Neurological symptoms can be one of the over-riding symptoms in patients with liver cirrhosis. Patients can present with subtle changes in mood or neurological function due to hepatic encephalopathy (HE), to more severe presentations including stupor and coma. While HE, in its severe form, can be clinically easy to diagnose, more subtle forms may be more difficult to recognize. Other neurological diseases may indeed be overlooked in the context of cirrhosis or confuse the physician regarding the diagnosis. Chronic acquired hepatocerebral degeneration (CAHD) is an uncommon problem occurring in patients with cirrhosis characterised by a Parkinsonian-like neurological presentation with damage to the brain secondary to manganese (Mn) deposition. Here we describe a case of a patient with a neurological presentation of liver disease with a review of the current CAHD literature. In conclusion, CAHD is a rare condition occurring in liver cirrhosis that should always be considered in patients with neurological manifestations of chronic liver disease.
A 55-year-old Chinese man presented to the emergency room with a two-year history of progressive neurological symptoms including dysarthria, gait ataxia, dysphagia, falls, tremors, and decreased visual acuity. His past medical history was significant for a previous cholecystectomy but no other operative history. He had a previous history of polio with residual right leg weakness, and untreated hypertension. There was no history of diabetes and he was not on regular medication and had never been so. He had no allergies, no family history of neurological disorders or liver disease. He was married and did not smoke or consume alcohol. Physical examination showed bilateral saccadic eye movements, lip smacking, mild bilateral upper limb cog-wheeling with decreased tone, bilateral finger to nose dysmetria and a resting hand tremor. There were no other ocular signs seen on examination and no cognitive impairment on testing. Investigations at admission revealed an AST of 48 IU/mL, ALT of 32 IU/mL, ALP 144 IU/mL, total bilirubin of 51 umol/L, albumin of 24 g/L, INR 1.5, Hg 119 g/L, WBC 2.3 x 109/L and platelets 77 x 109/L. A computed tomography (CT) scan of the abdomen revealed radiological evidence of cirrhosis (liver with a nodular contour and lobar redistribution) and splenomegaly. His blood panel for liver cirrhosis was initially unyielding with a negative virology screen, normal iron studies, negative autoimmune panel and metabolic screen aside from a low ceruloplasmin of 0.15 g/L (normal 0.2-0.6). MELD score was calculated as 15, MELD-Na 16. Whilst CT of the head was unremarkable, magnetic resonance imaging (MRI) revealed mild microangiopathic changes and mild diffuse atrophy. Furthermore, there was T1 hyperintensity in the basal ganglia (BG) bilaterally. His 24-h timed urine copper (Cu) level was elevated at 0.79 umol/d (normal 0.06-0.28), and a plasma Cu level of 9.3 umol/L (normal 11.2-20.6). He went on to have a liver biopsy, which confirmed cirrhosis without a clear aetiology but showed Cu accumulation within the hepatocytes. Subsequent quantitative copper measurement showed a high value of 4.12 umol/g (normal 0.16-0.55). Ophthalmology assessment revealed no Kayser-Fleischer (KF) rings.
Given the phenotypic probability of Wilson disease, he was started on zinc (Zn) monotherapy (50 mg PO TID) because of concern that a chelator may worsen his neurological symptoms. With no improvement in his symptoms or changes in his measured 24 hour urine copper parameters, he was then given a trial of D-penicillamine, however after 1 month of therapy he noted subjective worsening of neurological symptoms, thus treatment was discontinued. Given the non-response to chelation treatment, sequencing of the ATP-7B gene responsible for Wilson disease was performed and was reported negative for known mutations. He was subsequently reassessed by the Neurology service noting similar examination findings. An E.E.G was performed which was normal and he was prescribed Carbidopa/Levodopa (Sinemet) and suggested Mn serum testing. He had minimal improvement on Parkinsonian therapy and his condition continued to slowly deteriorate. The serum Mn level was modestly elevated at 25.7 mmol/ L (normal 8-20.7). A 24 h urine Mn collection off chelation therapy was 88.9 mmol/d (1.8-14.6) increasing to 273.2 mmol/d on chelation therapy. He was subsequently treated with Trientine (500 mg PO BID) with stabilization but no improvement of his neurological or hepatic status. He was also treated with Lactulose and Rifaximin to ensure there was no element of HE as a cause for his symptoms but he showed no improvement. On Trientine, there has been stabilization of his neurological status with no worsening of his liver disease. An additive diagnosis of presumed CAHD was made in view of lack of response to conventional treatments for Wilson’s disease. His MELD score has fluctuated from 12 to 19 with diuretic-sensitive ascites and small esophageal varices. He was evaluated for liver transplantation but has not been listed to date given concerns about his chance of neurological recovery and his relatively low MELD score.
DiscussionManganese (Mn) is the twelfth most abundant element and the fifth most abundant metal.1 As an essential nutrient, several enzyme systems have been reported to interact with, or depend on Mn for their catalytic or regulatory function. As such, Mn is required for the formation of healthy cartilage, bone and aids in the maintenance of mitochondria and the production of glucose.2,3 It also plays a key role in wound healing. The source of Mn intake is through the diet with vegetable and animal foods containing varying amounts of the mineral. Vegetarians often have diets richer in Mn than those who select omnivorous diets. Other potential sources of exposures include inhalation of air contaminated with particulate matter containing Mn. This is mostly seen in populations living in close proximity to mining activities and industries using Mn4 such as miners in manganese dioxide mines, workers in dry-cell battery factories, smelters and in welders.5 Other rare sources include ingestion of water, soil and consumer products that contain Mn (fish or shellfish) or exogenous sources of Mn such as in patients receiving long-term total parenteral nutrition (TPN).
In humans, Mn is excreted in the liver via bile,6 with 3% of ingested Mn absorbed and the remaining excreted via the biliary system.7 Acquired (non-Wilsonian) hepatocerebral degeneration also known as CAHD was first reported in 19198 and is a progressive debilitating neurological disorder.9,10 CAHD is an uncommon problem that can occur in cirrhosis characterised by a Parkinsonian-like neurological condition with damage to the brain thought to be secondary to Mn deposition. The formation of porto-systemic shunts may also aggravate the course of the disease by promoting increased shunting of Mn to the systemic circulation and deposition in the brain. The true prevalence in the general population is unknown but it is estimated to be 1-2% in patients with cirrhosis.11,12 Neurodegeneration may occur in the cerebral cortex, BG and cerebellum13,14 and there exists an overlap between regional vulnerability of the brain to the effects of Mn toxicity and Wilson disease-related Cu-toxicity. The deposition of Mn results from impaired hepatic removal of the metal, with porto-systemic shunting leading to deposition within the brain leading to oxidative/nitrosative stress, glutamate receptor-mediated excitotoxicity, neuro-inflammation15 and often secondary iron deposition.16 Once deposited in the brain, the effects of Mn on the central nervous system can include astrocyte mitochondrial dysfunction17 and an induction of mitochondrial peripheral type benzodiazepine receptor (MBR) sites, 18 which can lead to an increase in the synthesis of neuroactive steroids. Increased MBR sites have been reported in experimental animal models of chronic liver failure19 and also in the BG of patients dying from cirrhosis.20 Culture models have revealed Mn can affect cortical astrocytes leading to the decreased high affinity transport of glutamate,21 which in turn leads to a toxic effect on the glutamate transporter in such cells. This effect of Mn occurs in synergy with ammonia leading to reduced astrocyte glutamate uptake and thus an increase in extracellular glutamate concentrations, consequently resulting in excitotoxicity/nitro-sative stress-mediated neuronal cell damage or death15 through the activation of NMDA-receptor coupled NO-cGMP pathways.
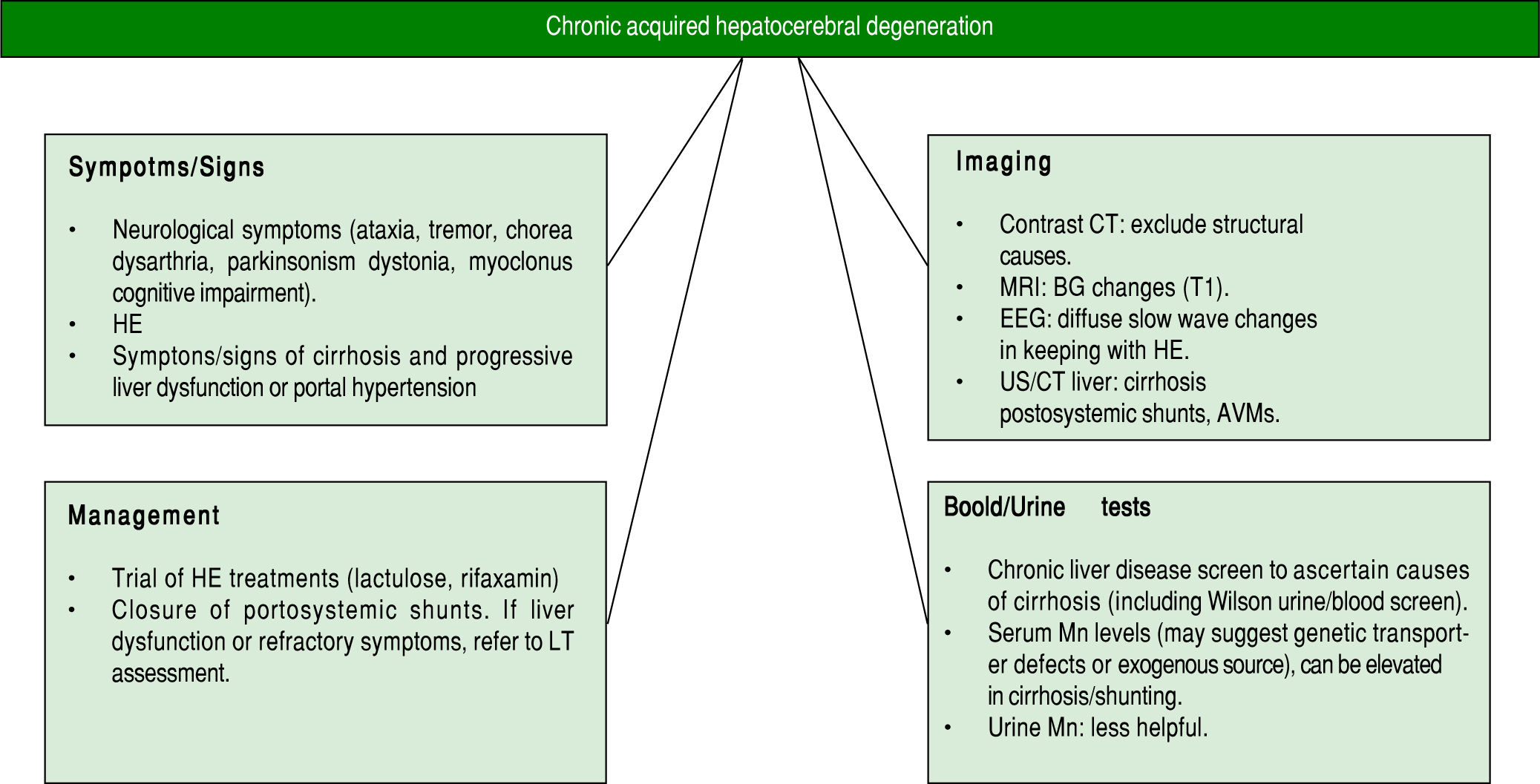
The clinical picture of CAHD includes neuropsychiatric movement disorders including ataxia, tremor, chorea, dysarthria, parkinsonism, dystonia and myoclonus.9,22 Most patients have a degree of cognitive impairment10 including difficulty with visuospatial attention.23 The term hepatic dementia has been used,24 however, there is an absence of global dementia and overt aphasia, apraxia or agnosia.10 Thus, the term dementia is best avoided as it can lead to misdiagnosis and labelling of patients that may preclude them from liver transplantation (LT) assessment. If profound neurological features, it is important to exclude other neurological conditions before the diagnosis of CAHD is made. The diagnostic modalities used to confirm or refute the diagnosis of CAHD can involve a combination of clinical examination, blood testing and neuro-imaging. Neuroradiological imaging such as contrast-CT or MRI can exclude other causes of neurological diseases such as small vessel disease, chronic subdural haematomas or other space occupying lesions. In CAHD specifically, MRI often reveals BG changes with hyper-intense signal changes on T1-weighted imaging mainly in the pallidum22,25 (with often normal T2 weighted images). T1-weighted MRI changes in the globus pallidus have been described in a large proportion of patients with cirrhosis, correlating with severity of liver disease, and have been found to be reversible post-LT suggesting that Mn deposition may be more common than generally recognized.26 Similar MRI changes occur in patients on long-term TPN but have also been seen in patients with Alagille’s syndrome who had high serum Mn levels-both of which normalised post liver transplantation.27
Blood tests can be less helpful in the diagnosis of CAHD. Blood Mn levels can vary in patients with CAHD and thus are not diagnostic. Very high blood Mn levels may suggest genetic causes for Mn overload or environmental exposures17 rather than CAHD. Intrahepatic Mn levels may also be elevated with high levels of hepatic Cu and Zn in patients with genetic overload conditions28-30 as opposed to CAHD. Mutations in the SLC30A10 transporter can lead to the onset of a familial Mn-induced parkinsonism and Mn retention in the liver (with subsequent liver damage), however these conditions are distinct from CAHD where shunting of Mn to the brain occurs rather than excess absorption/primary transporter defects. Serum Mn levels can be elevated in patients with cirrhosis even in the absence of CAHD. In a study from Spahr, et al.31 reported in 57 patients with cirrhosis, 67% had elevated Mn levels in the blood. Levels were significantly higher in patients with a previous portocaval anastomosis or transjugular intrahepatic porto-systemic shunt (TIPS) suggesting porto-systemic shunting of Mn may lead to increased blood levels and ultimately to deposition in the brain with potentially deleterious effects. There was a significant correlation between blood Mn levels with pallidal hyperintensity (PI) seen on MRI, however no correlations between blood Mn and liver synthetic function or neurological functions were seen. Also, there was no correlation between PI and neurological function. The importance of shunting, however, was highlighted by the fact that most patients with CAHD either have very advanced liver disease with established portal hypertension and/or surgical shunts, TIPS or spontaneous porto-systemic shunts.32 Other structural causes on imaging leading to shunting should be considered. These can include intrahepatic abnormal vascular malformations such as in conditions such as hereditary haemorrhagic telangiectasia where intrahepatic AVMs can lead to shunts bypassing the liver and resultant HE. Whilst the role of imaging is indeed helpful, there are no consensus recommendations in urine Mn testing in such patients, however, elevated levels often are see in patients with chronic Mn occupational/environ-mental exposure or ingestion. Although our patient had elevated levels there was no identifiable cause for this in his case. Ultimately, the diagnosis is made based on clinical suspicion and exclusion of other etiologies with no definitive diagnostic test.
Even if recognized, the treatment options for CAHD are limited (Figure 1). Trials of dopamine agonists in view of the Parkinson disease-like symptoms may be initiated, however, most patients do not respond to this approach, as was the case with our patient.33 There have been reports of improvement in symptoms with Rifaxamin - a non-absorbable antibiotic used for the treatment of HE although such studies are limited to small case series.34 With the link between porto-systemic shunting and CAHD, investigation for shunts is warranted with reported benefits in obliteration of spontaneous shunts.35 Liver transplantation has been reported to be effective for some patients with CAHD in small series,36-41 however, many patients present with advanced neurological symptoms, which often leads to exclusion from transplantation.37 There are no set guidelines to our knowledge to guide the role of liver transplantation in CAHD, and this option should be considered in the setting of refractory HE in the context of liver dysfunction due to cirrhosis. Stracciari, et al.10 described a series of 8 patients with CAHD, 3 of whom underwent transplantation over a 10-year period. Both clinical and neuro-radiologic abnormalities were reversed and improved after surgery, with prompt neurological recovery in all 3 individuals, and 2 of the 3 patients also showed improved cognitive performance post-transplantation. Large series of patients are still lacking in the field of transplantation for CAHD, making it difficult to assess the true efficacy of LT for this condition, however given the reported benefits in the cited examples, patients with decompensated cirrhosis not responding to medical therapies for HE should be considered for LT assessment, with the decision taking by individual units. As more individuals are transplanted, hopefully the transplant community will gain a better understanding of the potential for neurological recovery allowing for improved patient selection.
In summary, CAHD is a rare problem occurring in cirrhosis that can lead to debilitating neurological symptoms not readily amenable to pharmacological treatments. The formation of porto-systemic shunts may also aggravate the course of the disease by promoting increased shunting of Mn to the systemic circulation and the brain and thus shunts should be sought after and considered for closure if clinically appropriate. With limited effect of chelation therapy and dopamine agonists for CAHD, LT should be considered, however experience has been limited to small case series making it difficult to predict outcome. Increased awareness of CAHD will hopefully lead to increased recognition and ideally better therapeutic options for this challenging clinical entity.
Abbreviations- •
BG: basal ganglia.
- •
CAHD: chronic acquired hepatocerebral degeneration.
- •
HE: hepatic encephalopathy.
- •
Mn: manganese.
- •
MRI: magnetic resonance imaging.
- •
PI: pallidal hyperintensity.
- •
TIPSS: transjugular intrahepatic portosystemic shunts.
NR and MB wrote the review. JJF supervised project.
Conflicts of Interest StatementThe authors declares that there is no conflict of interest regarding the publication of this article.