Incidence of hepatotoxicity caused by the broad spectrum antibiotic combination amoxicillin-clavulanic acid (Co-amoxyclav) has been increasingly recognized and the mechanism of this toxicity remains undefined. On the other hand, Ursodeoxycholic acid (UDCA) has been suggested as efficient antioxidant therapy in various liver diseases. Therefore, the present study was designed to elucidate the possible role of oxidative stress in hepatotoxicity induced by Co-amoxyclav and the putative protective role of UDCA in rats. Effects of amoxicillin (Amox; 50 mg/kg, orally, 21 d) or clavulanic acid (Clav; 10 mg/kg, orally, 21 d) and their combined administration on the biochemical liver parameters, reduced glutathione (GSH), lipid peroxidation measured as hepatic malondialdehyde (MDA) levels. In addition, myeloperoxidase (MPO) activity and reactive oxygen species (ROS) production in liver homogenate were also evaluated. On the other hand, the protective effects of pretreatment with UDCA (20 mg/kg, orally, 21 d) on these parameters were also evaluated. Our results show that pretreatment with UDCA reduced the liver parameters that were enhanced by single or combined administration of Amox and/or Clav such as serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and serum bilirubin levels. Moreover, pretreatment with UDCA normalized the GSH level and inhibited the elevation in hepatic MDA concentration. The enhanced MPO activity and ROS production in liver homogenate of rats treated with Clav or Co-amoxyclav were also normalized by UDCA pretreatment. In conclusion, the present data suggest that UDCA acts as effective hepatoprotective agent against liver dysfunction caused by Co-amoxyclav and this effect is related to its antioxidant properties.
Liver injury caused by drugs and other chemicals accounts for approximately 5% of all cases of jaundice and encompasses a wide spectrum of diseases ranging from acute and chronic hepatitis to bile duct abnormalities.1,2 The antibiotic combination amoxicillin-clavulanic acid (Co-amoxyclav) has become one of the most widely prescribed antibiotics.3 Several reports incriminate Co-amoxyclav in the development of cholestatic hepatitis.4,5 A recent study has shown that Co-amoxyclav is the most common drug involved in drug-induced liver injury and is the most frequently prescribed drug leading to hospitalization for drug-induced liver disease.6
On the other hand, ursodeoxycholic acid (UDCA) is a naturally occurring tertiary dihydroxy hydrophilic bile acid used with considerable success in the treatment of primary biliary cirrhosis.7,8 UDCA a well-established therapy is used to treat liver dysfunction associated with various diseases, including cholestatic diseases and chronic active hepatitis.9-12 In the 1970s, the first prospective study of UCDA for the treatment of patients with gallbladder stones demonstrated gallstone dissolution13 and it is now recognized that UDCA dissolves gallstones by solubilizing cholesterol from the stone surface. UDCA also converts supersaturated bile to unsaturated bile, and such instauration enhances the transport capacity of bile for cholesterol.14 Clinically, long-term UDCA therapy improves liver function tests and delays the development and progression of cirrhosis.15,16 Moreover, it has been reported to have antiapoptotic activity on cholangiocytes and hepatocytes.17 A broad body of evidence indicates that the antioxidant properties of UDCA have been confirmed during the last two decades.18-21 Therefore, our study was conducted to explore whether there is a role for oxidative stress in Co-amoxyclav-induced liver toxicity and also to investigate the putative protective role of UDCA in this scenario.
Materials and methodsDrugs and chemicalsUDCA was purchased from Calbiochem (La Jolla, CA, USA). Lucigenin, NADPH, SOD were obtained from Sigma (Germany). Amoxicillin and clavulanic acid were a kind gift from Medical Union Pharmaceuticals (MUB), Cairo, Egypt. All other chemicals are of analytical grade.
AnimalsAdult male Sprague-Dawley rats (150-180 g) were used throughout the experiments. Rats were fed a standard diet of commercial rat chow and tap water ad libitum. Rats were left to acclimatize to the environment for at least one week prior to inclusion in the experiments. Experiments were conducted in accordance with the guidelines for animal care of the United States Naval Medical Research Centre, Unit No. 3, Abbaseya, Cairo, Egypt. Experimental animals randomly divided into 7 groups (n = 8-10/group). Groups were arranged as follows: group 1: received Amox (50 mg/kg, orally, 21 days), group 2: received Clav (10 mg/kg, orally, 21 days), group 3: received combined oral doses of Amox + Clav (50 mg/kg, 10 mg/kg, respectively, orally, 21 days), group 4: received UDCA + Amox (20 mg/kg, 50 mg/kg, respectively, orally, 21 days), group 5: treated with UDCA + Clav (20 mg/kg, 10 mg/kg, respectively, orally, 21 days), group 6: received UDCA + the combination of Amox and Clav (20 mg/kg, 50 mg/kg, 10 mg/kg respectively, orally, 21 days) and finally group 7 which was considered as a control group, received the same volume of saline. Oral doses of Amox/Clav (50/10 mg/kg/day) were selected from a range of doses as the minimal doses that produce a marked liver injury after 3 weeks. Also it was reported that these doses achieve high plasma concentrations of the two drugs during 24-hour period of therapy.22 UDCA was administered 1 hour before each drug. After 3 weeks all the rats were sacrificed by decapitation. De Haan and Stricker,23 reported that the latency period between first in-take of Co-amoxyclav and the onset of hepatic injury is three weeks on average. The blood was collected and centrifuged at 3,000 cycles/min for 10 min, and serum samples were collected for biochemical liver tests. The livers of rats were immediately dissected out and weighed. A part of the liver was immediately dipped into liquid nitrogen for determination of myeloperoxidase (MPO) activity and ROS production. The remaining liver tissue and serum samples were stored at – 80 °C until studied for other parameters. Thereafter, the liver tissues were homogenized in 0.25 M sucrose, 10 mM Tris-HCl, 1 mM EDTA medium, pH 7.4, centrifuged at 10,000 g for 10 min and supernatants were used for biochemical analysis.
Assessment of serum aminotransferases, alkaline phosphatase and bilirubinHepatic enzymes in the serum such as AST, ALT were used as biochemical markers of the hepatotoxicity and assayed by the method of Reitman and Frankel.24 Serum alkaline phosphatase (ALP) was determined according to the method of Eaton RH25 using colorimetric kit obtained from Diamond Co., Egypt. Total or direct serum bilirubin were determined spectrophotometrically according to the method of Merino et al.26 using kits purchased from Randox, Laboratory. Ltd., UK.
Determination of hepatic reduced Glutathione levelsGlutathione (GSH) levels were measured spectrophotometrically using the method of Sedlak and L’ Hanus.27 Results were calculated as nmol/g tissue.
Determination of hepatic malondialdehyde levelsLipid peroxidation was determined in liver homogenates as thiobarbituric acid reactive species (TBARS; sometimes referred to as malondialdehyde, MDA) as a marker of oxidative stress according to the method described by Buege and Aust.28
Determination of hepatic MPO activity in liver homogenate of ratsHepatic MPO activity was measured according to a previously described method.29 In brief, the livers were weighed and suspended in 6 mL of 50 mmol/L (mM) phosphate buffer (pH 6.0) containing 1% hexadecyltrimethylammonium bromide. The samples were homogenized and the homogenate was sonicated, freezethawed, and then centrifuged (4,500 g for 15 minutes at 4 °C). MPO activity in the supernatant (0.1 mL) was determined after the addition of 0.6 mL of phosphate buffer (pH 6.0) containing 0.167 mg/mL O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm over 10 minutes was measured in a spectrophotometer (Beckman, CA). One unit of MPO activity was defined as the amount of enzyme able to reduce 1 >mol of peroxide per minute. Results are expressed as units of MPO activity per gram of tissue.
Chemiluminescent measurement of ROS production in liver homogenate of ratsROS production in liver homogenates from each group was measured by lucigenin-enhanced chemiluminescence.30 Briefly, homogenates were resuspended in modified HEPES buffer containing (mM) NaCl 140, KCl 5, MgCl2 0.8, CaCl2 1.8, Na2HPO4 1, HEPES 25 and 1% glucose, pH 7. Homogenates were suspended in HEPES buffer (100 >g protein/mL). Immediately before recording chemiluminescence, NADPH (final concentration 100 >M) was added and dark-adapted lucigenin (5 >M) was added via an auto-dispenser. Light emission was recorded (Flurospectra, Germany) and expressed as mean arbitrary light units/min over 20 min. Each experiment was performed in triplicate. To verify the specificity of signals, in some experiments, tissue homogenates were pre-incubated with superoxide dismutase (SOD, 200 units/mL) before measurement of chemiluminescence.
Statistical analysisAll results are expressed as mean ± SEM. Biochemical parameters of groups were compared by ANOVA for repeated measures followed by Bonferroni’s test. P value > 0.05 was accepted as statistically significant.
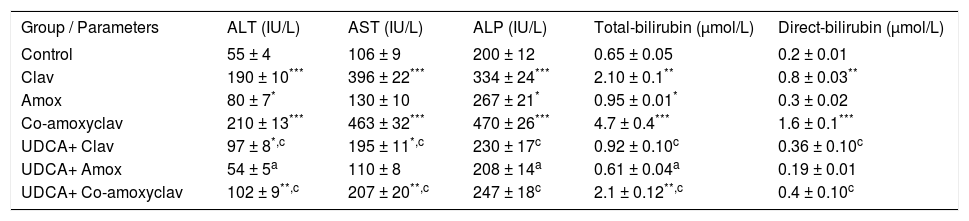
ResultsBiochemical serum parameters (ALT, AST, ALP and Bilirubin)Biochemical results of serum ALT, AST, ALP, total and direct bilirubin are summarized in Table I. As major criteria for liver dysfunction, serum ALT and AST, ALP, total and direct bilirubin levels in the serum of rats treated with Clav or Co-amoxyclav were found higher than control group. In Amox treated group serum ALT, ALP and total bilirubin levels were elevated significantly. The maximum liver injury was noticed with Co-amoxyclav followed by clavulanic acid then amoxicillin produced the least harmful effects on liver. However, pretreatment with UDCA reduced these values significantly compared to that of the control group and UDCA non-treated groups (Table I).
Biochemical results of serum ALT, AST, ALP, total and direct bilirubin in rats treated with amoxicillin (Amox), Clavulanic (Clav), or Co-amoxyclav with or without UDCA.
| Group / Parameters | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | Total-bilirubin (μmol/L) | Direct-bilirubin (μmol/L) |
|---|---|---|---|---|---|
| Control | 55 ± 4 | 106 ± 9 | 200 ± 12 | 0.65 ± 0.05 | 0.2 ± 0.01 |
| Clav | 190 ± 10*** | 396 ± 22*** | 334 ± 24*** | 2.10 ± 0.1** | 0.8 ± 0.03** |
| Amox | 80 ± 7* | 130 ± 10 | 267 ± 21* | 0.95 ± 0.01* | 0.3 ± 0.02 |
| Co-amoxyclav | 210 ± 13*** | 463 ± 32*** | 470 ± 26*** | 4.7 ± 0.4*** | 1.6 ± 0.1*** |
| UDCA+ Clav | 97 ± 8*,c | 195 ± 11*,c | 230 ± 17c | 0.92 ± 0.10c | 0.36 ± 0.10c |
| UDCA+ Amox | 54 ± 5a | 110 ± 8 | 208 ± 14a | 0.61 ± 0.04a | 0.19 ± 0.01 |
| UDCA+ Co-amoxyclav | 102 ± 9**,c | 207 ± 20**,c | 247 ± 18c | 2.1 ± 0.12**,c | 0.4 ± 0.10c |
Each value represents the mean ± SEM, (n = 8-10/ group). Data comparison was performed using ANOVA followed by Bonferroni’s test.
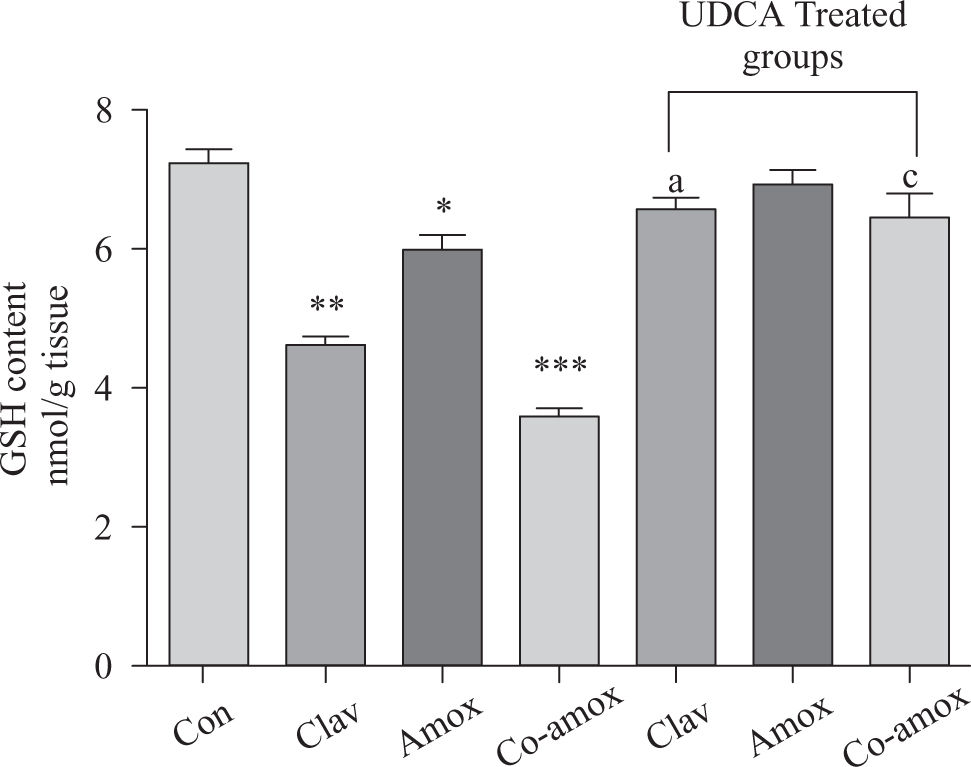
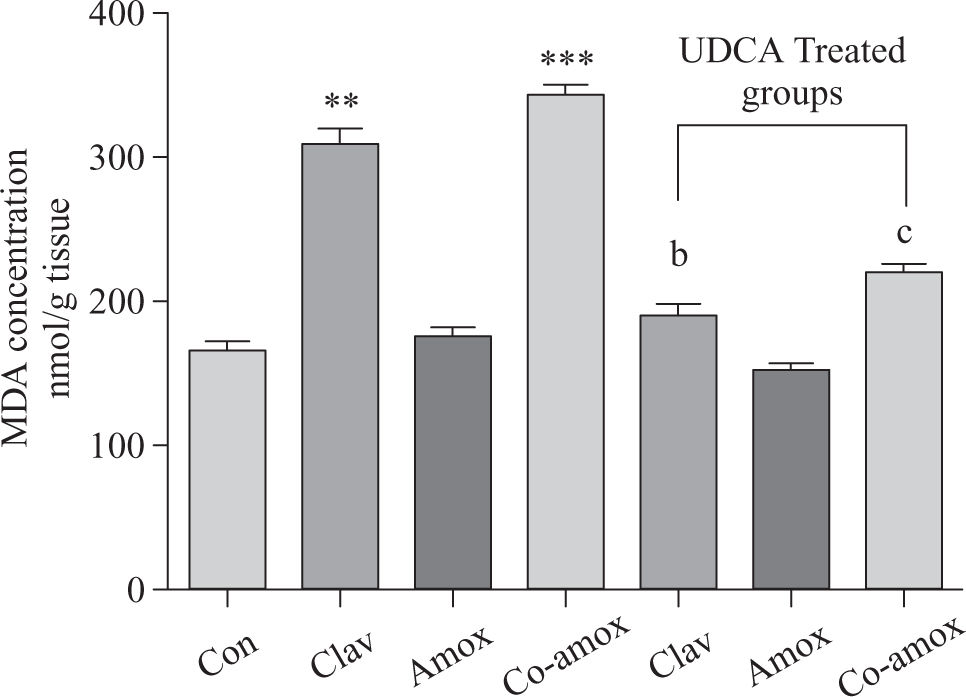
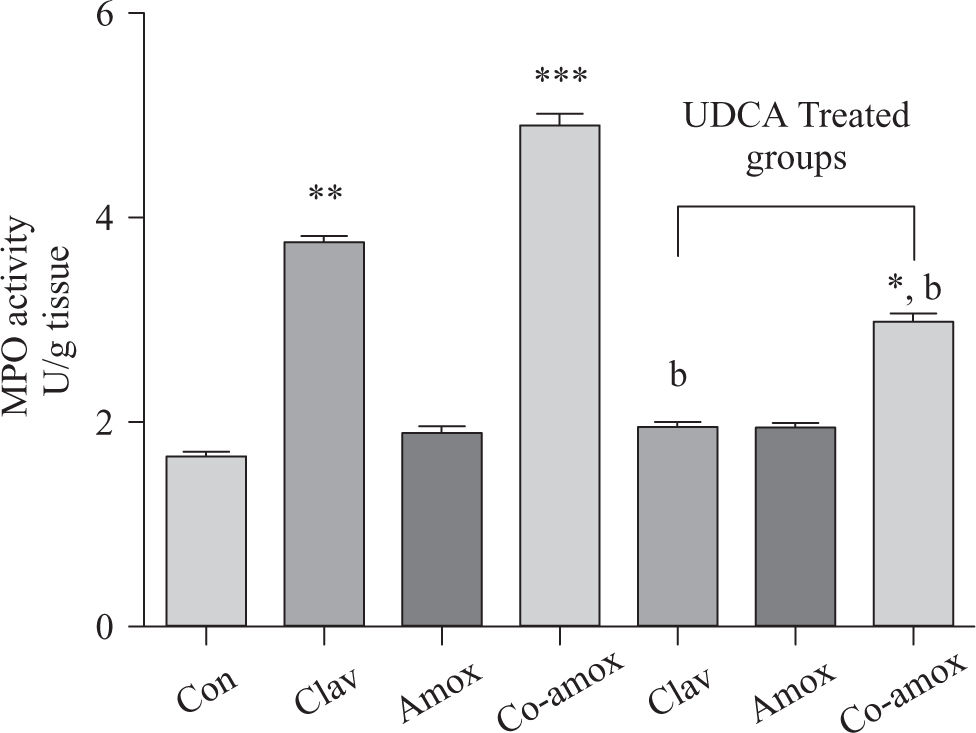
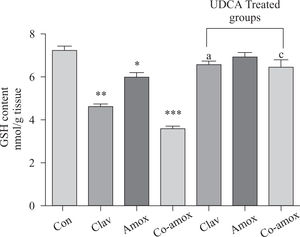
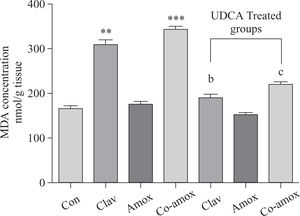
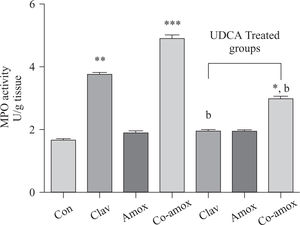
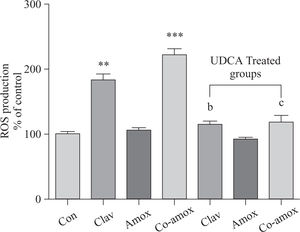
Hepatic GSH levels were significantly lower in the Amox, (p > 0.05), Clav (p > 0.01) treated rats and were more pronounced in Co-amoxyclav (p > 0.001) treated group in comparison with control group. However, pretreatment with UDCA markedly restored hepatic GSH to normal levels (Figure 1). The biochemical marker of oxidative stress; MDA contents and the marker of neutrophil infiltration expressed as MPO activity were significantly increased following to either of Clav (p > 0.01) and Co-amoxyclav (p > 0.001) and pretreatment of rats with UDCA markedly reduced these levels compared to that of UDCA non-treated groups and decreased their values to that of control as shown in Figures 2 and 3.
Hepatic GSH content in the study groups: Control (Con), Clav, Amox and Co-amoxyclav (Co-amox) in the absence or presence of UDCA. Values of each bar represent the mean ± SEM, (n = 8-10/group). Data comparison was performed using ANOVA followed by Bonferroni’s test. *p > 0.05; **p > 0.01; ***p > 0.001 compared with control value. Statistical difference between UDCA non-treated groups (Clav, Amox, Co-amoxyclav) and UDCA treated groups (Clav + UDCA, Amox + UDCA and Co-amoxyclav + UDCA) respectively was determined using multiple pair comparison Bonferroni’s test; ap > 0.05; cp > 0.001.
Hepatic MDA levels in the study groups: Control(Con), Clav, Amox and Co-amoxyclav (Co-amox) in the absence or presence of UDCA. Values of each bar represent the mean ± SEM, (n = 8-10/group). Data comparison was performed using ANOVA followed by Bonferroni’s test. **p > 0.01; ***p > 0.001 compared with control value. Statistical difference between UDCA non-treated groups (Clav, Amox, Co-amoxyclav) and UDCA treated groups (Clav + UDCA, Amox + UDCA and Co-amoxyclav + UDCA) respectively was determined using multiple pair comparison Bonferroni’s test; bp > 0.01; cp > 0.001.
Hepatic MPO activity in the study groups: Control (Con), Clav, Amox and Co-amoxyclav (Co-amox) in the absence or presence of UDCA. Values of each bar represent the mean ± SEM, (n = 8-10/group). Data comparison was performed using ANOVA followed by Bonferroni’s test. *p > 0.05; **p > 0.01; *** p > 0.001 compared with control value. Statistical difference between UDCA non-treated groups (Clav, Amox, Co-amoxyclav) and UDCA treated groups (Clav + UDCA, Amox + UDCA and Co-amoxyclav + UDCA) respectively was determined using multiple pair comparison Bonferroni’s test; bp > 0.01.
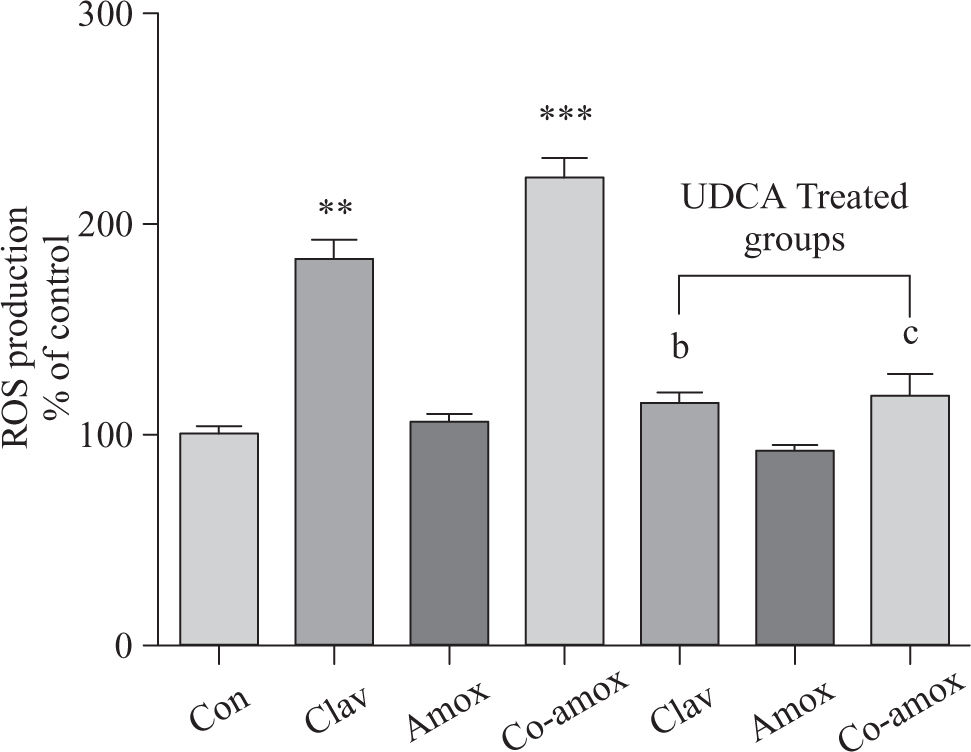
Similarly, (Figure 4) illustrates that UDCA inhibited ROS production that was augmented in rats treated with Clav or Co-amoxycalv. ROS production was measured by the chemiluminescence enhanced by lucigenin (5 >M) and was marked in liver homogenates of rats treated Clav and Co-amoxyclav. ROS production is more predominant in Co-amoxyclav (p > 0.001) compared to that of Clav (p > 0.01) alone. To verify the specificity of the chemiluminescence enhanced by lucigenin signal, in a separate experiment tissue homogenates were incubated with SOD (200 U/mL) for the indicated time, and the signal was completely inhibited.
ROS production in the study groups: Control (Con), Clav, Amox and Co-amoxyclav (Co-amox) in the absence or presence of UDCA. ROS production measured by Lucigenin-enhanced chemiluminescence (5 μM) and expressed as arbitrary light unit (% of control). Values of each bar represent the mean ± SEM, (n = 8-10/group). Data comparison was performed using ANOVA followed by Bonferroni’s test. **p > 0.01; ***p > 0.001 compared with control value. Statistical difference between UDCA non-treated groups (Clav, Amox, Co-amoxyclav) and UDCA treated groups (Clav + UDCA, Amox + UDCA and Co-amoxyclav + UDCA) respectively was determined using multiple pair comparison Bonferroni’s test; bp > 0.01; cp > 0.001.
It is well-known that amoxicillin and Co-amoxyclav are widely used as broad spectrum antibiotics. However, an increasing number of evidence indicates that they have risk of hepatotoxicity as adverse effect2,6 and the mechanism of their hepatotoxicity remains uncertain. UDCA stabilizes the mitochondrial and plasma membranes of hepatocytes that protect them from various outer injuries and constitute an antiapoptotic action.31 This protection effect is probably due to its antioxidant action.20 In this study, we have shown that the toxic effects of Co-amoxyclav on liver tissue of rats are induced by reactive oxygen molecules, therefore we evaluated the protective role of UDCA.
It is noteworthy that oxidative stress and lipid peroxidation that are mediated by oxygen free radicals has been implicated as a common link between chronic liver damage and hepatic fibrosis.32 Reactive oxygen metabolites are shown to mediate microvascular disturbances by various chemical substances.33 Hepatocytes are well recognized as being continuously exposed to reactive oxygen species (ROS) in various liver diseases including cholestasis. Antioxidant molecules such as glutathione (GSH) and antioxidative enzymes such as superoxide dismutase (SOD), and catalase, ordinarily provide hepatocytes with resistance to oxidative stresses.34 In the present study, administration of either Amox or Clav and their combination resulted in marked elevation of oxidative stress marker as evidenced by increased lipid peroxidation product MDA, ROS production and reduction in antioxidant molecules content such as reduced glutathione (GSH). An explanation to GSH depletion after administration of either Amox or Clav and their combination is increased consumption of GSH in non-enzymatic removal of oxygen-radicals. In addition, oxidation of GSH to GSSG by the oxidant stress, with efflux of GSSG being the major factor responsible for maintenance of the redox ratio. GSSG is of great biological importance, since it allows fine-tuning for the cellular redox environment under normal conditions and upon the onset of stress, and provides the basis for GSH stress signaling.35 Moreover, the myeloperoxidase enzyme activity was also increased in rat liver treated with either Clav or Co-amoxyclav indicating increased neutrophils infiltration and activation. These findings suggest that oxidative stress reactions might be an important contributing factor for the development of Co-amoxyclav liver dysfunction.
Interestingly, UDCA was able to normalize the elevated biochemical oxidative stress markers, ROS production and restored GSH normal level that plays an important role in the antioxidant defense mechanism in liver of Co-amoxyclav-treated rats, suggesting antioxidant properties of UDCA. This concept is consistent with several recent reports, confirming the antioxidant properties of UDCA.18-21 UDCA attenuates oxidative stress by 1) acting as ROS scavenger molecule itself,36 2) protecting the components of the mitochondrial electron chain against the uncoupling effect of endogenous hydrophobic bile salts37 and 3) by enhancing endogenous antioxidant defenses, including glutathione synthesis and antioxidant enzymes.38 UDCA increased hepatocytes levels of GSH and thiol-containing proteins, thereby protecting hepatocytes against oxidative injury,39 protected liver mitochondria from abnormalities induced by lipid peroxidation and minimized the elevation of lipid peroxides induced by hydrogen peroxide.40 Therefore, the normalization of GSH levels by UDCA may be due to improved GSH synthesis or preventing its consumption in non-enzymatic removal of oxygen-radicals.
Several reports incriminate amoxicillin-clavulanic acid therapy as a cause of intrahepatic cholestasis.4,5 This results in accumulation of toxic hydrophobic bile acids as evidenced from increase in serum cholestatic enzyme ALP. Palomero et al.41 have found a link between the induction of cholestasis by cyclosporine A in the liver of rats and free radicals formation as shown from depletion of GSH and increase of MDA levels. Moreover, it was reported that cholestasis resulted from bile duct ligation in rats enhances free radical formation that may play a role in portal hypertensive gastropathy.42 Therefore, accumulation of endogenous hydrophobic bile acid by Amox, Clav or their combination may impair mitochondrial function inducing release of ROS from mitochondrial origin. Our results showed also that the cholestatic effect of Co-amoxyclav is highly recognized (higher serum ALP level) compared to that of Amox alone. Consequently, free radicals formation and oxidative liver damage that produced by amoxicillin-clavulanic acid combination is more pronounced than in case of amoxicillin alone. It is well-established that clavulanic acid protects amoxicillin from degradation by beta-lactamases.43 This may lead to potentiating of therapeutic and harmful effects of Co-amoxyclav compared to amoxicillin alone. Owing to its susceptibility for degradation and mild cholestatic effect, amoxicillin may induce formation of few superoxide radicals that are directly neutralized by GSH without progression to sever toxic oxidative liver damage. This could explain the mild depletion of GSH content and the mild change in biochemical liver parameters after amoxicillin treatment.
Tsuji et al.44 have reported that neutrophils from bileduct ligated rats show significant activation leading to superoxide radicals generation compared with sham-ligated rats. In another study, it was clarified that when polymorphonuclear leukocytes (PMN) infiltrate, as a results of indomethacin treatment, it generates oxygen radicals and induces direct gastric mucosal injury, then increasing lipid peroxidation that further aggravates gastric damage.45 Therefore, it is obvious that the increase in PMN infiltration or activation (expressed as MPO activity) may generate free radicals. Based on this finding, we can conclude that cholestasis-induced by Co-amoxyclav or Clav may lead to neutrophils infiltration/activation and consequently ROS formation. This concept is further supported by our results since Co-amoxyclav or Clav administration markedly increased neutrophils infiltration as shown in the increase of MPO activity (a marker of polymorphonuclear infiltration/activation). This is accompanied by an increase in the levels of oxidative stress biomarkers.
UDCA has been used with considerable success in the treatment of chronic liver disease.46 The manner in which UDCA improves liver function is apparently diverse and several mechanisms have been proposed. Therapeutic dosing concentrations of UDCA enrich the bile acid pool with UDCA and, in doing so; shift the pool profile from one of hydrophobicity to hydrophilicity.47 Consequently, toxic hydrophobic bile acids are displaced by UDCA to the extent that UDCA becomes the major circulating bile acid.48 Secondly, UDCA can increase transcellular and canalicular transport of the bile acids thus reducing the hepatic retention of hydrophobic bile acids in patients.49 Based on the above mentioned data, the hypothesis was made that UDCA could ameliorate Co-amoxyclav-induced oxidative stress and liver damage through reducing the hepatic retention of hydrophobic bile acids. Our results are consistent with this finding since administration of UDCA to rats treated with Amox or Clav and their combination markedly reduced serum ALP (serum cholestatic enzyme) and hence bile acid retention in addition to amelioration of serum ALT, AST, bilirubin. This is accompanied by a normalization of oxidative stress parameters. UDCA inhibited the elevation in serum levels of AST, ALT and bilirubin without reducing them to normal control values that is in contrast to full normalization of oxidative stress markers. This may indicate that Co-amoxyclav may have additional mechanism (s) of hepatotoxicity other than free radical formation. The use of compounds with known antioxidant properties such as vitamin C or E may be of value to directly discriminate pro-oxidant mechanisms from other possible mechanism (s) like immuno-allergic drug-induced liver disease.50
In conclusion, our results suggest that UDCA has a hepatoprotective effect on liver dysfunction caused by Co-amoxyclav and this effect is attributed to its antioxidant properties and this could be clinically beneficial to reduce the hepatotoxic adverse effect of Co-amoxyclav.