Introduction. Extrahepatic portal vein obstruction is an important cause of portal hypertension among children. The etiology is heterogeneous and there are few evidences related to the optimal treatment. Aim and methods. To establish guidelines for the diagnosis and treatment of EHPVO in children, a group of gastroenterologists and pediatric surgery experts reviewed and analyzed data reported in the literature and issued evidence-based recommendations.
Results. Pediatric EHPVO is idiopathic in most of the cases. Digestive hemorrhage and/or hypersplenism are the main symptoms. Doppler ultrasound is a non-invasive technique with a high degree of accuracy for the diagnosis. Morbidity is related to variceal bleeding, recurrent thrombosis, portal biliopathy and hypersplenism. Endoscopic therapy is effective in controlling acute variceal hemorrhage and it seems that vasoactive drug therapy can be helpful. For primary prophylaxis of variceal bleeding, there are insufficient data for the use of beta blockers or endoscopic therapy. For secondary prophylaxis, sclerotherapy or variceal band ligation is effective; there is scare evidence to recommend beta-blockers. Surgery shunt is indicated in children with variceal bleeding who fail endoscopic therapy and for symptomatic hypersplenism; spleno-renal or meso-ilio-cava shunting is the alternative when Mesorex bypass is not feasible due to anatomic problems or in centers with no experience.
Conclusions. Prospective control studies are required for a better knowledge of the natural history of EHPVO, etiology identification including prothrombotic states, efficacy of beta-blockers and comparison with endoscopic therapy on primary and secondary prophylaxis.
Extrahepatic portal vein obstruction (EHPVO) is the most common cause of prehepatic portal hypertension in children. In most cases, variceal bleeding is the first clinical manifestation, often life-threatening and leading to anemia; other common presentation is splenomegaly and hipersplenism that often leads to hematologic work up including bone marrow biopsy. Guidelines exist for the diagnosis and treatment of portal hypertension in adults. Similar international guidelines for children have been based mainly on case reports and experts’ opinions due to lack of randomized controled studies reported in the literatura.1,2
AimTo develop clinical practice guidelines for the diagnosis and treatment of EHPVO in children in Mexico.
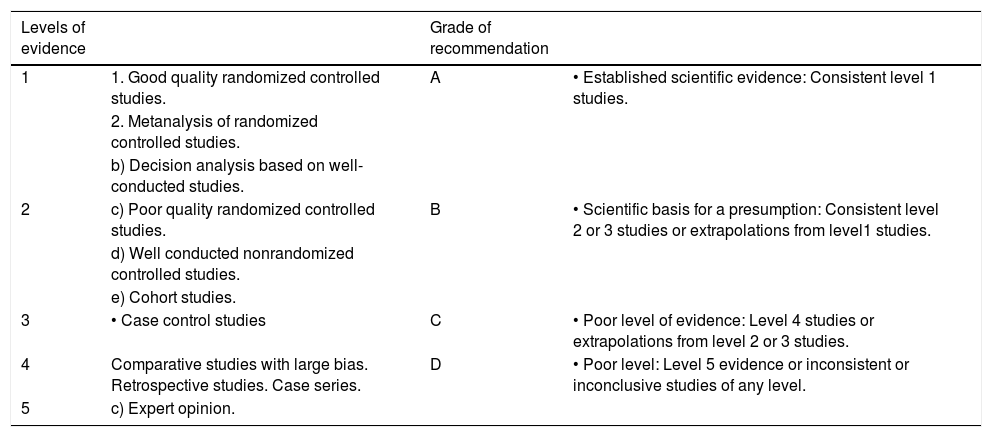
MethodsThese guidelines were developed during workshops that were held over three days during the 7th National Congress of Hepatology, which took place from June 22 to 24, 2011 in Cancun, Quintana Roo, Mexico. Pediatric gastroenterologists and pediatric surgeons with experience and interest in this subject, who work in Mexico’s leading centers for the treatment of children with portal hypertension (PHT), participated in the process. In order to make the current recommendations for the diagnosis and treatment of EHPVO in children, scientific evidence published using a modified version of the Oxford system for “Evidence-Based Medicine” (Table 1),3 was reviewed.
Modified Oxford System.
| Levels of evidence | Grade of recommendation | ||
|---|---|---|---|
| 1 | 1. Good quality randomized controlled studies. | A | • Established scientific evidence: Consistent level 1 studies. |
| 2. Metanalysis of randomized controlled studies. | |||
| b) Decision analysis based on well-conducted studies. | |||
| 2 | c) Poor quality randomized controlled studies. | B | • Scientific basis for a presumption: Consistent level 2 or 3 studies or extrapolations from level1 studies. |
| d) Well conducted nonrandomized controlled studies. | |||
| e) Cohort studies. | |||
| 3 | • Case control studies | C | • Poor level of evidence: Level 4 studies or extrapolations from level 2 or 3 studies. |
| 4 | Comparative studies with large bias. Retrospective studies. Case series. | D | • Poor level: Level 5 evidence or inconsistent or inconclusive studies of any level. |
| 5 | c) Expert opinion. |
Extrahepatic portal venous obstruction (EHPVO) occurs when a blockage of the portal vein prevents blood flow into the liver. This causes a formation of collateral vessels and bypasses known as a portal cavernoma to form around the obstructed site, which is detected in 40% of children with upper digestive tract bleeding. Approximately 79% of the affected children will have at least one serious bleeding episode during the course of its development.4,5 Variceal bleeding was the first symptom reported in 71% (10/14) Mexican children with EHPVO, none of these hemorrhage episodes were fatal.6
What is the definition of extrahepatic portal venous obstruction?- •
It is defined as an extrahepatic occlusion of the portal vein, with or without involvement of the intrahepatic portal veins, splenic vein or superior mesenteric vein.
- •
It is frequently characterized by the presence of a portal cavernoma.
- •
Isolated occlusion of the splenic vein or superior mesenteric vein do not constitute EHPVO and may require distinct management approaches.
- •
Portal vein obstruction associated with chronic liver disease or neoplasia does not constitute EHPVO.7,8
Level of evidence 1, grade of recommendation A.
How common is EHPVO in children?According to WHO, EHPVO fulfils the criteria for rare diseases, with a prevalence of < 5 per 10,000 inhabitants. It is the cause of PHT in more than 30% of cases of children with esophageal variceal bleeding.5,9
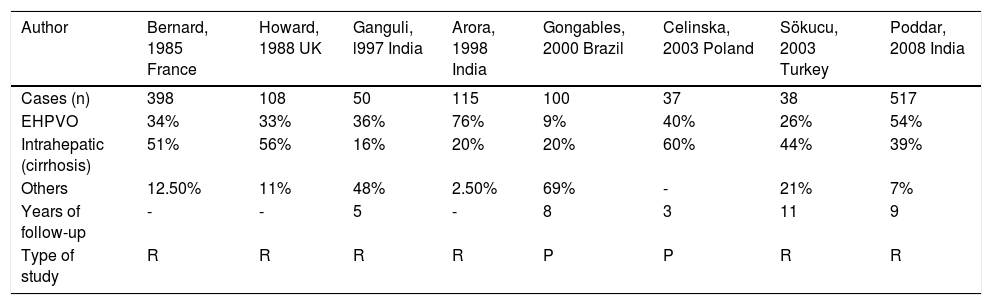
The frequency of EHPVO is variable and depends on the location of the obstruction to portal flow, being involved in 9% to 76% of all cases of portal hypertension in children (Table 2).10–17
Percentage of children with portal hypertension according to location of the obstruction.
| Author | Bernard, 1985 France | Howard, 1988 UK | Ganguli, l997 India | Arora, 1998 India | Gongables, 2000 Brazil | Celinska, 2003 Poland | Sökucu, 2003 Turkey | Poddar, 2008 India |
|---|---|---|---|---|---|---|---|---|
| Cases (n) | 398 | 108 | 50 | 115 | 100 | 37 | 38 | 517 |
| EHPVO | 34% | 33% | 36% | 76% | 9% | 40% | 26% | 54% |
| Intrahepatic (cirrhosis) | 51% | 56% | 16% | 20% | 20% | 60% | 44% | 39% |
| Others | 12.50% | 11% | 48% | 2.50% | 69% | - | 21% | 7% |
| Years of follow-up | - | - | 5 | - | 8 | 3 | 11 | 9 |
| Type of study | R | R | R | R | P | P | R | R |
R: Retrospective. P: Prospective.
Level of evidence 4, grade of recommendation C
What causes EHPVO?Several causes have been associated with EHPVO.2
- •
Direct injury to the portal vein due to omphalitis and umbilical vein catheterization, the latter is considered to be one of the major risk factors and is associated with: a delay in placement, catheterization for more than 3 days, misplacement, trauma and type of solution used.
- •
Portal vein abnormalities such as stenosis, atresia, and agenesis often are associated with congenital shunting and do not fit into the typical paradigm of EHPVO.
- •
Indirect factors: neonatal sepsis, dehydration, multiple exchange transfusions and prothrombotic states.
- •
Idiopathic, with no identifiable etiology.
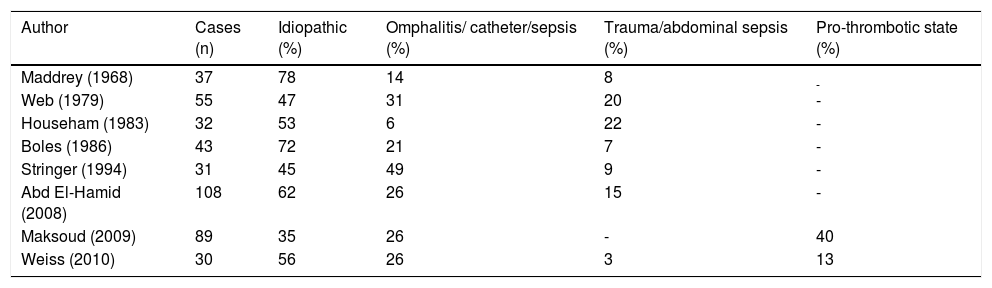
Causes of EHPVO, according to reports in the literature, are shown in table 3. Neonatal events such as omphalitis, umbilical vein catheterization or sepsis are present in 6% to 49% of cases; in those in which abdominal trauma or sepsis are present, the frequency varies between 3% and 22%.18–26
Frequency of causes associated with EHPVO in children.
| Author | Cases (n) | Idiopathic (%) | Omphalitis/ catheter/sepsis (%) | Trauma/abdominal sepsis (%) | Pro-thrombotic state (%) |
|---|---|---|---|---|---|
| Maddrey (1968) | 37 | 78 | 14 | 8 | - |
| Web (1979) | 55 | 47 | 31 | 20 | - |
| Househam (1983) | 32 | 53 | 6 | 22 | - |
| Boles (1986) | 43 | 72 | 21 | 7 | - |
| Stringer (1994) | 31 | 45 | 49 | 9 | - |
| Abd El-Hamid (2008) | 108 | 62 | 26 | 15 | - |
| Maksoud (2009) | 89 | 35 | 26 | - | 40 |
| Weiss (2010) | 30 | 56 | 26 | 3 | 13 |
Level of evidence 4, grade of recommendation C
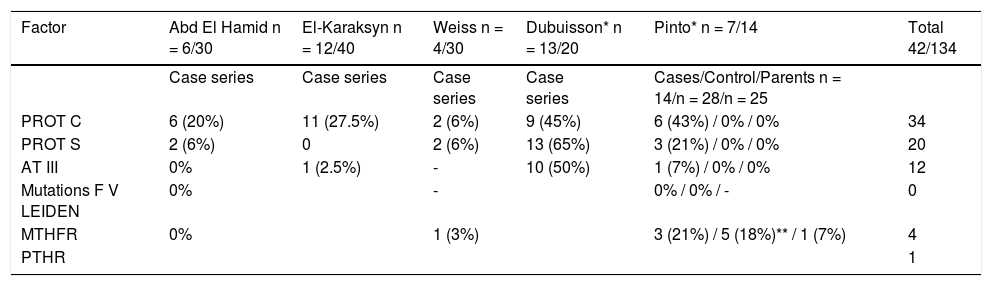
Prothrombotic states associated with EHPVO have been little studied and the results vary (Table 4).
Frequency of prothrombotic states in children with EHPVO.
| Factor | Abd El Hamid n = 6/30 | El-Karaksyn n = 12/40 | Weiss n = 4/30 | Dubuisson* n = 13/20 | Pinto* n = 7/14 | Total 42/134 |
|---|---|---|---|---|---|---|
| Case series | Case series | Case series | Case series | Cases/Control/Parents n = 14/n = 28/n = 25 | ||
| PROT C | 6 (20%) | 11 (27.5%) | 2 (6%) | 9 (45%) | 6 (43%) / 0% / 0% | 34 |
| PROT S | 2 (6%) | 0 | 2 (6%) | 13 (65%) | 3 (21%) / 0% / 0% | 20 |
| AT III | 0% | 1 (2.5%) | - | 10 (50%) | 1 (7%) / 0% / 0% | 12 |
| Mutations F V LEIDEN | 0% | - | 0% / 0% / - | 0 | ||
| MTHFR | 0% | 1 (3%) | 3 (21%) / 5 (18%)** / 1 (7%) | 4 | ||
| PTHR | 1 |
MTHFR (C677T): methylene tetrahydrofolate reductase. PTHR (G20210): prothrombin mutation.
There is evidence that deficiencies of coagulation inhibitors (protein C, protein S, antithrombin III), prothrombin (PTHR) gene G20210A mutation, methylene tetrahydrofolate reductase (MTHFR) gene C677T mutation and factor V Leiden (FVL) mutation may predispose to portal vein thrombosis. In 5 studies of children and adolescents thrombophilia was found in a total of 42 of 134 (31%) cases.23,25,27–29 Protein C (PC) deficiency appears to be the most frequent followed by deficiencies in protein S (PS) and antithrombin III (ATIII); approximately 20% of cases had combined deficiencies. A genetic origin has not yet been proven; the studies reported involved a small number of patients or were isolated case reports. In a prospective randomized trial in 17 children with EHPVO, seven had low levels of PC, PS and ATIII; none of the 24 controls nor any of the 25 parents of the children studied were found to have deficiencies in these factors and as for genetic mutations, there was no significant difference in the frequency of MTHFR between cases and controls; only a few isolated cases of mutant PTHR were seen.29
Level of evidence 3, grade of recommendation C
EHPVO is between 35% to 78% of cases idiopathic18–25 so that a greater number of studies seeking to identify the etiology are required, especially those aimed at detecting prothrombotic states.
Level of evidence 4, Grade of recommendation D
Non-Endoscopic DiagnosisThe most significant survey of diagnostic tests used for portal hypertension was reported in adult patients with cirrhosis, the aim of the tests being to:
- 1.
Establish the diagnosis.
- 2.
Detect the presence of esophageal varices.
- 3.
Identify the determinants of portal hypertension.
These objectives have been extrapolated to pediatric patients diagnosed with pre-hepatic portal hypertension.30–32
Which clinical criteria are needed for a presumptive diagnosis?1. Gastrointestinal bleeding and splenomegaly.EHPVO should be suspected in all pediatric patients presenting with unexplained gastrointestinal bleeding and splenomegaly. Shneider33 evaluated pediatric series with diagnosis of extrahepatic portal hypertension and reported that 46% to 90% of them presented with gastrointestinal hemorrhage and 25% presented with splenomegaly, and concluded that “the combination of gastrointestinal hemorrhage and splenomegaly should be suggestive of portal hypertension until proven otherwise”.
Hypersplenism and/or unexplained pancytopenia is other important clinical feature,4,5,10 that must be recognize earlier. Some of these cases undergo an extensive hematologic work up including bone marrow biopsy before EHVOP is suspected.
Level of evidence 3, grade of recommendation C.
2. Medical history.- •
Pediatric EHPVO may be present in the absence of any recognized risk factors.
- •
Umbilical vein catheterization at the time of birth. This is frequently associated with the presence of EHPVO,31,32,34,35 manifesting as gastrointestinal bleeding later in childhood. The thrombotic effect is induced by mechanical and chemical damage to the umbilical vein wall; Morag reported that 133 infants in the neonatal period and 5 during lactation developed portal vein thrombosis, 73% of them had had an umbilical vein catheter inserted, which was in an appropriate position in 46% of them.34
Level of evidence 3, grade of recommendation C.
- •
Associated diseases. EHPVO has been reported in association with perinatal events such as asphyxia, persistent pulmonary hypertension, sepsis and presence of congenital heart disease, a high percentage of which are cyanotic.34
- •
Hypercoagulability syndromes:
- a)
Hereditary thrombophilia: risk factors for EHPVO include mutations in the prothrombin gene, factor V Leiden (FVL), methylene tetrahydrofolate reductase (MTHFR) gene and deficiencies of proteins C and S.36–39 A prospective case-control study that included 31 patients between the ages of 11 months and 18 years and a control group of 26 children, reported an incidence of FVL mutation in 7%, heterozygous G20210A mutation in the prothrombin gene in 10% and MTHFR-C667T mutation in 69% of the patients.36 It is recommended that hereditary thrombophilia be rouled out in cases of unknown etiology or family history of prothrombotic disorders. Anti-coagulation therapy should be considered in cases with a well-documented prothrombotic state.2
- a)
Level of evidence 3, grade of recommendation C.
b) Acquired thrombophilia: it has been reported in association with anti-phospholipid syndrome and paroxysmal nocturnal hemoglobinuria.33,34
Level of evidence 4, grade of recommendation D.
3. Physical examination.Collateral venous network and splenomegaly are the common manifestations. There may be stunted growth and developmental delay. Ascites is a rare condition but it may appear after a bleeding episode, associated to decreased serum albumin levels, aggressive fluid management or in cases with EVH-PO associated to intrinsic liver disease that should be ruled out. Clinical signs of hypersplenism are petechiae or ecchymoses; other less common are jaundice caused by cholestasis associated with external compression of the bile ducts due to the cavernous degeneration of the portal vein and dyspnea, exercise intolerance and finger clubbing due to hepatopulmonary syndrome.31–33
Level of evidence 4, grade of recommendation C.
4. Laboratory tests.- a)
Complete blood count: assesses the presence of secondary hypersplenism (anemia, leukopenia and thrombocytopenia).33
- b)
Liver function tests: to rule out associated liver disease, including: alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase and serum bilirubin levels, serum proteins (albumin and globulin).34
- c)
Clotting tests and coagulation factor assays.
- d)
Thrombophilia testing: mutations in the prothrombin gene, factor V Leiden (FVL), methylene tetrahydrofolate reductase (MTHFR) gene and deficiencies of proteins C, S and AT III, it is advisable to perform when no other etiology can be found or in cases where there is a family history of thrombophilia.36–38
Level of evidence 3, Grade of recommendation C.
5. Follow up studies.Follow-up of children with esophageal varices has shown that variables that are significant as non-invasive predictors include the platelet: spleen diameter ratio (p < 0.001), platelet count (p < 0.001), INR (international normalized ratio) (p = 0.001), aspartate aminotransferase: alanine aminotransferase ratio (p = 0.002) and serum albumin levels (p = 0.003). These variables have been studied in children with both extra- and intrahepatic portal hypertension.39,40
Level of evidence 3, grade of recommendation C.
What are the different methods for non-endoscopic diagnosis of EHPVO in children?- 1.
Liver ultrasound: allows assessment of liver size and echogenicity, spleen size and presence of cavernous degeneration of the portal vein (vessels or collateral veins around the thrombosed portal vein appear to become recanalized). In children with history of PVT in the neonatal period, it has been shown that abdominal ultrasounds is the most sensitive method for detecting progression of portal hypertension caused by PVT. It is therefore advisable to perform periodic abdominal ultrasound examinations in patients with a history of neonatal PVT.31,34,38,41 Ultrasound is also recommended during follow up in order to detect portal biliopathy (irregular diltation of the biliary tree) and associated colelithiasis and cholecystitis.2
- 2.
Doppler ultrasound: used to assess patency of the portal and the splenic vein, the hemodynamic status of the portal system, distinguish the portal vein from the inferior vena cava and the hepatic artery and determine the direction of blood flow: hepatopetal or hepatofugal.42
- 3.
Splenoportography: allows evaluation of the portal venous system, extent and location of the obstruction and presence of collateral vessels; its invasive nature limits its use.5
- 4.
Other techniques: computed tomography and MRI angiography should be the first option to assess the anatomy of the portal system and it have been recommended in cases where surgery is required.5,7
Level of evidence 3, grade of recommendation C.
- 5.
Measurement of portal pressure/hepatic venous pressure gradient. Assesses type and severity of portal hypertension; it is an invasive technique and published clinical experience reports discuss its use in children with intrahepatic portal hypertension.43 It is of no value in EHPVO as hepatic vein pressure gradient is normal in this condition and will not detect the portal hypertension otherwise it is associated with intrahepatic disease.
Level of evidence 4, grade of recommendation D.
Which is the non-endoscopic diagnostic method of choice?Abdominal Doppler ultrasound, because it evaluates portal blood flow velocity and arterio-portal ratio, is a non-invasive technique that has been reported in the evaluation of EHPVO in children, with a sensitivity of 91% and a specificity of 100%.39,40,42,43
Level of evidence 3, grade of recommendation C.
Endoscopic DiagnosisEsophagogastroduodenoscopy (EGD) is the best procedure to screen for esophageal and gastric varices and should be done in every suspected case of portal hypertension; the variceal grade findings such as large tense varices, red spots and gastric varices will help to identify those cases at risk of gastrointestinal bleed.44
What is the classification of esophageal varices?Soehendra’s classification of esophageal varices is the one most widely used in endoscopic practice45 (Table 5) although at the most recent workshops on methodology of diagnosis in Baveno in 2005 and 20108,46 it was recommended that varices be defined according to size (Table 6) as:
- a)
Small varices. Minimally elevated veins above the esophageal mucosa surface.
- b)
Medium varices. Tortuous veins occupying less than one third of the esophageal surface, and
- c)
Large varices. Those occupying more than one third of the esophageal surface.
Esophageal varices. Soehendra classification.
| • Grade I. Mild dilatation, diameter < 2 mm, barely rising above relaxed esophagus, more marked in head down position. |
| • Grade II. Moderate dilatation, tortuous, diameter 3–4 mm, limited to the lower part of the esophagus. |
| • Grade III. Total dilatation, taut, diameter > 4 mm, thin-walled, varices upon varices, in gastric fundus. |
| • Grade IV. Total dilatation, taut, occupy the entire esophagus, frequent presence of gastric or duodenal varices. |
Recommendations for medium-sized varices are the same as for large varices because this is how they were grouped in prophylactic trials47 as well as in a meta-analysis of randomized controlled clinical trials.44,45,48,49
Other classification used in children to evaluate the severity of the esophageal varices by endoscopic findings had been reported as:
- •
Grade I. When varices are flattened by insufflations.
- •
Grade II. When varices are not flattened by insufflations but are separated by healthy mucosa.
- •
Grade III. When varices are not flattened by insufflations and had mucosa red signs.50
Level of evidence 5, grade of recommendation D.
What is the classification of gastric varices?Risk factors for gastric variceal hemorrhage include the size of fundal varices which are classified by size into large (> 10 mm), médium-sized (5–10 mm) and small (< 5 mm), respectively.45,51
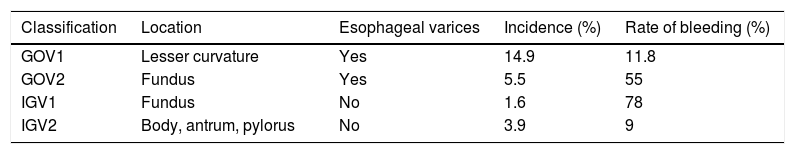
According to Sarin, gastric varices are commonly classified based on the presence or absence of esophageal varices and their location in the stomach (fundus or lesser curvature)52 (Table 7). They are divided into GOV-1, with esophageal varices extending along the lesser curvature; GOV-2, with esophageal varices located in the gastric fundus; IGV-1 located in the fundus, without presence of esophageal varices, and, IGV-2, with varices in the lesser curvature but no esophageal varices. The rate of bleeding depends on location, varices located in the gastric fundus cause bleeding more often. Sarin’s classification continues in use up to the present and has been useful in helping to determine the type of treatment in adults, according to the findings.
Level of evidence 5, grade of recommendation D.
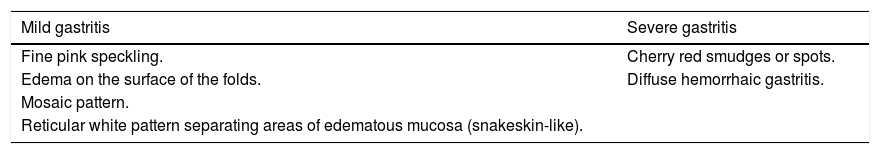
What is the definition and classification of hypertensive gastropathy?Hypertensive gastropathy is a mucosal lesion characterized by ectatic gastric mucosal vessels, mainly in the fundus and body of the stomach. It is classified as mild or severe (Table 8).53 In mild gastritis there is fine pink speckling, erythema on the surface of the folds, presence of a reticular white mosaic pattern separating areas of erythematous and edematous mucosa that resemble snakeskin. Severe gastritis presents with cherry red smudges or spots with diffuse hemorrhagic gastritis. The presence of gastro-esophageal varices is a predictive factor for hypertensive gastropathy and the presence of gastropathy is high in patients with esophageal varices who have undergone endoscopic treatment.53,54
Classification of portal hypertensive gastropathy.
| Mild gastritis | Severe gastritis |
|---|---|
| Fine pink speckling. | Cherry red smudges or spots. |
| Edema on the surface of the folds. | Diffuse hemorrhaic gastritis. |
| Mosaic pattern. | |
| Reticular white pattern separating areas of edematous mucosa (snakeskin-like). |
Level of evidence 4, grade of recommendation C.
II. TreatmentPrimary ProphylaxisWhat medications exist for primary prohylaxis of EHPVO?Propranolol and nadolol are the most widely studied non-selective beta-blockers in adult patients with cirrhosis; their mechanism of action involves blocking the β1 receptors that causes decreased cardiac output and splanchnic vasoconstriction by blocking β2 receptors. Reduction of the hepatic venous pressure gradient (HVPG) to less than 12 mmHg or a reduction greater than 20% from baseline is the only appropriate parameter for evaluating reduction in portal pressure; however performing this invasive procedure in children raises ethical issues, so it is not routinely performed.1,44 To assess the effective dose, a 25% reduction in heart rate (HR) has been recommended however in adults not even a 40% reduction in the HR has produced a significant decrease in HVPG.55 The decrease in HR only seems to be useful for determining tolerance to the drugs and not response to treatment. The dose used in children was between 1 to 2 mg/kg/day and in some cases up to 8 mg/kg/day, to achieve a 25% reduction in HR from baseline.56 Reported adverse effects in adults are: bradycardia, heart failure, behavioral disturbances, bronchospasm and hypoglycemia, which have been reported in 10 to 20% of the cases treated.57 There is not enough information to recommend their use in children with EHPVO.
Level of evidence 4, grade of recommendation D.
Is primary prophylaxis with NSBB justified in children with EHPVO who have not yet developed esophageal varices?There are no reports available in the reviewed literature, in either children or adults, to justify use of non-selective beta-blockers (NSBB) in preventing the development of varices.
Level of evidence 4, grade of recommendation D.
Is primary prophylaxis with NSBB justified in children with esophageal varices?There are few studies in children that prove BBs are effective in reducing the risk of bleeding. Retrospective and prospective case series in children treated with propranolol with or without cirrhosis, first bleeding occur in 16% and 35% of cases in a follow-up time between 3 and 5 years respectively,58–60 these percentage is similar for the first bleeding that occurs in 30% of the cases in the natural course of the disease over a follow-up period of 24 months.60,61 In adult patients with cirrhosis controlled studies showed that propranolol reduces the risk of bleeding between 30% and 50%; this effect is limited to those with medium or large varices.62,63
Based on the limited evidence, we cannot recommend propranolol for primary prophylaxis in children with EHPVO.64
Level of evidence 4, grade of recommendation D.
Is sclerotherapy and variceal ligation justified for primary prophylaxis?Endoscopic therapy (sclerotherapy or variceal ligation) in adult patient with medium and large varices had been reported to reduce the risk of bleeding by 30% without altering mortality.65 In children sclerotherapy for primary prophylaxis has been reported in 26 cases, 42% had variceal bleeding within an average of 2.4 years after the endoscopic therapy;66 in a prospective study of 100 children with portal hypertension with different etiologies, variceal hemorrhage appeared in 6% with sclerotherapy vs. 42% (p < 0.05) of those who did not undergo the procedure during a mean follow-up of 36 months. However the rate of variceal gastric hemorrhage and hypertensive gastropathy (HTG) seems to be higher in those who had sclerotherapy 38% vs. 6% who did not have sclerotherapy (p < 0.05),14 this is an important aspect to consider before initiate an esclerotherpay as primary prophylaxis treatment. In most of these cases the patients had cirrhosis, so that on the basis of these studies, prophylactic sclerotherapy cannot be recommended in children with EHPVO.
Variceal ligation for primary prophylaxis has been reported in only two series of children with and without cirrhosis and showed a bleeding rate of 0%–10% during a follow-up of 16 and 23 months, respectively, this procedure was well tolerated without major complications.15,67 There are still few studies in children with EHPVO to allow recommendation of sclerotherapy and ligation for primary prophylaxis. However, in children with large varices who are at a high risk of bleeding or living far from the hospital, endoscopy therapy is justified, otherwise it must be done in a protocolized study.
Level of evidence 4, grade of recommendation D.
Is combined therapy (propranolol + sclerotherapy/ ligation) justified for primary prophylaxis?There is no information regarding the combined use of propranolol and sclerotherapy/ligation in children, so therefore it cannot be recommended.
Level of evidence 4, grade of recommendation D.
What are the time intervals of endoscopic surveillance?It is recommended that endoscopy be performed every 2–3 years in adult patients with cirrhosis without varices. Patients with small varices should have one every 1–2 years and patients with big varices should have one every year.46
Nothing has been published to support endoscopic screening in children with EHPVO or to define appropriate follow-up times so that studies are necessary to do so.56 Extrapolating from adults to children and taking the natural course of the disease into consideration,61 we believe that an endoscopy should be performed initially in every child with EHPVO and follow up must be under a study protocol every 1–2 years in children with esophageal varices with no history of bleeding.
Level of evidence 4, grade of recommendation D.
Control of Acute Variceal BleedingWhat therapeutic approaches are there for controlling acute variceal bleeding?- 1.
Stabilization and restitution of blood volume to be done cautiously to maintain hemoglobin between 8–10 mg/dL.1
- 2.
Vasoactive drugs. Terlipressin, somatostatin and octreotide, they should be maintained between 48 h and 5 days.46
- 3.
Endoscopic treatment. Ligation is the treatment of choice for bleeding from esophageal varices (in older children) within the first 24 hours after admission and after the child is hemodynamically stable.2 For bleeding gastric varices the procedure is obturation with cyanoacrylate.1,2
- 4.
Sengstaken-Blakemore tube. Stops bleeding in almost 90% of cases, to be used as a temporary measure and should be inserted by trained staff because of the high risk of complications.68
Level of evidence 1, grade of recommendation A.
What drugs are used to treat acute variceal bleeding?Vasoactive drugs cause splachnic vasoconstriction, thereby reducing portal pressure and controlling variceal bleeding in 75% to 80% of patients. They should be started as soon as possible, prior to endoscopy in patients with suspected variceal hemorrhage.46,67
Level of evidence 1, grade of recommendation A.
a) Octreotide. It is a somatostatin analogue that is equally effective; control of bleeding was reported in 70% of 3 pediatric case series. The recommended dose is a 1 to 2 mcg/kg bolus followed by infusion of 1 to 2 µcg/kg/h. The infusion is adjusted according to response. When the bleeding is under control, the dose is reduced by 50% every 12 to 24 h.69,70
Level of evidence 1, grade of recommendation A.
b) Terlipressin is a synthetic analog of vasopressin, with a longer shelf life and fewer side effects. Control of bleeding within the first 48 h is achieved in 75% to 80% of adults and of 67% within 5 days. It is administered as an initial IV dose of 1 mg for < 50 kg, 1.5 mg for 50–70 kg, 2 mg for > 70 kg followed by 1–2 mg every 4 h for 5 days. In pediatrics, use of terlipressin has been reported as rescue treatment in patients with refractory septic shock and bleeding in the digestive system in a case series in which control of bleeding was achieved in 8/15 (53%) of cases.71,72 More information is required before recommendations for its routine use in the treatment of esophageal varices in children can be made.56
Level of evidence 5, grade of recommendation D.
What are the endoscopic techniques for the control of acute variceal bleeding?Depending on the age of the patient and skill of the endoscopist, both sclerotherapy and ligation have been proven effective in controlling acute variceal bleeding. Endoscopic therapy should be performed within the first 12 to 24 h of the onset of bleeding, after the child is stable and hemodynamically compensated.1,2,46
Level of evidence 1, grade of recommendation A.
- •
Sclerotherapy. The obliteration of varices can be achieved in approximately 92% of children with extrahepatic portal hypertension and this technique can be used in infants, children and adolescents.11,73–75
- •
Ligation of esophageal varices. Has been reported to be effective for controlling acute bleeding in 96% of cases, similar to sclerotherapy, which has a 92% rate.73,75,76
Level of evidence 1, grade of recommendation A.
- •
Combination therapy. A meta-analysis of adults treated with a combination of vasoactive drugs and endoscopic therapy showed better control of initial bleeding and at day 5.77 A case report in children showed control of bleeding control in 90%.78
Level of evidence 4, grade of recommendation D.
What are the criteria for failure of endoscopic treatment?- •
Adults. Persistence of hematemesis > 2 h after endoscopic therapy is considered a failure to control acute variceal bleeding, Hb decrease of > 3 g/dL.8
- •
Children. Fresh hematemesis or nasogastric aspiration of > 2 mL/kg/h or 100 mL of fresh blood > 2 h after the therapeutic endoscopy.2,46
Level of evidence 1, grade of recommendation A.
What are the criteria for using Sengstaken-Blakemore tube?The device consists of two balloons, one for the esophageal varices and one for gastric varices; it stops bleeding in nearly 90% of cases. However, it is only temporary measure and should not be left in place for more than 24 h; it has a high rate of complications such as mucosal ischemia, perforation, aspiration and airway obstruction. It is recommended for use in cases when drug and/or endoscopic treatment fail, or as a temporizing measure until surgery.68,79
Level of evidence 4, grade of recommendation D.
What is the mortality after the first episode of variceal bleeding?It is unknown in children with EHPVO, it has been reported to be between 0% to 8% in cases with intra- and extrahepatic portal hipertensión.58,74,80–82
Level of evidence 4, grade of recommendation D.
What are the treatment failure criteria of acute variceal bleeding?The criteria have not been clearly defined for children with EHPVO; extrapolating from adults to children, the following criteria should be taken into account:1,2
The time frame for the acute bleeding episode is 120 h (5 days).
Treatment failure signifies the need to change therapy of at least one of the following criteria:
- 1.
Fresh hematemesis is ≥ 2 h after the start of a specific drug or endoscopic treatment.
- 2.
For patients with a nasogastric tube, aspiration of more than 100 mL of fresh blood or more than 2 mL/kg.
- 3.
Three gram drop in Hb and 9% drop in Hct if no blood transfusion is administered.
- 4.
Death.
- 5.
Some people consider a score ≥ 0.75 on the ABRI index at any time point denotes failure, there is no data related to the use of ABRI in children.
ABRI = blood units transfused/(final hematocritinitial hematocrit)+ 0.01)
Level of evidence 5, grade of recommendation D.
Secondary Prophylaxis1. Drugs.Which drugs are used for secondary prophylaxis in EHPVO patients?The most widely used non-selective beta-blocker is propranolol. The evidence is insufficient to recommend its use in adults with prehepatic portal hiypertension;1 most studies are in cirrhotic patients and it has not been shown to be superior to endoscopic treatment. In children, most of who presented with intrahepatic portal hypertension, variceal rebleeding has been reported in between 25% to 53% of cases, in a follow-up time between 3 and 5 years.58,59,83
To date, there are insufficient data to allow recommendation for use of propranolol as standard therapy for the secondary prophylaxis of bleeding esophageal varices in children with EHPVO.
Level of evidence 5, grade of recommendation D.
Is use of beta-blockers in combination with sclerotherapy/ligation indicated?Non-selective beta-blockers (especially propranolol) have been used in combination with ligation for secondary prophylaxis in adult patients with prehepatic portal hypertension.1
A meta-analysis of adults with both prehepatic and intrahepatic portal hypertension showed that a combination of propranolol and ligation or sclerotherapy is superior to endoscopic treatment alone for the control of bleeding, which is why it is recommended as first line management.84
To date, combination therapy for secondary prophylaxis cannot be recommended, as there are no data regarding its use in children. Studies in this regard are needed and it could be used within a protocol.
Level of evidence 5, grade of recommendation D.
What is the mortality rate from rebleeding with use of beta-blockers?There are no data about mortality following re-bleeding from esophageal varices in pediatric patients with portal hypertension due to EHPVO.
Level of evidence 5, grade of recommendation D.
2. Endoscopic Procedures.What are the endoscopic procedures for secondary prophylaxis in EHPVO patients?a) Sclerotherapy.
- •
Description. Endoscopic procedure that involves intra- or paravariceal injection of pharmacological agents in order to obliterate esophageal varices and/or control active bleeding.
- •
Types of sclerosing solutions. The most common are: 1% and 3% polidocanol, 5% ethanolamine oleate, 100% ethanol, sodium morrhuate. There are no studies comparing the different sclerosing agents for efficacy, adverse effects or with recommendations for their use in children, so we cannot recommend any drug in particular.85
- •
Indications. During acute bleeding episodes and for the eradication of esophageal varices. 3 to 7 sessions are required to eradicate the varices.11,73,74,86
- •
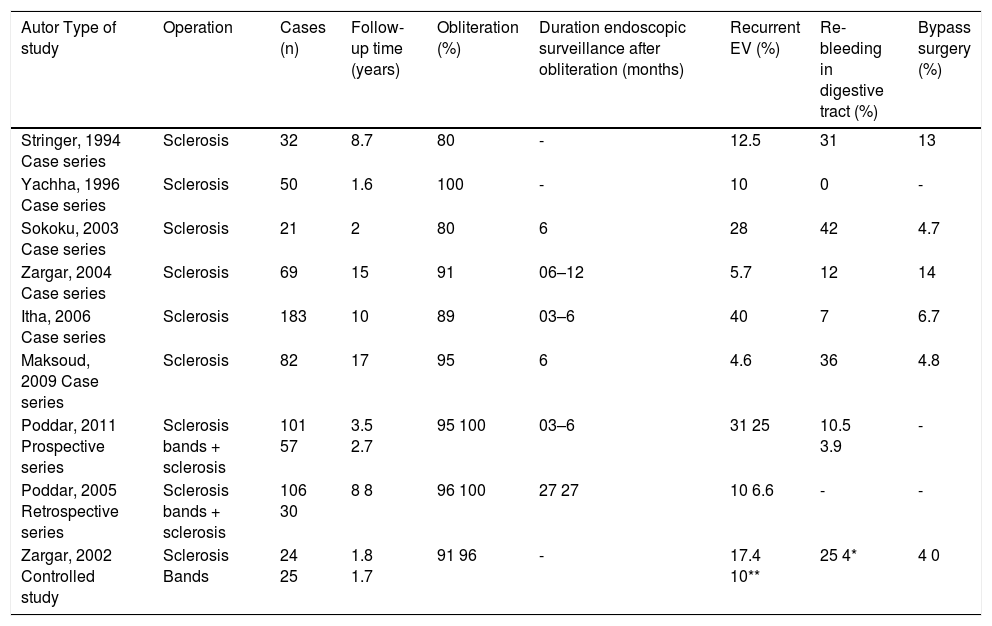
Response to treatment. In pediatric patients with pre-hepatic PHT, variceal eradication by sclerotherapy is achieved in 80 to 100% of cases,16,66,73,80,87–90 and the rate of recurrence of esophageal varices is 10% to 40% after eradication. Gastrointestinal rebleeding is frequently caused by gastric varices and occurs in 0 to 42% of cases. Bypass surgery is necessary in 4% to 13% of cases (Table 9).
Table 9.Secondary prophylaxis in children with variceal bleeding due to extrahepatic portal hypertension.
Autor Type of study Operation Cases (n) Follow-up time (years) Obliteration (%) Duration endoscopic surveillance after obliteration (months) Recurrent EV (%) Re-bleeding in digestive tract (%) Bypass surgery (%) Stringer, 1994 Case series Sclerosis 32 8.7 80 - 12.5 31 13 Yachha, 1996 Case series Sclerosis 50 1.6 100 - 10 0 - Sokoku, 2003 Case series Sclerosis 21 2 80 6 28 42 4.7 Zargar, 2004 Case series Sclerosis 69 15 91 06–12 5.7 12 14 Itha, 2006 Case series Sclerosis 183 10 89 03–6 40 7 6.7 Maksoud, 2009 Case series Sclerosis 82 17 95 6 4.6 36 4.8 Poddar, 2011 Prospective series Sclerosis bands + sclerosis 101 57 3.5 2.7 95 100 03–6 31 25 10.5 3.9 - Poddar, 2005 Retrospective series Sclerosis bands + sclerosis 106 30 8 8 96 100 27 27 10 6.6 - - Zargar, 2002 Controlled study Sclerosis Bands 24 25 1.8 1.7 91 96 - 17.4 10** 25 4* 4 0
Level of evidence 3, grade of recommendation C.
b) Ligation of esophageal varices.
- •
Description. Ligation is an endoscopic procedure that consists in the use of ties or bands to obliterate the varix and/or control active bleeding. The main limitation in pediatric patients is the size of the overtube for band placement, as it can measure as much as 11 mm in diameter. There are no reports of its use based on the age and weight of children, in our setting it is performed after the age of one year or weight > 10 kg. Nevertheless, it must be individualized for each patient.91
- •
Indications. For controlling acute bleeding and for the obliteration of varices already receiving sclerotherapy.
- •
Response to treatment. Ligation controls acute EV bleeding in 96% of cases.73,90 Obliteration of the varices is achieved in 16 to 100% of cases treated with 2 to 4 sessions being necessary to achieve obliteration.91–94 Recurrence of EV in pediatric patients has been reported to be from 5% to as much as 75%.90–95
Level of evidence 4, grade of recommendation D.
Which is the best endoscopic treatment?By comparison with sclerotherapy, ligation requires fewer sessions (3–9 vs. 6.1) and as such, fewer anesthetic procedures; there are fewer major complications (25% vs. 4%), whereas there is no difference in control of bleeding or in the obliteration of esophageal varices, which is achieved in 91.7% and 96% respectively (p < 0.61).70,73
Level of evidence 1, grade of recommendation A.
Which are the criteria of endoscopic treatment failure?- a)
Failure to control active bleeding or a re-bleeding episode after two separate attempts of the same endoscopic treatment.
- b)
Three or more re-bleeding episodes requiring endoscopic treatment and blood transfusion.73
Level of evidence 5, grade of recommendation D.
What are the complications of endoscopic treatment?- •
Sclerosis. The most common complications of sclerotherapy are bleeding, ulceration, odynophagia and dysphagia. Other possible complications are perforation, pneumonia, sepsis, esophagopleural fistula, gastroesophageal reflux and esophageal motility disorders.87–90
- •
Ligation. The most common complications are chest pain, dysphagia, odynophagia and ulceration. Esophageal perforation, fever and post-ligation bleeding can occur.90–95
Level of evidence 1, grade of recommendation A.
Management of Gastric and Duodenal VaricesIs primary prophylaxis for gastric and duodenal varices justified?
It has been documented that gastric varices can be seen in 5% to 33% of patients with portal hypertension.96
Studies performed in adults show they are related to presence of more severe hemorrhage, with a greater need for blood transfusion and mortality.52,97
There have been no studies in the pediatric population to assess the role of beta-blockers or endoscopic treatment in primary prophylaxis for gastric varices due to extrahepatic portal hypertension.
To date, there is insufficient data to allow recommendation of a standard therapy for primary prophylaxis of gastric and duodenal varices.
Level of evidence 5, grade of recommendation D.
The management criteria for the primary prophylaxis of GOV 1 gastric varices will be the same as for the prophylaxis of esophageal varices.
Level of evidence 5, grade of recommendation D.
Which are the indications for the use of cyanoacrylate in gastric varices?In the adult population, the use of tissue adhesives such as N-butyl-cyanoacrylate or isobutyl-2-cyanoacrylate has proven more effective than ligation and sclerotherapy for the control of bleeding, in preventing rebleeding and also in reducing mortality.4,98
In case series of pediatric patients there have been reports of good response in controlling acute bleeding.99,100
It is recommended for active bleeding from gastric varices.
Level of evidence 4, grade of recommendation D.
Several complications have been reported from the use of tissue adhesives (cyanoacrylate) such as cerebral and pulmonary embolism, damage to endoscopic equipment and adhesion of the endoscopic needle.93–95
Level of evidence 4, grade of recommendation D.
Is secondary prophylaxis for gastric and duodenal varices justified?To date, the use of drug therapy for secondary prophylaxis of gastric or duodenal varices cannot be recommended, as there are no data regarding its use.
Level of evidence 5, grade of recommendation D.
The use of cyanoacrylate is useful for secondary prophylaxis of gastric variceal bleeding; although its use in children has been limited, results appear to have been good.98–101
Level of evidence 4, grade of recommendation D.
III. Diagnosis and Treatment of the Clinical Complications of Ehpvo in Children- •
Secondary hypersplenism.
- •
Hepatopulmonary syndrome.
- •
Portopulmonary hypertension.
- •
Portal biliopathy.
Secondary hypersplenism refers to the sequestration of blood cells that is associated with splenomegaly and causes a decrease in all blood cell lines (thrombocytopenia, anemia, leukopenia). It can cause gastrointestinal bleeding and episodes of epistaxis in patients with portal hypertension.102–104
Level of evidence 1, grade of recommendation A.
How is secondary hypersplenism diagnosed?- •
Splenomegaly.
- •
Decrease in one or more cell lines.
- •
Bone marrow hyperplasia.
- •
Clinical evidence of bleeding.
- •
Improvement in cytopenias following splenectomy.
- •
Imaging: abdominal Doppler ultrasound and computed axial tomography scan.
Level of evidence 1, grade of recommendation A.
What is the treatment for secondary hypersplenism?Surgical treatmentSplenctomy alone is not recommended as treatment of choice. Spleno-renal shunt should be performed after taking into account the following:
- •
Thrombocytopenia with systemic involvement (bleeding).
- •
Splenic infarction.
- •
Infections associated with enlargement of the spleen.
- •
Growth retardation.
Level of evidence 5, grade of recommendation D.
Splenic embolization:There is little experience in children, it was performed in 8/15 children that underwent sclerotherapy and embolization and 7/15 who only underwent embolization, rebleeding occurred in 2 cases, fever in 2 cases and there were no major complications or deaths related to the procedure.105,106 Indication for this procedure reported are hipersplenism who present with recurrent thrombocytopenia, with a platelet count less than or equal to 100,000/mm3, hypersplenism with clinical evidence of bleeding: gingival bleeding, epistaxis and in patients with extrahepatic portal hypertension and massive splenomegaly.
What are the post-embolization complications?Pain associated with the procedure and fever, pleural effusion, ascites, splenic abscess, splenic rupture, sepsis.107,108
Level of evidence 1, grade of recommendation A.
2. Hepatopulmonary Syndrome.When to suspect and how to diagnose hepatopulmonary syndrome?Hepatopulmonary syndrome (HPS) is characterized by the triad hypoxemia, intrapulmonary vascular dilatation and liver disease.110,111 It should be suspected clinically in patients who present with dyspnea, orthodeoxia (decrease in the partial pressure of oxygen in arterial blood ≥ 4 mmHg or 5% from the supine to upright position), clubbing and cyanosis.
HPS can occur in the absence of intrinsic liver disease; cases of prehepatic portal obstruction with no detectable predisposing factors have been reported in adults;112 in children it has been described as complications of congenital portosystemic shunts, polysplenia syndrome with interruption of the inferior vena cava and absence of portal vein.113–116
Pulse oximetry monitoring can be used to detect oxygen saturation (SaO2) ≤ 96% and help select patients who should then undergo further evaluation to confirm HPS.104,117
The criteria for the diagnosis of HPS106,107,112 are:
- a)
Oxygenation defect. PaO2 < 80 mmHg or alveolar-arterial oxygen gradient (PA-aO2) ≥ 15 mmHg with FiO2 of 21%.
- b)
Intrapulmonary vascular dilatation. Through echocardiography with agitated saline contrast; is considered positive for HPS when micro bubbles are seen in the right atrium after injection into a peripheral vein and within 3 to 6 cardiac cycles appear in the left atrium, consistent with intrapulmonary shunting. Tc99m-macroaggregated albumin scintigraphy, presence of intrapulmonary shunt to be considered if it shows ≥ 6% activity in the brain or liver, or cardiac catheterization demonstrates pulmonary vascular dilatation.
- c)
Portal hypertension with or without cirrhosis.
Level of evidence 4, grade of recommendation C.
What is the treatment for HPS?The primary medical treatment for HPS is longterm supplemental oxygen.117
Studies with various drugs have been conducted but results have been inconclusive.
Liver transplantation (LT) is the only effective treatment, resulting in gradual improvement of arterial oxygenation and resolution of HPS.118–120
Level of evidence 4, grade of recommendation C.
3. Portopulmonary Hypertension.When to suspect and how to diagnose portopulmonary hypertension?Portopulmonary hypertension (PPH) is a pulmonary vascular complication of liver disease and results from the obliteration of the pulmonary artery. It is defined by elevated mean pressure in the pulmonary artery (PAP) > 25 mmHg at rest, increased vascular resistance and normal pulmonary capillary wedge pressure < 15 mmHg in the presence of portal hypertension.117,121,122
PPH and HPS have been reported in children with intrahepatic portal hypertension due to cirrhosis and extrahepatic portal hypertension due to congenital and acquired anomalies of the portal venous system, but the incidence is unknown.112,115,123
Clinical symptoms are nonspecific and insidious, making the diagnosis requires a high degree of suspicion; syncope is the symptom that is most clearly linked to this condition, a new heart murmur on auscultation and progressive dyspnea.121
Echocardiographic measurement of PAP is a suggestive diagnostic technique; right heart catheterization can confirm the diagnosis. Chest X-ray may show cardiomegaly or prominent pulmonary artery and ECG may show right ventricular hypertrophy.124,125
Level of evidence 5, grade of recommendation D.
What is the treatment for portopulmonary hypertension?Liver transplantation (LT) is the only effective treatment, although it is contraindicated in cases of severely elevated pulmonary arterial pressure > 50 mmHg in which the definitive treatment would be heart, lung and liver transplantation.126
The goal of medical treatment is to transform a borderline candidate for LT into an acceptable one, and includes providing supplemental oxygen to maintain SaO2 > 92%, diuretics to control hypervolemia, calcium-channel blockers if it can be demonstrated that the patient has a good vasodilator response during cardiac catheterization, continuous infusions of prostaglandins such as Epoprostenol as a bridge to LT.119,121
Level of evidence 5, grade of recommendation D.
4. Portal Biliopathy.When to suspect and how to diagnose portal biliopathy?Portal Biliopathy (PB) is a term introduced in 1992 that refers to abnormalities of the extrahepatic and intrahepatic bile ducts in association with an external compression mechanism by the portal cavernoma, ischemia and long-term portal hypertension with development of collaterals in the biliary region.127 It occurs in 80 to 100% of adult patients with extrahepatic portal hypertension128 but there are few reports in children; 4% in a series of 69 cases followed up for 15 years and 8 of 121 children with EHPVO.7,129 Reported complications related to PB are: cholangitis, secondary biliary cirrhosis, gallstones, hemobilia, hypoalbuminemia, coagulation disorders.7
Level of evidence 3, grade of recommendation C.
PB should be suspected in any patient with EHPVO who presents with jaundice, chronic abdominal pain, and recurrent cholangitis and is shown to have alterations of the intrahepatic or extrahepatic bile ducts by a Doppler ultrasound.128
Level of evidence 4, grade of recommendation D.
How is portal biliopathy diagnosed?- •
Clinical features:
Level of evidence 4, grade of recommendation D.
- •
Biochemical data: liver function tests may be normal or show levels of bilirubin and/or alkaline phosphatase as high as 30%.129
- •
Imaging:
- a)
Endoscopic retrograde cholangiopancreatography (ERCP) is an invasive method for the diagnosis and is recommended in symptomatic cases that may require medical intervention.1,7
Level of evidence 1, grade of recommendation A.
- b)
Magnetic resonance cholangiography: Is the alternative non-invasive method for the first line of investigation.2
Level of evidence 3, grade of recommendation C.
- c)
Doppler ultrasound: useful for demonstrating the presence of gallbladder varices.7
Level of evidence 3, grade of recommendation C.
- d)
Liver biopsy: not the diagnostic method of choice but if cirrhosis or other disease is suspected, it may be performed.7
Level of evidence 3, Grade of recommendation B
- a)
- 1.
“NO” treatment for asymptomatic patients is recommended, but if asymptomatic stones are present at the time of portal hypertension surgery they must be removed; if stones develop after surgery, it should be observed and treated only if symptoms develop.2
Level of evidence 5, grade of recommendation D.
- 2.
Treatment in symptomatic patients must be individualized according to symptoms:128
- a)
ERCP with sphincterotomy and stone extraction.
- b)
Biliary stent with or without dilatation.
- c)
Bypass surgery-hepaticojejunostomy in patients with persistent obstruction.
- d)
Shunt surgery allows improve portal biliopathy by decompression of the portal system.
- a)
Level of evidence 3, grade of recommendation C.
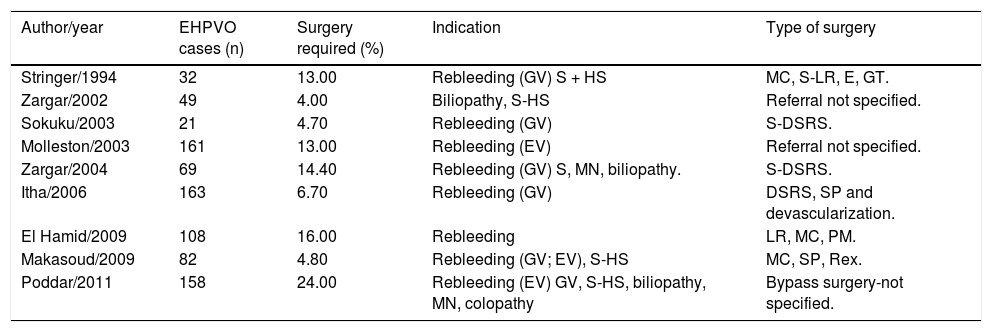
Surgical Treatment for EHPVOThe aim of surgery is to divert portal blood flow into the systemic circulation. It has been reported that between 4% and 24% of children with EHPVO require bypass surgery after failure of medical and endoscopic therapy, with recurrence of bleeding being the main indication (Table 10).16,23,24,73,85,88,89,130,131
What are the indications for surgical treatment?- •
Persistent bleeding after medical and endoscopic treatment.
- •
Large fundal varices.
- •
Massive splenomegaly with hypersplenism.
- •
Splenomegaly with infarction.
- •
Portal biliopathy.
- •
Colonic varices.
- •
Massive bleeding.
Level of evidence 1, grade of recommendation A.16,23,24,85,88,89,130,131
What are the criteria for determining which type of surgery to perform?- •
Patients for bypass surgery shall be those without intrahepatic diseases unless no alternative treatments are available.
- •
The patient’s weight. The type of bypass surgery will depend on the surgeon and his experience; nevertheless it has been found that the lower the patient’s weight, the greater the risk for thrombosis from the bypass.
- •
Vascular evaluation. Computed tomographic angiography is recommended for evaluation of the morphology of the portal vein and collaterals; if a computed tomographic scan is not possible, then a magnetic resonance angiography should be done. Splenoportography or conventional angiography should be limited to special or difficult areas.
- •
Urgency. There is no absolute indication for emergency surgery; the first concern is to stabilize the patient with acute variceal bleeding and in case of improvement, consider bypass surgery.
Frequency and Indications for bypass surgery in children with EHPVO.
| Author/year | EHPVO cases (n) | Surgery required (%) | Indication | Type of surgery |
|---|---|---|---|---|
| Stringer/1994 | 32 | 13.00 | Rebleeding (GV) S + HS | MC, S-LR, E, GT. |
| Zargar/2002 | 49 | 4.00 | Biliopathy, S-HS | Referral not specified. |
| Sokuku/2003 | 21 | 4.70 | Rebleeding (GV) | S-DSRS. |
| Molleston/2003 | 161 | 13.00 | Rebleeding (EV) | Referral not specified. |
| Zargar/2004 | 69 | 14.40 | Rebleeding (GV) S, MN, biliopathy. | S-DSRS. |
| Itha/2006 | 163 | 6.70 | Rebleeding (GV) | DSRS, SP and devascularization. |
| El Hamid/2009 | 108 | 16.00 | Rebleeding | LR, MC, PM. |
| Makasoud/2009 | 82 | 4.80 | Rebleeding (GV; EV), S-HS | MC, SP, Rex. |
| Poddar/2011 | 158 | 24.00 | Rebleeding (EV) GV, S-HS, biliopathy, MN, colopathy | Bypass surgery-not specified. |
EV: esophageal varices. GV: gastric varices. S: splenomegaly. HS: hypersplenism. MN: malnutrition. MC: Mesocaval. SP: splenectomy. DSRS: distal splenorenal. GT: gastric transaction. PC: portacaval. PM: portomesenteric.
Different surgical techniques have been used in the surgical treatment of EHPVO (Table 11); Mesocaval and distal splenorenal or “Warren” shunts are the types most often used in children, with no difference as far as postoperative complications shunt patency and mortality are concerned.134–138
Surgical procedures for the treatment of EHPVO.
| Bypass Non bypass | |
|---|---|
| a) Non selective: • Total: Portacaval. Mesocaval. Proximal splenorenal. • Partial: Portacaval H-graft. Mesocaval H-graft. | a) Direct: • Esophagogastric resection. • Ligation of esophageal varices. • Esophageal transection. b) Indirect: • Sugiura, Omentopexy and transposition of the spleen into the thoracic cavity. |
| b) Selective: • Distal splenorenal (Warren). • Coronary caval (Inokuchi). | |
Level of evidences 1, grade of recommendation A.
It has recently been suggested that the meso-Rex procedure is the best alternative; it involves recanalization of the regular portal system, creating a bypass between the superior mesenteric vein and the intrahepatic left portal vein using an autograft; experience in Mexico is limited. Although there are few reports in the literature, results are encouraging. Restoration of venous flow is achieved in most cases, but this shunt can only be performed when a patent left branch is available and the thrombosis is higher.132,139,140
This technique can be recommended for those who have experience in performing this type of bypass.
Level of evidence 3, grade of recommendation C.
What are the postoperative complications?- •
Immediate. Bleeding, encephalopathy, thrombosis, infection.
- •
Delayed. Variceal rebleeding.
- •
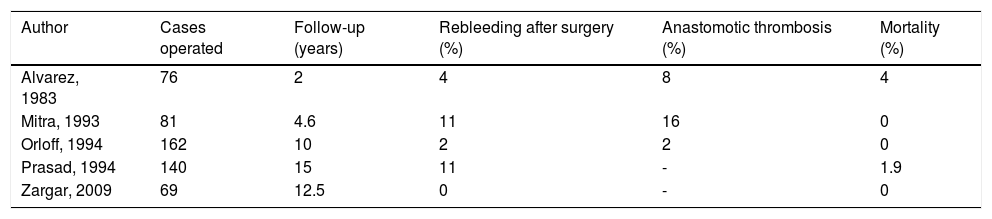
Recent studies have reported 0% to 11% recurrence of bleeding, 2% to 16% anastomotic thrombosis and 0% to 4% mortality134–138 (Table 12).
Table 12.Results of bypass surgery in children with EHPVO.
Author Cases operated Follow-up (years) Rebleeding after surgery (%) Anastomotic thrombosis (%) Mortality (%) Alvarez, 1983 76 2 4 8 4 Mitra, 1993 81 4.6 11 16 0 Orloff, 1994 162 10 2 2 0 Prasad, 1994 140 15 11 - 1.9 Zargar, 2009 69 12.5 0 - 0
Level of evidence 1, grade of recommendation A.
What are the postoperative monitoring procedures?- •
Clinical. Disappearance of bleeding, gradual reduction of splenomegaly and hypersplenism.
- •
Imaging. Doppler ultrasound or angiography to assess shunt patency.
- •
Endoscopy. Follow-up endoscopy to evaluate the reduced size or disappearance of the varices (3 to 6 months after surgery).133
Level of evidence 1, grade of recommendation A.
When is indicated the use of Sengstaken-Blakemore balloon?This is a measure of last resort when everything else has failed to control acute bleeding. It is not without serious complications and experienced staff must carry out placement; it may be used as a bridge to stabilize the patient until surgical bypass can be performed. There is very little experience in children.135
Level of evidence 4, grade of recommendation D.
ConclusionsRandomized clinical trials are needed to guide decisions about the optimal management of children with EHPVO. Betablockers and endoscopy treatment for primary and secondary profilaxis have been studied in few randomized studies, most of them are case reports and data from clinical trials providing support for their use are lacking.
Guidelines for management in children have been published,1,2,7,8 these guidelines predominantly reflect expert opinion, since there are only limited data from randomized trials to guide management, the recommendations in this article are generally consistent with these guidelines and case reports.
AknowledgementsWe are grateful to the President of the Mexican Hepatology Association (AMH), Dr. Francisco Sánchez Avila, and to the ex presidents of the AMH, Dr. Eduardo Marín López and Dr. Nahum Méndez-Sánchez for all their help on the organization of the meetings related to the preparation and propagation of the guidelines. Our special gratitude to Dr. Benjamin Shneider for reviewing the final version of the guidelines.
Conflict of InterestNone for all authors.