Background and aim:The pharmacokinetics of acemetacin, a non-steroidal anti-inflammatory drug which is biotransformed to indomethacin by hepatic first-pass effect, was examined during the necrotic and regeneration phases resulting from acute hepatitis induced by carbon tetrachloride (CCl4).
Material and methods: Acute hepatitis was induced by oral CCl4 administration to male Wistar rats. On days 0, 1 and 3 after the insult, liver histological analysis was performed, biochemical markers of liver damage and regeneration were measured, and the pharmacokinetics of oral acemetacin and of its active metabolite, indomethacin, were determined.
Results: One day after CCl4 administration, liver necrosis was apparent and there was an increase in the circulating levels of indicators of liver damage and regeneration with regard to control conditions. Acemetacin bioavailability was increased, although not in a statistically significant manner. On the other hand, indomethacin bioavailability was significantly reduced. By day 3, histological analysis revealed liver recovery, although not complete, while biochemical indicators of hepatic damage had reverted either totally or partially. Markers of liver regeneration were still increased. Bioavailability acemetacin and indomethacin was comparable to control values.
In conclusion: Indomethacin bioavailability after oral administration of its precursor, acemetacin, is significantly reduced by acute hepatitis produced by CCl4. Pharmacokinetic alterations, as liver damage, are reversible, but do not require complete liver regeneration to return to basal conditions.
Acemetacin, a carboxymethyl ester derivative of indomethacin, is currently used in the treatment of inflammation and pain.1-3 It has been shown that equimolar doses of both agents result in comparable analgesic and anti-inflammatory effects.1,2 Notwithstanding, clinical studies have shown that acemetacin produces significantly less gastric damage than indomethacin.3,4 Both, experimental and clinical studies, have shown that acemetacin is metabolized to indomethacin by sterolytic cleavage after oral administration with a relevant participation of hepatic first-pass effect after oral administration.5-9 Notwithstanding, we have provided evidence that acemetacin is not only a prodrug of indomethacin, but an agent endowed of gastric-sparing mechanisms, probably involving a reduction in leukocyte adherence.10,11
Liver diseases could change drug disposition, as hepatic damage can produce a reduction in the metabolic activity of several enzymes and therefore decreases intrinsic drug clearance.12 It has been documented that numerous therapeutic agents exhibit pharmacokinetic changes during hepatic injury. Particularly, our group has reported that experimental liver damage results in alterations in the bioavailability of several non-steroidal anti-inflammatory drugs (NSAIDs), including naproxen,13 ketorolac14 and diclofenac.15 It should be noted, however, that liver status after an acute damage is continuously changing, as the necrotic phase is followed by an active regeneration phase.16 Hence, pharmacokinetic alterations after acute hepatic injury are not permanent, but revert.13 Such alterations are particularly relevant for drugs submitted to a substantial hepatic first-pass effect after oral administration.17
As mentioned above, oral acemetacin is biotransformed to indomethacin by liver first pass effect.5-9 However; there are no available reports on acemetacin pharmacokinetics during acute liver injury and subsequent liver regeneration. Thus, the aim of this study was to examine the pharmacokinetics of acemetacin and of its active metabolite, indomethacin, during the necrotic and regenerative processes occurring after the induction of acute hepatitis by CCl4 in the rat, a widely accepted experimental model of liver damage.15
Material and methodsChemicalsIndomethacin and mineral oil were purchased from Sigma Chemical Company (Saint Louis, MO, USA). CCl4 and methanol (chromatographic grade) were obtained from J.T. Baker (Xalostoc, Mexico). Acemetacin was a gift from Bayer de México (Mexico City). All other reagents were of analytical grade. High-quality water, employed to prepare solutions, was obtained using a MilliQ Reagent Water System (Continental Waters Systems, El Paso, TX, USA).
AnimalsMale Wistar rats (250-300 g) from our own breeding facilities were used in this study. All animals received human care and the study complied with the institution’s guidelines and the Mexican official regulation regarding technical specifications for production, care and use of laboratory animals (NOM-062-ZOO-1999).18 Animals were housed under controlled conditions at 22 ± 2 °C temperature, 50-60% relative humidity and 12 h light-dark cycles. Food was withdrawn 12 h before initiation of experiments, but animals had free access to drinking water.
Study designRats received either CCl4 (4 g/kg) dissolved in mineral oil or vehicle. One or three days after either CCl4 or vehicle (control) administration, acemetacin (35 mg/kg) was administered orally by gavage. The drug was suspended in carboxymethyl cellulose (4 mL/kg). 200-μL blood samples were drawn from the caudal artery, as described previously,14 at 0, 0.08, 0.16, 0.25, 0.33, 0.5, 0.75, 1, 2, 4, 8, 10, 24, 27 and 30 h after drug administration. Plasma was obtained and acemetacin and indomethacin were determined in plasma samples as described below. Once blood sampling was finished, animals were anesthetized with ether and submitted to exsanguinations by cardiac puncture. This blood was used to determine enzyme markers of liver function. Finally, animals were sacrificed by an excess of anesthesia and the liver was immediately extracted for histological analysis.
Liver function and regeneration assaysLiver damage was determined by measuring the activities of γ-glutamyltranspeptidase (γ-GTP) and alanine amino-transferase (ALT), as described previously.15 ALT is considered an indicator of liver necrosis, whereas γ-GTP is an indicator of cholestasis.19,20 Liver regeneration was estimated by the determination of ±-fetoprotein by enzyme immune analysis (EMIT) using a commercial kit (AxSYM AFP, Abbott, Mexico City), as previously described.13 Histological analysis of the liver was performed by hematoxilineeosin staining as described by Mourelle and coworkers.21
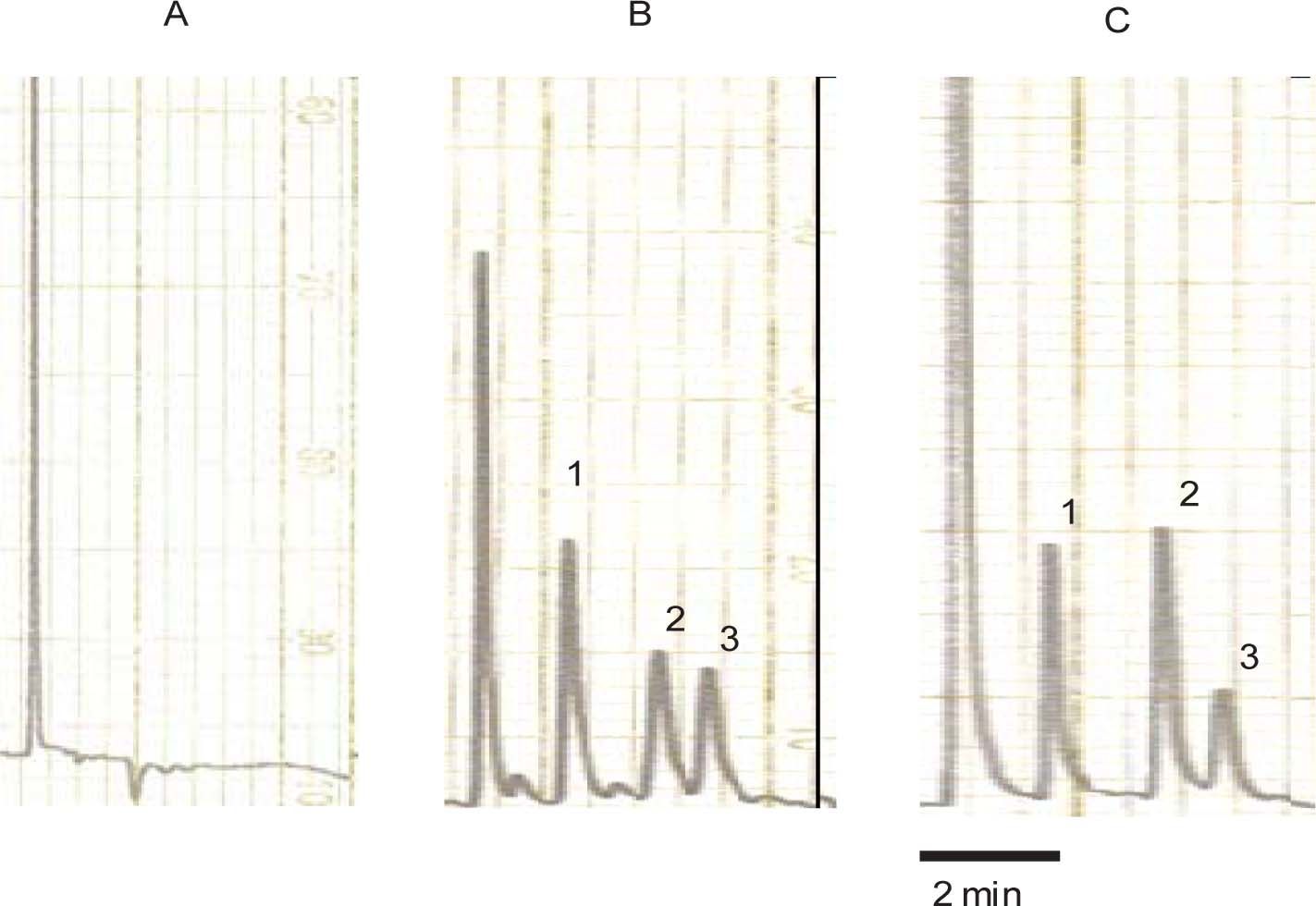
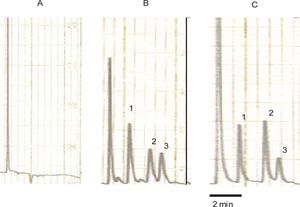
Determination of acemetacin and indomethacin in plasmaAcemetacin and indomethacin concentrations in plasma were determined by reverse-phase high-performance liquid chromatography with ultraviolet detection method developed in our laboratory. Briefly, 100 μL of plasma was spiked with 30 μg/mL of carbamazepine (internal standard) and 1,100 μL of methanol was added. Extraction was carried out by vortex agitation during 1 min at maximum speed; then samples were centrifuged. Aliquots of 60 μL of the supernatant were injected into the chromatographic system. The chromatographic system consisted of a Novapak C-18 column (150 × 3.9 mm ID, particle size 4 μm, Waters Assoc., Milford, MA, USA) eluted with a mobile phase consisting of a mixture of 0.025 M phosphate buffer (pH 6.0) with methanol, 45:55 v/v at constant flow of 1.0 mL/min at room temperature. The effluent from the column was monitored spectrophotometrically at 260 nm. Retention times were 2.30, 4.25 and 5.10 min for the internal standard, indomethacin and acemetacin respectively. Typical chromatograms from a blank rat plasma sample, a blank rat plasma sample spiked with acemetacin, indomethacin and the internal standard carbamazepine, and a plasma sample from a rat treated with acemetacin spiked with the internal standard are shown in Figure 1. It can be clearly appreciated that there were no peaks due to endogenous compounds which could interfere with the assay. Acemetacin and indomethacin recovery from plasma samples, as established by comparison with standard solutions, was 85-95%. Calibration curves were constructed for acemetacin and indomethacin in the 5-100 μg/mL range, being linear (r2 > 0.98) for both compounds. Accuracy and precision of the assay, determined by replicate analysis of spiked plasma samples of known concentration, was within the 100 ± 15% range.
Typical chromatograms. A: blank rat plasma sample. B: rat blank plasma sample spiked with carbamazepine (internal standard), indomethacin and acemetacin. C: plasma sample from a rat treated with acemetacin spiked with the internal standard. Peaks indicated by the numerals 1, 2 and 3 correspond to carbamazepine, indomethacin and acemetacin, respectively.
Individual plasma concentration against time plots were constructed. The peak concentration (Cmax), the time to reach this peak (Tmax) were determined graphically from these plots. The area under the curve (AUC) was estimated by the trapezoidal rule. The terminal half-life was determined from the slope obtained by linear regression of the terminal phase of semi logarithmic concentration versus time plots. All pharmacokinetic parameters were obtained by non-compartamental analysis using the Win NONLIN software, version 3.0 (Pharsight Corp., Mountain view, CA, USA).
Comparisons of indicators of liver function or pharmacokinetic parameters among different experimental groups were made using a one-way ANOVA followed by the Tukey’s test. Differences were considered to achieve statistical significance when p α 0.05.
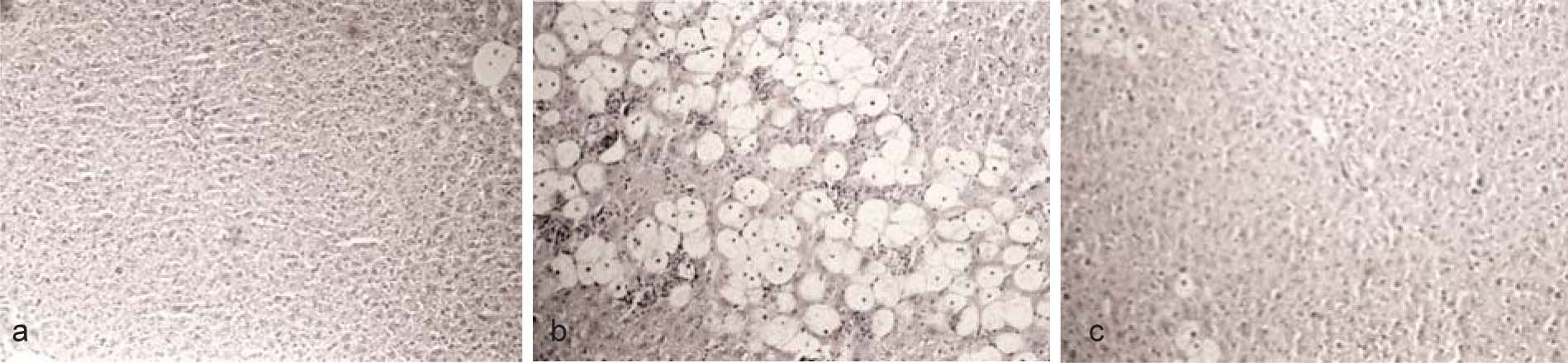
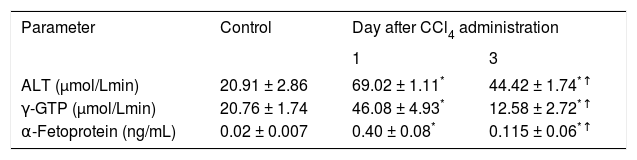
ResultsAdministration of CCl4 induced a reversible liver damage (Figure 2, Table I). One day after a single dose of CCl4 histological analysis clearly showed the presence of necrosis while the activity of ALT and γ-GTP in plasma was increased compared to control conditions. The levels of α-fetoprotein, an indicator of liver regeneration,13 were also increased. By day 3, histological analysis reveled that liver recovery had occur. However, as it can be appreciated in Figure 1, this was not yet complete, as necrosis zones, although scarce, could still be detected. ALT decreased, but remained above control values, while γ-GTP decreased to levels even lower than in control animals. Levels of α-fetoprotein exhibited a further increase with regard to day 1, suggesting that the regeneration process was fully active.13
Images (x 125) of liver preparations, stained with hematoxiline-eosine, from rats which received a single dose of CCl4 (4 g/kg). A) Before administration of CCl4. B) One day after CCl4. The presence of necrosis can be clearly appreciated. C) Three days after CCl4. Liver has regenerated, but not completely. Minor zones of necrosis can still be appreciated.
Indicators of liver damage and regeneration observed in control rats and in animals studied one and three days after CCl4 administration.
| Parameter | Control | Day after CCl4 administration | |
|---|---|---|---|
| 1 | 3 | ||
| ALT (μmol/Lmin) | 20.91 ± 2.86 | 69.02 ± 1.11* | 44.42 ± 1.74*↑ |
| γ-GTP (μmol/Lmin) | 20.76 ± 1.74 | 46.08 ± 4.93* | 12.58 ± 2.72*↑ |
| α-Fetoprotein (ng/mL) | 0.02 ± 0.007 | 0.40 ± 0.08* | 0.115 ± 0.06*↑ |
Data are presented as the mean ± SEM (n = 6-9).
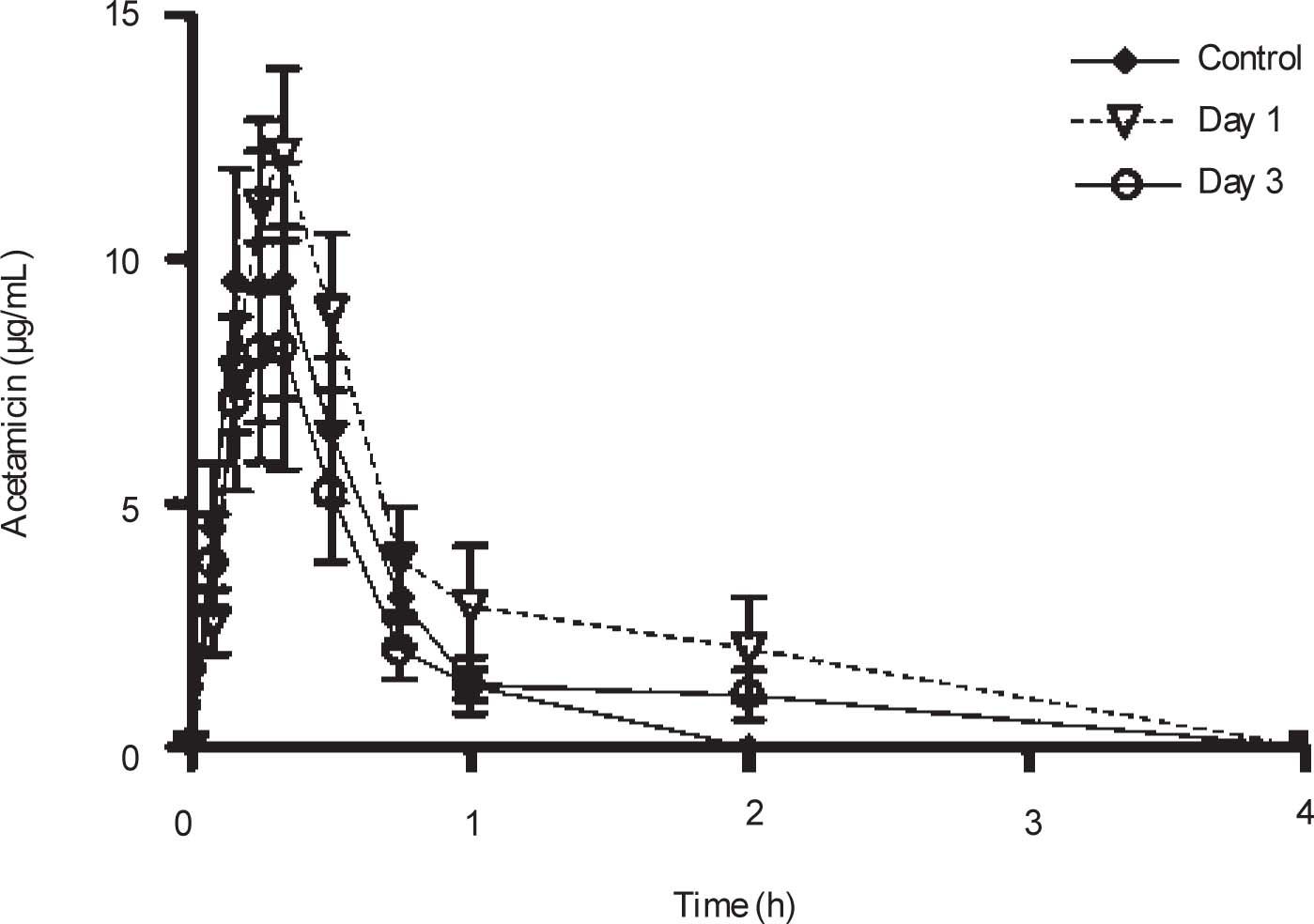
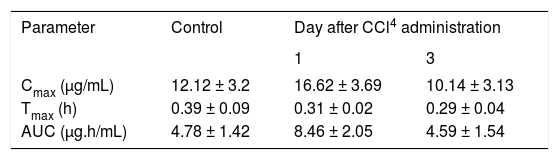
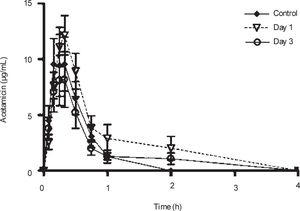
Plasma concentrations against time curves of acemetacin one and three days after CCl4 administration and before the insult are shown in Figure 3 Acemetacin concentrations were low and remained above detection levels for only short time in all the animals studied. Hence, it was not possible to accurately estimate half-life values due to an insufficient number of experimental points for linear regression. Pharmacokinetic parameters which could be estimated with enough precision and accuracy are given in Table II One day after CCl4 administration, acemetacin plasma levels were augmented. Notwithstanding, there was no significant difference with regard to controls in AUC, Cmax and Tmax. By day 3 both, plasma acemetacin levels and pharmacokinetic parameters were comparable to control values.
Acemetacin pharmacokinetic parameters observed after a single oral dose (35 mg/kg) in control rats and in animals studied one and three days after CCl4 administration.
| Parameter | Control | Day after CCl4 administration | |
|---|---|---|---|
| 1 | 3 | ||
| Cmax (μg/mL) | 12.12 ± 3.2 | 16.62 ± 3.69 | 10.14 ± 3.13 |
| Tmax (h) | 0.39 ± 0.09 | 0.31 ± 0.02 | 0.29 ± 0.04 |
| AUC (μg.h/mL) | 4.78 ± 1.42 | 8.46 ± 2.05 | 4.59 ± 1.54 |
Data are presented as mean ± SEM (n = 6-9).
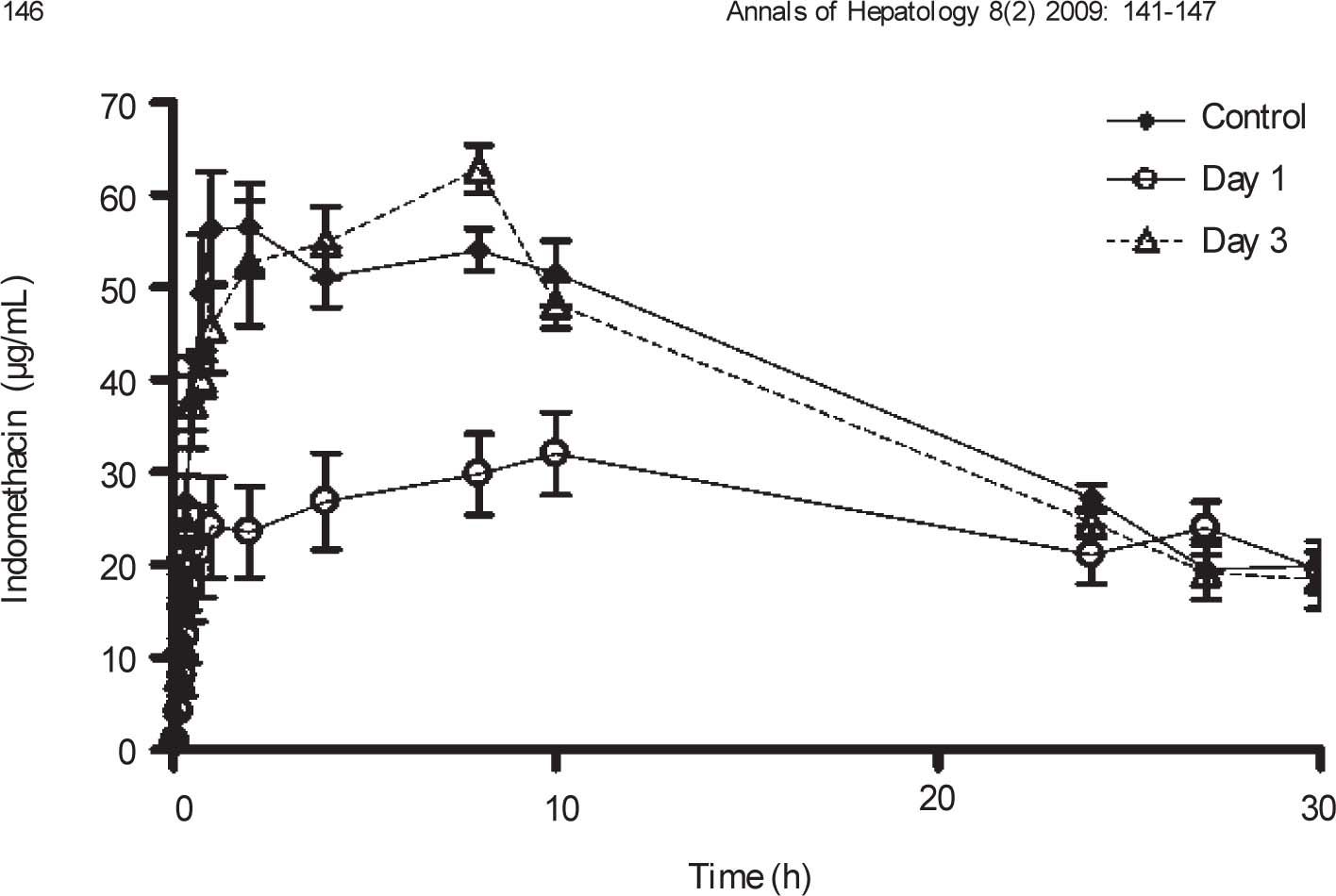
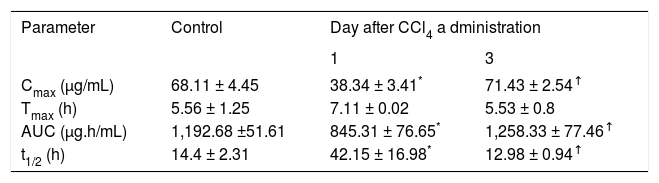
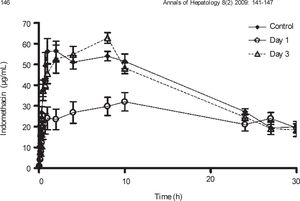
Plasma concentration against time curves for indomethacin are shown in Figure 4 Pharmacokinetic parameters are shown in Table III One day after CCl4 intoxication, indomethacin plasma levels were lower than control values. As a result, AUC and Cmax were reduced in a statistically significant manner, despite a prolongation in half-life. There was no significant difference in Tmax. By day 3, indomethacin plasma levels and all derived pharmacokinetic parameters were comparable to control values.
Effect of CCl4 on indomethacin plasma concentrations. Rats received an oral dose of 35mg/kg acemetacin by the oral route under control conditions, as well as one and three days after acute administration of CCl4. Indomethacin is a metabolite of acemetacin. Data are presented as the mean ± SEM (n = 6-9).
Pharmacokinetic parameters of indomethacin resulting from a single oral dose of acemetacin (35 mg/kg) in control rats and in animals studied one and three days after CCl4 administration.
| Parameter | Control | Day after CCl4 a dministration | |
|---|---|---|---|
| 1 | 3 | ||
| Cmax (μg/mL) | 68.11 ± 4.45 | 38.34 ± 3.41* | 71.43 ± 2.54↑ |
| Tmax (h) | 5.56 ± 1.25 | 7.11 ± 0.02 | 5.53 ± 0.8 |
| AUC (μg.h/mL) | 1,192.68 ±51.61 | 845.31 ± 76.65* | 1,258.33 ± 77.46↑ |
| t1/2 (h) | 14.4 ± 2.31 | 42.15 ± 16.98* | 12.98 ± 0.94↑ |
Data are presented as mean ± SEM (n = 6-9).
Acemetacin is a drug which exerts its effects, at least in part, by its biotransformation to indomethacin by hepatic first-pass effect.5-9 It is well known that liver damage can alter the pharmacokinetics of a wide variety of drugs,12-16 being particularly relevant for agents submitted to an extensive first-pass effect.17 On the other hand, it has been described that a single CCl4 administration is a suitable model of hepatitis15 which mimics clinical acute liver damage.21-23 Therefore, we decided to examine the effect of CCl4-induced acute hepatitis on the pharma-cokinetics of acemetacin and of its active metabolite, indomethacin.
CCl4 intoxication induced a reversible hepatic injury. Histological analysis showed that a considerable hepatic damage with abundant necrosis was present one day after the insult. Notwithstanding, three days after the insult liver recovery was apparent, although scarce zones of necrosis were still present, suggesting that liver regeneration was not still completed. Consistently with histological data, biochemical indicators of liver damage, ALT and γ-GTP, were increased one day after intoxication, but decreased by day 3. On the other hand, α-fetoprotein, an indicator of liver regeneration,13 was increased by day 1, being further increased by day 3 after CCl4 intoxication. Taken together, these results suggest that, in this experimental model, there is a significant liver damage one day after CCl4 administration, while liver regeneration has already started. Three days after CCl4 administration the necrotic phase has almost concluded, whereas the regeneration process appears to be fully active.
CCl4 administration resulted in an increase of acemetacin plasma levels. However, we were unable to observe any significant difference in acemetacin bioavailability between the compared experimental groups. This is probably due to the fact that acemetacin is rapidly absorbed, but also very rapidly eliminated by biotransformation to indomethacin.5-9 Hence, plasma concentrations were measurable only during a short period of time and showed a considerable variability. As a result, there is considerable inaccuracy in the determined pharmacokinetic parameters, and thus it is not possible to detect statistically significant differences between diverse experimental conditions. This has also been observed for other drugs which are extensively and rapidly biotransformed. We have reported that unchanged aspirin bioavailability cannot be estimated with precision in rats after oral administration due to the fact that it aspirin is readily metabolized to salicylic acid by hepatic first-pass effect.24
Conversely, the appearance and disappearance of indomethacin in plasma could be followed in an adequate manner, due to the high and long lasting plasma concentrations observed. Indomethacin plasma levels, observed after acemetacin administration, were decreased one day after CCl4 administration; that is, during the necrotic phase of liver damage. AUC and Cmax were reduced. This max data suggest that indomethacin formation from acemetacin is decreased due to an impaired first-pass effect resulting from liver damage. Notwithstanding the reduction in AUC, indomethacin half-life was prolonged. It should be noted that indomethacin is also eliminated by metabolic clearance, particularly by hepatic CYP2C9.25 Hence, indomethacin clearance also appears to be affected by CCl4 liver damage. It should be mentioned; however, that the decrease in indomethacin bioavailability could also be due to impaired acemetacin absorption, as we have previously demonstrated that oral ketorolac absorption is reduced in hepatic damage by bile duct ligation.14 Further investigation is required to determine the actual of CCl4 liver damage on oral acemetacin absorption.
By day 3, indomethacin pharmacokinetic parameters were similar to control values. Notwithstanding, histological data as well as results obtained with α-fetoprotein suggest that, at this time, the liver regeneration process was fully active. Thus, it appears that pharmacokinetic alterations due to impairment of the biotransformation of both, acemetacin and indomethacin, and eventually of acemetacin absorption, revert quite rapidly and do not require complete liver regeneration to return to basal levels. Therefore, conventional indicators of liver damage are not directly associated to acemetacin pharmacokinetic changes and thus are not useful for eventual dosing adjustments.
It is concluded that pharmacokinetic alterations after acute hepatitis are not permanent, but reversible due to liver regeneration. Changes are particularly important for drugs which are being generated from hepatic first-pass metabolism of a parent drug, such as indomethacin derived from acemetacin. However, pharmacokinetic alterations fully reverted while liver regeneration was not yet complete as determined by histological analysis and biochemical markers. Hence, conventional liver damage indicators are not adequate predictors of changes in acemetacin and indomethacin disposition. The present results show that the CCl4 intoxication model is a useful tool for the understanding of the time course of alterations in the pharmacokinetics of acemetacin and its biotransformation to indomethacin. Hence, this model can be used to study the effect of acute liver damage and regeneration on the pharmacokinetics of drugs submitted to an important first-pass effect.
AcknowledgmentsThe authors thank to Lourdes González-Flores, Mario Gil Moreno, Ramón Hernández and Benjamín Salinas for highly skilled technical assistance.