7 vs. 8.1 months, respectively; HR 0.64; 95% CI 0.52-0.8; p< 0.001) and better disease control rate (DCR) for the combo [75]; the results were confirmed in the Japanese phase II BT22 trial [76]. In the recent ASCO-Gastrointestinal Cancer Symposium was presented the TOPAZ-1 study, the first phase III trial that demonstrated positive results with the addition of an immune checkpoint inhibitor to the standard of care in an unselected population [77,78]. In 685 previously untreated patients, treatment with durvalumab, a programmed death-ligand1 (PD-L1) inhibitor, plus cisplatin and gemcitabine, conferred a 20% reduction in the risk of death compared with cisplatin and gemcitabine alone (HR 0.80, 95% CI [0.66,0.97] p=0.021); also progression-free survival (PFS) and objective response rate (ORR) were better in the chemo-immunotherapy arm. Due to these results, the association cisplatin plus gemcitabine plus durvalumab is likely to become the new standard of care for advanced biliary cancer in the first-line setting.
Treatment strategies for unresectable CCA. First line treatment for unresectable CCA is chemotherapy (CT), with gemcitabine and cisplatin; different agents have been proposed as second line chemotherapy and a clinical trial with FOLFOX is still ongoing in this setting. Target therapies against different molecular targets and immunotherapy have been proposed in the last few years as second and third line of treatment, alone or in combination (i.e. chemotherapy and target therapies, chemotherapy and immunotherapy, target and immunotherapy together). FGFR, fibroblast growth factor receptors; IDH, Isocitrate Dehydrogenase 1 and 2; ERBB2, HER familiy receptors; MAPK, Mitogen-Activated Protein Kinases; TRK, Tyrosine receptor kinase.
In a metastatic setting, the combination of gemcitabine with oxaliplatin was also investigated: the overall response rate varies from 15% to 50%, while oxaliplatin exhibits more favorable toxicity profile compared with cisplatin [79]. Furthermore, fluoropyrimidine-based chemotherapy has shown efficacy in advanced biliary tract cancers [80].
Although combination treatment has to be preferred in advanced disease, due to the prognostic relevance of performance status (PS) ECOG in this disease [81], in PS 2 patients, monotherapy remains the best choice.
Unanswered questions in advanced biliary disease comprise whether more intensive treatment is superior to a two-drug s standard combo. Some interesting trials addressed this issue, such as the aBTCs trial, a phase II trial focused on triplet therapy cisplatin, gemcitabine, and nab-paclitaxel [82], as well as the phase III trial of cisplatin, gemcitabine plus S1 [83].
Another question is about the possibility of increasing the activity of chemotherapy by overcoming the mechanisms of resistance. Acelarin (NUC-1031) is a first-class nucleotide analog that presents a good safety profile in association with cisplatin for the first-line treatment in the phase Ib trial ABC-08 [84] at the recommended dose of 725 mg/m2 NUC-1031 is under evaluation in phase III trial NuTide-121.
Regarding patients who fail first-line chemotherapy, a good PS ECOG is the most important selection factor for the activation of second-line therapy [85].
A systematic review of several phase II or retrospective trials was published by Lamarca et al. in 2014. In this analysis, the treatment regimens included fluoropyrimidine, irinotecan, docetaxel, gemcitabine, and platinum compounds. The calculated median OS in these trials ranged between 6.6 and 7.7 months, while the median PFS was 2.8 months and the median response rate was only 7.7% [86].
By the same Author is the publication of the first randomized phase III study ABC-06 in the second-line setting. There were 162 patients randomized to receive active symptom control (i.e., antibiotic therapy, corticorticosteroid therapy, biliary drainage) versus FOLFOX regimen (oxaliplatin/fluorouracil) after cisplatin-gemcitabine failure. Although the reported median survival benefit of the FOLFOX regimen over active symptom control was small (6.2 versus 5.3 months, adjusted HR 0.69), the FOLFOX regimen obtained a more significant survival rate at 6 (50.6% versus 35.5%) and 12 months (25.9% versus 11.4%) [87]; the regimen is now considered the reference second-line treatment.
Other trials are focusing on second-line chemotherapy, such as the recently closed recruitment NALIRICC, in which nal-IRI (liposomal irinotecan) is compared to fluorouracil (NCT03043547).
6Target therapiesMOSCATO-01 trial by Massard C et al. [88] firstly showed, in a largescale evaluation of hard-to-treat cancers, that molecular alterations could be matched to appropriate targeted molecular therapy [89] . This trial included 43 BTC cases (29 iCCA, 10 eCCA, 4 gallbladder cancer) and showed that the proportion of patients with actionable alterations was higher in patients with BTC than in the MOSCATO trial overall, suggesting that BTC may comprise target-rich tumors. Moreover, molecular target-directed allocation to appropriate targeted therapies significantly increased PFS when compared with patients with BTC not allocated to a targeted therapy trial, confirming the benefit of precision medicine for BTC.
In the last years, lots of basic studies identify many molecular targets potentially useful as target-directed therapies, i.e. fibroblast growth factor receptor (FGFR), epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), metabolic regulators as isocitrate dehydrogenase 1 and 2 (IDH1/2), BRAF, tyrosine kinase receptors inhibitors, transcription factor FOSL1 [90–95].
Among them, different target therapies have been studied in clinical trials during the last few years.
6.1FGFR AntagonistsFibroblast growth factors (FGFs) signaling has a role in cell development and angiogenesis; they are moreover largely expressed in different cell types; these are the reasons why FGFs and their associated fibroblast growth factor receptors (FGFRs) have been studied extensively, with a focus to exploit the therapeutic potential of FGF-FGFR signaling [96]. FGFRs comprise a family of receptor tyrosine kinases (FGFR1–4). FGF-FGFR signaling is triggered by the ligand-dependent receptor dimerization following the binding of FGF at the cell surface. This leads to intracellular phosphorylation of receptor kinase domains, a cascade of intracellular signaling, and gene transcription that activates a number of intracellular survival and proliferative pathways [97]. In detail, the binding of FGFR by its ligand results in multiple downstream signaling pathways activation, including JAK-STAT, RAS–BRAF– MEK–ERK, and PI3K–AKT–mTOR, leading to cell proliferation, differentiation, and survival [98].
Alterations in FGFR genes, including activating mutations, chromosomal translocations, gene fusions, and gene amplifications, can result in ligand-independent signaling, which, in turn, leads to constitutive receptor activation [99] and pathologic cell proliferation.
Several FGFR inhibitors have been developed and studied in clinical trials. Both reversible ATP-competitive FGFR inhibitors (eg, derazantinib [ARQ 087], infigratinib [Truseltiq], erdafitinib [JNJ-42756493], and pemigatinib [Pemazyre]) and irreversible non-ATP-competitive FGFR inhibitors (eg, futibatinib [TAS-120]) have shown promising clinical activity [2].
6.1.1DerazantinibDerazantinib is an oral ATP-competitive, pan-FGFR inhibitor with strong activity against FGFR1–3 kinases. It also inhibits a number of other kinases, including RET, DDR2, VEGFR1, and KIT [100].
A multicenter, phase I/II, open-label study (NCT01752920) enrolled 29 adult patients with unresectable iCCA with an FGFR2 fusion, who progressed on, were intolerant to, or not eligible for first-line chemotherapy; Derazantinib provided an overall response rate of 20.7% and the DCR was 82.8% with a median PFS of 5.7 months [101].
6.1.2InfigratinibInfigratinib (BGJ398, Novartis AG) is an oral, ATP-competitive pan-FGFR inhibitor. Infigratinib efficacy was assessed in a phase II trial in patients with different FGFR alterations [FGFR2 fusions (n = 48), FGFR2 mutations (n = 8), FGFR2 amplification (n = 3)] after first-line chemotherapy. The overall response rate was 14.8%, almost all with FGFR2 fusions, and median PFS was 5.8 months, and interestingly DCR was 75.4%; however, the durability of response was limited [102,103].
PROOF 301 (NCT03773302), a phase III multicenter, open-label, randomized trial of infigratinib in comparison to standard of care gemcitabine and cisplatin in advanced/metastatic cholangiocarcinoma with FGFR2 translocations is still ongoing [104,105].
6.1.3ErdafitinibErdafitinib (JNJ-42756493, Jansenn) is a pan-FGFR small molecule kinase inhibitor being tested in clinical trials. In a phase I study Erdafitinib was tested in different advanced solid tumors; CCA was mostly responsive to erdafitinib, with ORR of 27.3% (3/11). All patients with cholangiocarcinoma who responded to erdafitinib carried FGFR mutations or fusions. The median response duration was 11.4 months for cholangiocarcinoma [106].
An interim analysis of an open-label phase II, a study conducted in China, Korea, and Taiwan (NCT02699606), conducted on adults with advanced CCA containing FGFR alterations who had failed at least one prior systemic treatment, showed that 15 of the 17 treated patients had an evaluable response: 7 (46.7%) achieved partial response (PR); 5 (33.3%) had stable disease (SD); and progression disease was seen in 3 (20.0%) patients. The ORR was 7/15 (47%) and the DCR was 12/15 (80%) [107].
6.1.4PemigatinibPemigatinib (Pemazyre) is an oral selective inhibitor of FGFR1–3.
In FIGHT-202, 146 enrolled patients were assigned to one of three cohorts: patients with FGFR2 fusions or rearrangements (N = 107), patients with other FGF/FGFR alterations (N = 20), or patients with no FGF/FGFR alterations (N = 18). The primary endpoint was centrally-assessed ORR among those with FGFR2 fusions or rearrangements. After a median follow-up of 17.8 months, 38 (35.5%) of patients with FGFR2 fusions or rearrangements achieved an objective response (3 had complete responses, 35 had PRs). The median duration of response was 7.5 months [108]. According to these data, in April 2020, the US FDA approved pemigatinib as the first targeted drug for patients with advanced refractory CCA with an FGFR2 fusion or rearrangement.
6.1.5FutinatinibFutibatinib is an oral, highly selective, irreversible FGFR1-4 inhibitor [109] that showed promising results in phase I trial (FOENIX-101; NCT02052778): among patients treated with futinatinib, 5.8 achieved PRs and 46% achieved SD. Responses were rapid (mostly occurring within 3 months) and lasted for >12 months in 2 of the 5 responders, indicating durable clinical benefit [110].
These promising results led to FOENIX-CCA2, an open-label, multicenter phase II registrational trial in patients with iCCA harboring FGFR2 gene fusions or other rearrangements (NCT02052778). Interim results from the FOENIX-CCA2 study (NCT02052778) were reported after the enrolment of 103 patients, who had progressed on previous standard therapies, or for whom standard therapy was not tolerated. Among the 67 patients having ≥ 6 months of follow-up included in this analysis for efficacy and safety, the ORR was 37.3% and the DCR was 82.1% [111].
While other molecules are under investigation in phase III clinical trial, FGFR-inhibitors' adverse events are reported to be similar and they are, as a class, very well tolerated. The most common adverse events are hyperphosphatemia (55-81% of patients), which is secondary to inhibition of FGF23, and determines inhibition of phosphate absorption in the intestine and reducing phosphate reabsorption in the kidney [112,113].
Ophthalmologic toxicity: retinal toxicities such as retinal pigment epithelial detachment (RPED) and central serous retinopathy (CSR) may cause symptoms such as blurred vision, visual floaters, or photopsia. RPED and CSR occur in around 4% and 9%, respectively, of patients with CCA treated with FGFR inhibitors, and these are generally of grade 1 or 2 [108,111]. Other FGFR toxicities are blepharitis, cataract development, increased lacrimation, trichiasis, trichomegaly, and blurred vision.
Nail toxicities also occur on FGFR inhibitors, especially with the increased duration of treatment; most are grade 1 and 2, and grade 3 nail toxicity rarely occurs. Onycholysis, the painless detachment of the nail from the nail bed, occurs in 5–7% of patients. Paronychia, an often tender bacterial or fungal infection that develops at the nailbed, occurs in 5–7% of patients. Other nail toxicities include nail discoloration, nail disorder, nail dystrophy, nail hypertrophy, nail infection, onychalgia, and paronychia [102,108].
6.2Inhibitors of IDH1/2 mutantIDH catalyzes decarboxylation of isocitrate to α-ketoglutarate. Mutated IDH converts α-ketoglutarate to 2-hydroxyglutarate, an oncometabolite. The accumulation of 2-hydroxyglutarate leads to epigenetic changes, impaired DNA repair, and aberrant cell metabolism, promoting tumourigenesis [114].
Several inhibitors of mutant IDH1 protein have been developed and evaluated in clinical trials. Most notably, ivosidenib (AG-120), an oral, targeted, small-molecule inhibitor of IDH1-mutant, was studied in a phase 1 trial of 73 previously treated patients with IDH1-mutant CCA, showing a median PFS 3.8 months (95% CI 3.6–7.3) and median OS of 13.8 months (95% CI 11.1–29.3) [115,116]. In the follow-up phase 3 ClarIDHy trial [117], patients with advanced, unresectable IDH1-mutant CCA, who had one to two previous lines of therapy, were randomized to ivosidenib versus placebo. The ORR with ivosidenib was 2.4%, the DCR was 50.8%. Median PFS was longer with ivosidenib (2.7 months) versus placebo (1.4 months; HR 0.37 [95% CI 0.25–0.54]; p<0.0001). Median OS was 10.3 months for ivosidenib versus 7.5 months for placebo (p=0.093), which included 70% of patients who crossed over from placebo. Although the clinical activity of ivosidenib has been encouraging, the challenge of acquired resistance has been reported [115]. The treatment appeared to be well tolerated, the most common treatment-related adverse events being nausea (38%), diarrhea (32%), and fatigue (28%).
In addition to ivosidenib, many other IDH mutant inhibitors and IDH pathway target therapy are under evaluation in clinical trials (i.e. NCT02381886, NCT02481154, NCT03684811) [114].
6.3Agents Targeting HER Family (ERBB2) ReceptorsThe HER family includes 4 members: epidermal growth factor receptor (EGFR/HER1), HER2, HER3, and HER4; they are type I transmembrane growth factor receptors that function to activate intracellular signaling pathways in response to extracellular signals; HER2 overexpression has a well-demonstrated tumorigenic potential [118].
The combination of pertuzumab and trastuzumab was investigated in 11 patients with previously treated BTC, with HER2 amplification or HER2 mutations; ORRs of 7.5% and 33.3% were reported, respectively [119]. Targeting gene mutations rather than amplifications may also have potential (e.g., neratinib [120]. An ORR of 10% was reported in the biliary subgroup (20 patients) of the SUMMIT clinical trial exploring the role of neratinib in patients whose tumors harbored HER2 mutations [121].
Altogether, HER represents the most frequent targetable aberration for CCA and GBC, but only a few data exist regarding their utility as treatment of BTC; work is still needed for this target to be ready for prime time use in clinical practice.
6.4MAPK (Mitogen-Activated Protein Kinases) Pathway InhibitorsBRAF is a serine/threonine protein kinase activating the MAP kinase/ERK-signaling pathway, leading to cell proliferation, differentiation, and survival mutational activation of BRAF favors its hyper-activation, which promotes cell proliferation, differentiation, and survival [122,123]. Mutations of BRAF are described in iCCA, with a prevalence of 1–3% [124].
BRAF mutations at codon 600, mostly V600E, are of interest because they are potentially targetable with BRAF inhibitors. Unfortunately, single-agent BRAF inhibitors seemed to have limited activity in CCA, and it could be due to feedback EGFR activation as in colorectal cancer. The inhibition of MEK could be an alternative strategy to target MAPK [2].
The dual inhibition of BRAF and MEK is an alternative and potentially more efficient strategy to target the RAS-ERK pathway. In two independent reports, the combination of Dabrafenib and Trametinib showed durable clinical responses [125,126]. Finally, the preliminary results of a basket trial involving patients with BRAF mutation showed, in a cohort of pretreated BTC, a response rate of 42% with a median OS of 11.7 months [127]
6.5Tyrosine receptor kinase (TRK) inhibitorsThere are three TRK tyrosine kinase receptors, TRKA, TRKB, and TRKC. These are encoded by the genes NTRK1, NTRK2, and NTRK3, respectively. Neurotrophin ligand binding and TRK activation result in homodimerization of the receptor, followed by transactivation of the intracellular domains, and recruitment of cytoplasmic adaptors. These adaptors, in turn, activate downstream signaling via the MAPK, PI3K, and/or PKC pathways, involved in cycle cell progression, cell proliferation, and cell survival [128].
Actionable oncogenic TRK activation is primarily mediated by NTRK gene fusion. The NTRK inhibitors larotrectinib (VITRAKVI; Loxo Oncology Inc, San Francisco, CA, USA) and entrectinib (Rozlytrek; Hoffmann-La Roche, Basel, Switzerland) have shown high response rates and durable responses in early phase trials of NTRK-fusion-positive advanced solid tumors, which included patients with CCA. Larotrectinib yielded an ORR of 75% and a median duration of response of 10 months. Entrectinib showed an ORR of 57% with a median duration of response not reached [129,130]. Both larotrectinib and entrectinib have been approved by the US FDA for patients with NTRK-fusion-positive solid tumors who have no alternative treatment or progressed after treatment. The National Comprehensive Cancer Network (NCCN) guidelines also recommend NTRK inhibitor as first or subsequent-line therapy in NTRK-fusion-positive biliary tract cancer.
6.6Further perspectivesSignaling pathways involved in CCA development and progression are still under investigation, i.e., transcription regulators as NOTCH genes and FOSL1; their inhibition showed in vitro promising results [131,132]. Tumor microenvironment role is under investigation as a promising target for future therapies development; it is constituted by CCA stroma, made by cancer-associated endothelial cells, inflammatory cells (macrophages, neutrophils, natural killer (NK), and T cells), cancers associated fibroblasts and extensive network of proteins such as collagens, laminin, and fibronectin [7].
Epithelial to mesenchymal transition (EMT) is a cell plasticity-promoting phenomenon initially reported to occur during embryogenesis, but that also takes place in cancer, enabling epithelial cancer cells to acquire mesenchymal features with invasive properties that lead to metastatic colonization. The prototype inducer of EMT is the tumor growth factor-β (TGFβ)-dependent pathway and is under investigation to become a target for new drugs fibronectin [7].
7Immunotherapy for metastatic biliary tract cancerRecent advances in immunotherapy have changed the therapeutic chances in BTC, which are considered ‘immune-cold’ due to low-moderate tumor mutational burden [7,133]; moreover, the tumor microenvironment encourages the immune escape and inhibition of immune response [134].
In this context, the immune checkpoints inhibitors, targeting programmed death 1 (PD-1), PD-L1, cytotoxic T-lymphocyte antigen-4 (CTLA-4), and novel therapeutic approaches like cancer vaccines and adoptive cell therapy (ACT), can empower antitumor activity.
7.1Immune checkpoint inhibitorsPD-1, PD-L1, and CTLA-4 are immune checkpoints expressed by activated T cells: they dampen the immune response downregulating the function of activated T cells and the tumor cells enhance their survival. In this contest, the immune checkpoint inhibitors targeting PD-1, PD-L1, and CTLA-4 block this pathway in order to maintain the immunologic balance [135].
At present, the data on immunotherapy in the BTC is limited, but several trials are currently investigating the role of anti-CTLA-4 (such as ipilimumab or tremelimumab), anti-PD-1 (such as pembrolizumab or nivolumab) and anti-PD-L1 (such as durvalumab) [136,137].
PD-L1 expression has been detected in 10-70% of patients with BTC, a wide range depending on different factors [138–140] . Patients with a high expression of PD-L1 have a poor prognosis but can better respond to immune checkpoint inhibitors [140].
PD-L1 positive tumors are linked with: microsatellite instability increased tumor mutational burden, and expression of biomarkers, e.g. BRCA2, TP53, BRAF, RNF43, TOP2A mutations [141]. In addition, in particular in GBC, there is an association between the expression of ERBB2/ERBB3 mutants and the PD-L1 expression [142].
Monotherapy with PD1/PD-L1 inhibitors does not obtain satisfactory results in unselected patients with BTC.
Pembrolizumab is a highly selective, humanized monoclonal antibody against PD-1 which has been designed to block the interaction between PD-1 and its ligands, PD-L1, and PD-L2. It was approved by FDA for DNA mismatch repair (MMR) deficiency and/or microsatellite instability-high (MSI-H) advanced solid tumors, thus including biliary tract cancers. Of note, MMR deficiency has been reported to occur in 5% to 10% of BTC [143]. We can consider MSI-H and deficient mismatch repair (dMMR) as predictive biomarkers of immunoresponse like PD-L1 tumor expression [144–146].
KEYNOTE-028 was a phase Ib trial testing the activity of pembrolizumab in heavily treated BTC patients [147].
In the phase II trial KEYNOTE-158 pembrolizumab obtained an ORR has been 5.8% (6/104, 95% CI: 2.1%-12.1%), the median OS of 7.4 months (95% CI: 5.5-9.6) and the median PFS of 2 months (95%CI: 1.9-2.1). All responders had microsatellite stable (MSS) tumors [147].
Nivolumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2. It has been evaluated in a phase II trial single-arm [148]. With the ORR was 22%, the median OS was 14.24 months (95% CI: 5.98 months to not reached) and the median PFS was 3.68 months (95% CI: 2.30-5.69 months). All the responder patients had MSS tumors. Moreover, there was a correlation between PD-L1 expression and better PFS but no correlation with OS.
Due to unsatisfactory results of monotherapy, recent strategies include the combination of checkpoint inhibitors with different checkpoint inhibitors, anti-angiogenic therapies, multi-target tyrosine kinase inhibitors (TKI), poly ADP-ribose polymerase inhibitors, and chemotherapy (Table 1).
Some of the many clinical trials of combination therapies
| Trial name | Agent | Target | Setting | Outcomes |
|---|---|---|---|---|
| NCT04211168 [176] | ToripalimabLenvatinib | PD-1TKI | 2° Line and subsequent | ORR |
| NCT04010071 [177] | ToripalimabAxitinib | PD-1TKI | 2° Line and subsequent | PFSORR |
| NCT03684811 [178] | FT-2102Nivolumab or GEMCIS | IDH1 PD-1 CT | 2° line in IDH1 mutated | SafetyORR |
| NCT04895046 [179] | DostarlimabNiraparib | PD-1PARPi | Maintenaince after 1° line platinum based therapy | PFS |
| NCT03639935 [180] | NivolumabRucaparib | PD-1PARPi | Maintenaince after 1° line platinum based therapy | PFS |
| NCT03991832 [181] | DurvalumabOlaparib | PD-L1PARPi | IDH1 mutated and pretreated tumors | ORROveral disease control rate |
PD-1: programmed death 1; PD-L1: programmed death ligand 1; PFS: median progression free survival; ORR: overall response rate; TKI: Tyrosine kinase inhibitors; PARPi: Poly (ADP-ribose) polymerase inhibitor; CT: chemotherapy; IDH1: Isocitrate dehydrogenase 1.
The combinations of PD-1 and CTLA-4 blockade have shown good results in several tumor types [118,149,150] due to synergistic effects resulting in increased numbers of Tumor-infiltrating lymphocytes (TILs), decreased regulatory T (Tregs) cells, and overall improved inhibition of tumor growth [151,152].
The phase II trial, CA209-538, has investigated the combination of anti-CTLA-4 ipilimumab with nivolumab [153]. ORR has been reported at 23% with a DCR of 44%. The median PFS was 2.9 months (95% CI: 2.2-4.6 months) while the median OS was 5.7 months (95% CI: 2.7-11.9 months). All patients who responded to treatment had MSS tumors. Treatment has been well tolerated (only 15% of patients reported adverse events > grade 2).
A phase II study has evaluated the efficacy of PD-L1 durvalumab alone or in combination with anti CTLA-4 remelimuab in 42 pretreated patients [154]. In the monotherapy arm, the ORR was 4.8%, the median PFS was 2 months and the median OS was 8.1 months.
The median OS for the combination was 10.1 months (95% CI: 6.2-11.4 months) versus 8.1 months with durvalumab in monotherapy, the median duration of response was 8.5 months, and the ORR was 10.8%.
The association of double immunotherapy did not show favorable results.
7.1.2Other combinatory strategiesAnti-angiogenetic therapies inhibit tumor growth and have an immune-modulatory role [155–157].
The combination of anti-vascular endothelial growth factor receptor 2 (VEGFR2) ramucirumab with pembrolizumab failed in a phase I study that enrolled patients with advanced BTC showing an ORR of 4%, median PFS and OS of 1.6 months, and 6.4 months respectively [158,159].
Pembrolizumab has been assessed with an anti-angiogenic multikinase inhibitor lenvatinib in phase II study LEAP-005 on 31 pretreated patients with BTC [160]. The ORR was 10%, and the median PFS and OS were 6.1 months and 8.6 months, respectively. The most observed adverse events have been hypertension, dysphonia, and diarrhea. At present, an ongoing phase II study is investigating the association pembrolizumab/levantinib in 100 patients (NCT03797326).
Another combination study was toripalimab and levatinib with gemcitabine and oxaliplatin in 30 patients at the first line in metastatic iCCA. In a follow-up of 16.6 months, the study showed an ORR of 80%, (with one complete response), a median PFS of 10 months, while the median OS was not reached. Expression tumor of PD-L1 was associated with significant responses [161].
The IMbrave 151 trial has involved 150 patients with biliary tract cancers at first or following lines of therapy treating with gemcitabine plus cisplatin with or without bevacizumab (anti- vascular endothelial growth factor (VEGF)). The trial is still ongoing [162] and the results are awaited.
In a single-arm, multicentre phase II trial, the antitumor activity of regorafenib (anti-VEGF) in combination with avelumab (anti-PD-L1) has been assessed in 34 pretreated patients with metastatic BTC [163]. The median PFS and OS were 2.5 months (95% CI: 1.9-5.5) and 11.9 months (95% CI: 6.2-NA), respectively. It is interesting to note how, on tumor biopsies, high baseline levels of PD-L1 and indoleamine 2,3 dioxygenase 1 (IDO1), which is an enzyme that plays a crucial role in modulating the tumor microenvironment were associated with improved outcomes. Further studies should investigate in a selected population (high PD-L1 and/or high IDO1 expression).
Only 1-7% of BTC are BRCA1-2 mutated, but DNA damage repair mutation occurs in 60% of this type of cancer [164]. This genetic alteration seems to be sensible to poly ADP-ribose polymerase inhibitors (PARPi). Prior studies suggest that PARPi may promote responsiveness to immune checkpoint inhibitors by increasing neoantigens and tumor mutational burden, recruiting T cells through particular pathways and upregulating PD-L1 expression [165]. There are several ongoing clinical trials that are evaluating different combinations of PARPi and anti-PD-1 like nivolumab plus rucaparib (NCT03639935) or dostarlimab ant-PD-1 plus niraparib (NCT04895046).
7.2Adoptive immunotherapyAdoptive immunotherapies with chimeric antigen receptor (CAR) T-cells [166,167] have found a rationale in the treatment of BTC due to its harsh tumor immune microenvironment.
This immunotherapeutic strategy provides T cells genetically modified to express CAR or tumor antigen-specific T cell receptors (TCR) in order to enhance their ability to recognize and kill cancer cells.
The use of the adoptive transfer of ex-vivo-expanded tumor-reactive T cells has created a challenge in the development of a novel therapy. Injecting a specific population of T cells, which have an affinity for patients’ cancer cells helps to contrast the immune inhibitory tumor microenvironment. Anti-CD133 CAR T-cells have shown efficacy in ex-vivo tissue models [168]. Over 50% of biliary tracts express CD133. Feng et al., have tested in a patient with metastatic BTC CAR-T targeting CD133 and EGFR with PR of 8.5 months with CAR-T EGFR therapy and a PR of 4.5 months with CAR-T 133 treatment [169]. Moreover, a phase I clinical study (NCT01869166) studied CAR-T cells in EGFR-positive metastatic BTC. The study has shown 5.8% of complete responses and 58.8% (10/17) of SD [170]. The ongoing trial (NCT04660929) is necessary for validating the activity and the safety of CAR-T cells. Moreover, several studies have suggested that the addition of PD-1/PD-L1 blockade could improve the anti-tumor efficacy of CAR T cells in solid tumors, representing another potential strategy that warrants further evaluation [171].
7.3Tumor vaccinesThe use of tumor vaccines in BTC is an attractive therapeutic approach as they can introduce antigens capable of activating the immune system, promoting the proliferation of memory T cells, and enhancing the immune response. Single peptide-based vaccines can induce an immune response to Wilms Tumor 1 (WT1) and Mucin 1 (MUC1) which are overexpressed cell surface molecules in BTC. A study has combined gemcitabine with WT1 peptide vaccine in 16 patients with advanced BTC reporting a DCR of 50% and a median OS of 9.5 months [172] . In a phase I study MUC1 vaccine in 3 patients with BTC proved to be safe and well-tolerated, but 2 of 3 patients had PD [173].
Multiple peptide-based vaccines improved the efficacy in patients with biliary tract cancers. A study has shown in 9 patients the use of peptide vaccines for cell division cycle-associated 1 (CDCA1), cadherin 3 (CDH3), and kinesin family member 20A [174].The results registered stable disease in 5 patients and a median OS of 9.7 months. In a clinical trial (UMIN000005820) dendritic cell vaccines have been combined with adoptive cellular therapy in patients with BTC in post-operative [175]. The median OS for surgery plus immunotherapy was 31.9 months versus 17.4 months for only surgery. Several ongoing studies (NCT04853017, NCT03942328) are necessary to improve responses with tumor vaccines in BTC.
The role of immunotherapy in the treatment of BTC is under investigation. Although immunotherapies have demonstrated limited efficacy in the unselected population, the combination of new therapeutic strategies could be the next therapeutic frontier. Further translational studies are needed to define more specific biomarkers predictors of tumor response.
8ConclusionsThe burden of CCA is steadily growing with increasing incidence worldwide. Although great advances have been made in the understanding of CCA's pathogenetic mechanisms leading to the growing availability of new therapeutic molecules, the prognosis of CCA is still invariably poor.
Indeed, many critical questions are still unsolved. Among risk factors, the presence of chronic biliary inflammation and bile stasis seems to be the main drivers of cholangiocarcinogenesis. However, the trigger of this pathogenetic mechanism is not always clearly identifiable and thus preventable.
In the subgroup of patients with recognized risk factors for CCA, the lack of biomarkers for early diagnosis is a critical issue.
The treatment of choice, when feasible, is surgery with the goal of radical resection (R0). Nonetheless, how to determine which patients will have long-term benefits from surgery is still debated.
Many systemic therapies (Figure 1), traditional chemotherapy and target therapies are currently available or under investigation for unresectable CCA; however, pre-treatment biomarkers that can reliably predict response to a specific treatment regimen have not yet been identified.
Therefore, more scientific and clinical efforts are warranted in order to identify targetable molecular mechanisms of cholangiocarcinogenesis, develop innovative therapeutic strategies and improve our ability to stratify patients to offer them the best therapeutic strategy available.
FundingThe authors received no financial support for the research and authorship. The publication of this article was funded by Fundación Clínica Médica Sur.
Authors’ contributionsAll persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. All the authors gave their final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. A.E. and S.M. gave substantial contributions to the conception and design and had a main role in drafting the article. A.L, M.S., R.L., A.M.S., L.C, and A.C revised the literature and contributed to drafting and editing the article. D.C., P.I., and S.M critically revised the manuscript for important intellectual content.
Data availability statementData sharing is not applicable to this article as no new data were created or analyzed in this study.
Declaration of interestPietro Invernizzi and Sara Massironi are members of the European Reference Network on Hepatological Diseases (ERN RARE LIVER), and they thank AMAF Monza ONLUS and AIRCS for the unrestricted research funding. The remaining authors have no conflicts of interest to declare.