Ilntroduction and aims. We aimed to investigate the clinical and pathological differences between low-AFP-secreting (AFP < 20 ng/mL) and high-AFP-secreting (AFP ≥ 20 ng/mL) hepatocellular carcinomas in patients who undergo liver transplant (LT).
Material and methods. We evaluated 145 patients who underwent deceased donor LT for HCC from January 1, 2005 until August 1, 2015 at the Johns Hopkins Hospital.
Results. Median pre-LT AFP in the entire cohort was 13 ng/mL (IQR 6-59). Using serum AFP cutoff of 20 ng/mL, 61 (42%) patients had high-AFP-secreting tumors and 84 (58%) had low-AFP-secreting tumors. Patients with high-AFP-secreting tumors had larger lesions (3 cm vs. 2.4 cm, p = 0.024), and were more likely to have microvascular-invasion (36.1% vs. 20.2%, p = 0.02) and poor-differentiation (18% vs. 4.8%, p = 0.01), and tumor recurrence following LT (28% vs. 6%, p < 0.001). The 1-year, 3-year, and 5-year recurrence-free survival for patients in the low-AFP-secreting group compared to the high-AFP-secreting group were 100%, 92%, 92% vs. 81.3%, 71.3%, 68.5% respectively (p = 0.0003).
Conclusion. AFP is a suboptimal predictor of tumor recurrence following liver transplant in HCC patients. However, it can have some value in distinguishing more aggressive forms of HCC (high-AFP-secreting) that are associated with higher tumor recurrence. Novel tumor biomarkers are needed that can enhance predicting tumor recurrence following LT based on tumor biology.
Liver transplant (LT) has emerged as one of the primary treatment options for cirrhotic transplant candidates with hepatocellular carcinoma within the widely accepted Milan criteria (MC).1 Since the development of the MC in 1996, there have been additional extended criteria models proposed that would further expand access to LT for candidates with HCC with similar post-transplant survival and tumor recurrence compared to MC.2,3 However, criteria relying on tumor size and number alone have been suboptimal in predicting HCC recurrence following LT.4,5 As a result, various groups have sought to better characterize other factors that may influence tumor behavior, including tumor biology.
Alpha fetoprotein (AFP) is a major plasma protein produced by the liver during fetal development and can be elevated in patients with HCC. The role of serum AFP in screening for HCC remains controversial, with studies demonstrating a sensitivity of only 60% for a cutoff of 20 ng/mL.6,7 Furthermore, the optimal cutoff for serum AFP in detecting HCC is undefined, with studies reporting high false positive rates with low cutoff values and high false negative rates with higher cutoff values.8,9
AFP has been shown to have prognostic value in both the transplant and non-transplant settings.10,11 Elevated AFP levels have been associated with tumor recurrence and poor survival outcomes.12,14 AFP has also been demonstrated to be a valuable tool in monitoring response to HCC treatment.15,16 Furthermore, studies have shown worse outcomes following liver transplant in patients with elevated serum AFP, including higher rates of tumor recurrence and worse post-LT survival.17–19
However, a considerable proportion of patients with hepatocellular carcinoma have minimally elevated or even normal AFP levels.20 There is limited data in the literature to compare clinical outcomes of “low-AFP-secreting” vs. “high-AFP-secreting” HCCs in patients undergoing LT. In this study, we aimed to better characterize outcomes in patients with low-AFP-secreting HCCs compared to high-AFP-secreting tumors following LT and to identify any additional clinical or pathologic risk factors that may aid in predicting tumor recurrence in this population.
Materials and MethodsStudy populationAfter approval by the institutional review board (IRB), we retrospectively evaluated 170 adult patients with a diagnosis of HCC who underwent LT at the Johns Hopkins University Comprehensive Liver Transplant Center between January 1, 2005 to August 1, 2015. We excluded 4 patients who underwent living donor LT, 5 patients who died within 30 days following LT, 15 patients who did not have serum AFP levels checked within 120 days prior to LT, and 1 patient due to the quality of data. A total of 145 patients were included in the final analysis. Patients were divided into having low-AFP-secreting tumors versus high-AFP-secreting tumors based on AFP value of 20 ng/ mL. This cut-off was selected based on prior published data.21 The clinical and pathological characteristics, tumor recurrence, and survival data were reviewed between these two groups.
PathologyAll explant pathology was reviewed retrospectively, and the following data were collected: tumor size, number of lesions, lobar involvement, presence of microvascular invasion (MVI), and tumor differentiation. Based on tumor size and number of lesions on explant pathology, it was determined if each patient was within Milan or UCSF criteria. Additionally, data was gathered on locoregional therapy and percentage of tumor necrosis to calculate the number of patients that subsequently met Milan and UCSF criteria after downstaging.
Statistical analysisCategorical variables are reported by percentages and continuous variables are reported as medians and interquartile ranges (IQRs). We compared patients with low-AFP-secreting versus high-AFP-secreting tumors as well as patients with and without HCC recurrence. Continuous variables were compared using the Wilcoxon ranksum test and are reported as medians and IQRs, and categorical variables were compared using the Fisher’s exact test and are reported as percentages. To evaluate the diagnostic value of AFP we determined the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). The overall predictive performance of AFP was measured by area under the receiver operating characteristic (ROC) curve (AUC). Patient survival curves were computed using Kaplan-Meier method and were compared by log-rank tests. Statistical analyses were performed using STATA V.13 (StataCorp college station, TX). Logistic regression analysis was used to determine the association between AFP and tumor recurrence following deceased donor LT.
ResultsStudy population- a)
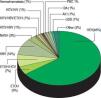
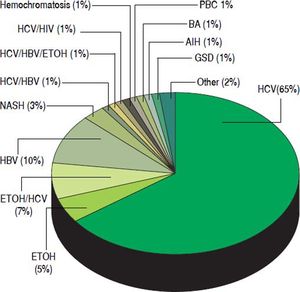
Baseline characteristics. The characteristics of the 145 HCC patients are summarized in table 1. The median age was 59 years. Among the transplant recipients, 110 (75.9%) were male. The majority of LT recipients were white (N = 79, 54.5%), followed by 31% African American. Chronic hepatitis C was the most prevalent etiology of liver disease in our patient population accounting for 75% of patients (HCV was defined as HCV antibody and/or HCV RNA positivity) (Figure 1). Chronic hepatitis B as the sole diagnosis was present in 10% of the patients (defined as HBS Antigen and/or HBV DNA positivity). Only 3% of patients had NASH as the etiology of cirrhosis (Figure 1).
Table 1.Clinical, laboratory, and pathologic characteristics of study population.
Variable N = 145 Clinical features: Male sex, n (%) 110 (75.9%) Age (years) 59 (55-63) Ethnicity, n (%) White 79 (54.5%) African American 45 (31.0%) Asian 8 (5.5%) Hispanic 4 (2.8%) Other 9 (6.2%) Days from listing to LT 153 (50-299) Laboratory: Pre-LT AFP (ng/mL) 13.0 (6.0-59.0) Post-LT AFP (ng/mL) 3.0 (2.0-4.9) MELD 10 (8-16) WBC (109/L) 5.1 (3.9-6.2) Hgb (g/dL) 12.1 (10.5-14.0) MCV (fL) 93.5 (89.3-97.9) PLT (103/microL) 85 (55-115) BUN (mg/dL) 14 (11-18) Creatinine (mg/dL) 0.9 (0.7-1.1) TP (g/dL) 7.0 (6.3-7.4) Alb (g/dL) 3.4 (2.9-3.8) ALP (U/L) 128 (98-165) AST (U/L) 63 (42-108) ALT (U/L) 39 (25-76) AST:ALT ratio 1.5 (1.1-1.8) T.Bili (mg/dL) 1.3 (0.8-2.6) PT (sec) 12.6 (11.5-14.7) INR 1.2 (1.1-1.4) Explant Pathology:Number of lesions, n (%) 1 72 (49.7%) 2 32 (22.1%) 3 21 (14.5%) 4 3 (2.1%) 5 6 (4.1%) 6 4 (2.8%) > 6 7 (4.8%) Largest lesion (cm) 2.5 (2.0-3.6) Total tumor size (cm)* 3.5 (2.3-5.8) Tumor location, n (%) Right lobe 84 (57.9%) Left lobe 16 (11.0%) Multi-lobar 42 (29.0%) Caudate lobe 2 (1.4%) Unknown 1 (0.7%) Tumor differentiation, n (%)** Well 19 (13.1%) Moderate 89 (61.4%) Poor 15 (10.3%) Unknown 22 (15.2%) Microvascular invasion, n (%)*** Yes 39 (26.9%) No 90 (62.1%) Bile duct invasion 1 (0.7%) Unknown 15 (10.3%) Total number of loco-regional therapies, n (%) 0 52 (35.9%) 1 66 (45.5%) 2 21 (14.5%) 3 4 (2.8%) 4 2 (1.4%) Patients with viable tumor, n (%) Yes 129 (89.0%) No 16 (11.0%) Within Milan, n (%) Yes 117 (80.7%) No 28 (19.3%) Downstaged to Milan, n (%) 17 (11.7%) Within UCSF, n (%) Yes 121 (83.4%) No 24 (16.6%) Downstaged to UCSF, n (%) 6 (4.1%) Quantitative data are expressed as median (25%-75% Interquartile ranges [IQRs]). Categorical variables are reported as percentages. LT: liver transplant. AFP: alpha-fetoprotein. MELD: model for end stage liver disease score. WBC: white blood cell count. Hgb: hemoglobin. MCV: mean corpuscular volume. PLT: platelet count. BUN = blood urea nitrogen. TP: total protein. Alb: albumin. ALP: alkaline phosphatase. AST: aspartate aminotransferase. ALT: alanine aminotransferase. T.Bili: total bilirubin. PT: prothrombin time. INR: international normalized ratio. UCSF: University of California San Francisco.
Figure 1.Distribution of etiologies of liver disease in 145 patients who underwent liver transplant for hepatocellular carcinoma at the Johns Hopkins University Comprehensive Liver Transplant Center, expressed in percentages. HCV: hepatitis C virus. ETOH: Alcoholic liver disease. HBV: hepatitis B virus. NASH: non-alcoholic steatohepatitis. HIV: human immunodeficiency virus. PBC: primary biliary cholangitis. BA: biliary atresia. AIH: autoimmune hepatitis. GSD: glycogen storage disease.
- b)
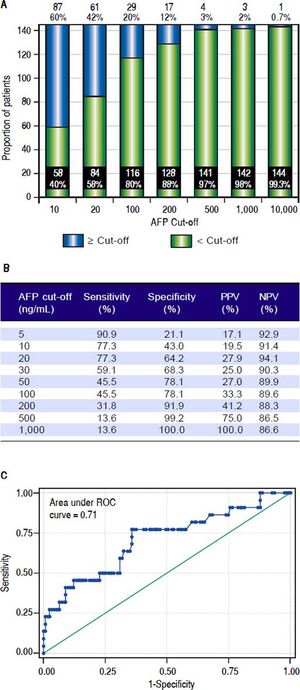
Laboratory results. The laboratory data of our transplant recipients are reported in table 1. The median biological MELD score was 10. The serum AFP values were available for all 145 patients. The median pre-LT AFP in the entire cohort was 13 ng/mL (IQR 6-59). When the cut off for serum AFP was determined at 10 ng/mL, 60% of patients had elevated AFP vs. 40%. When the cut off value for serum AFP was set at 20 ng/ mL, 42% of patients had elevated AFP vs. 58% (Figure 2A).
Figure 2.A.Distribution of patients based on different AFP cut-offs, expressed in total number n and percentages. AFP: aiplia-fetoprotein. B. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) based on various AFP cut-offs for prediction of postliver transplant HCC recurrence. C. Area under the receiver operating characteristic (ROC) curve (AUC) of AFP for prediction of post-liver transplant HCC recurrence.
- c)
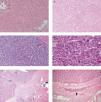
Pathology findings. Among LT recipients, 49.7% of patients had only one lesion on explant. The median largest tumor size was 2.5 centimeters (Table 1). The tumors were categorized into well, moderate and poorly differentiated by our local liver pathologist (Figure 3) In total, 19 (13.1%) had well-differentiated tumors compared to 89 (69.5%) and 15(10.3%) with moderate and poor differentiation, respectively. Overall 39 (26.9%) patients had MVI on the explant (Table 1). The differentiation and MVI could not be determined in a small percentage of patients due to the effect of prior locoregional therapy (Figure 3). Most patients (64.1%) received at least one locoregional treatment prior to LT. A total of 17 patients were downstaged to Milan, and 6 patients were downstaged to UCSF. Therefore 80% of patients were within MC, and 83.4% of patients were within UCSF criteria after locoregional therapy. Only 16 (11%) patients did not have any viable tumor on explant compared to 129 (89%) of patients who had viable tumor (Table 1). In other words, among 17.2% of patients who received at least one locoregional therapy, there was no evidence of viable tumor on explant pathology.
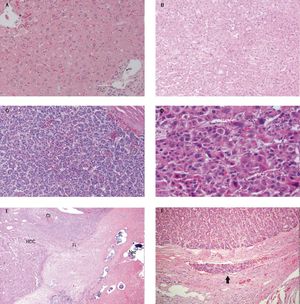
Figure 3.Pathology description. A. Normal liver 20x. B. Well differentiated HCC 10x. C. Moderately differentiated HCC 10x. D. Poorly differentiated HCC 20x. E. Histology of TACE treated hepatocellular carcinoma demonstrating regions of cirrhotic liver (Ci), viable hepatocellular carcinoma (HCC), fibrosis (Fi) and tumor necrosis (Nec). Arrows show collections of embolic beads. F. Microvascular invasion. Arrow illustrates the tumor cells inside the vessel.
Based on the AFP cut off of 20 ng/mL, 84 (58%) patients had low-AFP-secreting tumors and 61 (42%) patients had high-AFP-secreting tumors (Figure 2). The clinical and pathological differences between these two groups are shown in table 2. There was no difference in terms of age, sex, ethnicity, and days from listing to LT between the two groups. Chronic hepatitis B virus (HBV) was more common in the high-AFP-secreting group compared to low-AFP-secreting group (16.4 vs. 4.8%, P = 0.068). Patients with high-AFP-secreting HCC had higher alkaline phosphatase compared to low-AFP-secreting group, median 142 vs. 124 respectively (P = 0.032). Remaining labs, including the biological MELD score, were comparable between the two groups (Table 2).
Clinical, laboratory, and pathologic differences between low-secreting-AFP vs. high-secreting-AFP tumors.
| Variable | AFP < 20 (n = 84) | AFP ≥ 20 (n = 61) | P value |
|---|---|---|---|
| Clinical features: | |||
| Male sex, n (%) | 66 (78.6%) | 44 (72.1%) | 0.43 |
| Age (years) | 59 (54-63) | 60 (56-64) | 0.28 |
| Ethnicity, n (%) | 0.34 | ||
| White | 48 (57.1%) | 31 (50.8%) | |
| African American | 25 (29.8%) | 20 (32.8%) | |
| Asian | 2 (2.4%) | 6 (9.8%) | |
| Hispanic | 2 (2.4%) | 2 (3.3%) | |
| Other | 7 (8.3%) | 2 (3.3%) | |
| Etiology of liver disease, n (%) | 0.068 | ||
| HCV | 53 (63.1%) | 41 (67.2%) | |
| HCV/ETOH | 7 (8.3%) | 3 (4.9%) | |
| ETOH | 5 (6.0%) | 3 (4.9%) | |
| HBV | 4 (4.8%) | 10 (16.4%) | |
| NASH | 5 (6.0%) | 0 (0%) | |
| Other | 10 (11.9%) | 4 (6.6%) | |
| Days from listing to LT | 161 (83-307) | 118 (26-284) | 0.14 |
| Laboratory: | |||
| Pre-LT AFP (ng/mL) | 6.3 (4.0-10.8) | 88.0 (39.1-203.0) | < 0.001 |
| Post-LT AFP (ng/mL) | 2.9 (2.0-4.0) | 4.1 (2.6-11.7) | < 0.001 |
| MELD | 10 (8-16) | 10 (8-14) | 0.42 |
| WBC (109/L) | 5.2 (3.9-6.2) | 4.9 (3.9-6.5) | 0.54 |
| Hgb (g/dL) | 12.1 (10.4-14.3) | 12.1 (10.6-14.0) | 0.93 |
| MCV (fL) | 94.0 (89.3-98.1) | 92.8 (89.4-96.8) | 0.47 |
| PLT (103/microL) | 85 (54-116) | 85 (57-113) | 0.99 |
| BUN (mg/dL) | 14 (11-20) | 15 (11-17) | 0.66 |
| Creatinine (mg/dL) | 0.9 (0.8-1.1) | 0.9 (0.7-1.0) | 0.39 |
| TP (g/dL) | 7.0 (6.0-7.4) | 6.9 (6.4-7.5) | 0.52 |
| Alb (g/dL) | 3.3 (2.8-3.8) | 3.4 (3.0-3.8) | 0.39 |
| ALP (U/L) | 124 (96-155) | 142 (106-191) | 0.032 |
| AST (U/L) | 62 (41-82) | 66 (42-124) | 0.12 |
| ALT (U/L) | 36 (25-66) | 48 (29-94) | 0.11 |
| AST:ALT ratio | 1.5 (1.1-1.9) | 1.4 (1.2-1.8) | 0.69 |
| T.Bili (mg/dL) | 1.4 (0.8-2.8) | 1.3 (0.8-2.3) | 0.47 |
| PT (sec) | 12.9 (11.6-15.5) | 12.5 (11.5-14.4) | 0.40 |
| INR | 1.3 (1.1-1.5) | 1.2 (1.1-1.4) | 0.51 |
| Explant PathologyP:athology: | |||
| Number of lesions, n (%) | 0.72 | ||
| 1 | 42 (50.0%) | 30 (49.2%) | |
| 2 | 21 (25.0%) | 11 (18.0%) | |
| 3 | 11 (13.1%) | 10 (16.4%) | |
| 4 | 1 (1.2%) | 2 (3.3%) | |
| 5 | 4 (4.8%) | 2 (3.3%) | |
| 6 | 1 (1.2%) | 3 (4.9%) | |
| > 6 | 4 (4.8%) | 3 (4.9%) | |
| Largest lesion (cm) | 2.4 (1.8-3.5) | 3.0 (2.0-4.0) | 0.024 |
| Total tumor size (cm)* | 3.3 (2.1-5.0) | 4.4 (2.8-6.5) | 0.031 |
| Tumor location, n (%) | 0.61 | ||
| Right lobe | 45 (53.6%) | 39 (63.9%) | |
| Left lobe | 11 (13.1%) | 5 (8.2%) | |
| Multi-lobar | 26 (31.0%) | 16 (26.2%) | |
| Caudate lobe | 1 (1.2%) | 1 (1.6%) | |
| Unknown | 1 (1.2%) | 0 (0%) | |
| Tumor differentiation, n (%)** | 0.01 | ||
| Well | 15 (17.9%) | 4 (6.6%) | |
| Moderate | 51 (60.7%) | 38 (62.3%) | |
| Poor | 4 (4.8%) | 11 (18.0%) | |
| Unknown | 14 (16.7%) | 8 (13.1%) | |
| Microvascular invasion, n (%) | 0.02 | ||
| Yes | 17 (20.2%) | 22 (36.1%) | |
| No | 59 (70.2%) | 31 (50.8%) | |
| Bile duct invasion | 0 (0%) | 1 (1.6%) | |
| Unknown | 8 (9.5%) | 7 (11.5%) | |
| Total number of loco-regional therapies, n (%) | 0.64 | ||
| 0 | 30 (35.7%) | 22 (36.1%) | |
| 1 | 41 (48.8%) | 25 (41.0%) | |
| 2 | 11 (13.1%) | 10 (16.4%) | |
| 3 | 1 (1.2%) | 3 (4.9%) | |
| 4 | 1 (1.2%) | 1 (1.6%) | |
| Patients with viable tumor, n (%) | 1.00 | ||
| Yes | 75 (89.3%) | 54 (88.5%) | |
| No | 9 (10.7%) | 7 (11.5%) | |
| Within Milan, n (%) | 0.40 | ||
| Yes | 70 (83.3%) | 47 (77.0%) | |
| No | 14 (16.7%) | 14 (23.0%) | |
| Downstaged to Milan, n (%) | 9 (10.7%) | 8 (13.1%) | 0.79 |
| Within UCSF, n (%) | 0.50 | ||
| Yes | 72 (85.7%) | 49 (80.3%) | |
| No | 12 (14.3%) | 12 (19.7%) | |
| Downstaged to UCSF, n (%) | 0 (0%) | 6 (9.8%) | 0.005 |
Quantitative data are expressed as median (25%-75% Interquartile ranges (IQRs)). Categorical variables are reported as percentages.
On explant pathology, there was no difference between the two groups regarding the number of lesions and the location of the tumors. However, the median size of the largest HCC lesion was greater in the high-AFP-secreting group than the low-AFP-secreting group: 3 cm vs. 2.4 cm respectively (P = 0.024). More patients in the high-AFP-secreting group had MVI on explant compared to the low-AFP-secreting group, 36.1% vs. 20.2% (P = 0.02). In total, 18% of patients in high-AFP-secreting group had poor differentiation on pathology compared to 4.8% in low-AFP-secreting group (P = 0.01). Milan and UCSF criteria distribution were comparable between the two groups. However, all of the 6 patients that were downstaged to UCSF were in the high-AFP-secreting group (P = 0.005) (Table 2).
HCC recurrenceThe overall HCC recurrence in our patient population was 15.2% (22 out of 145). The overall rate of tumor recurrence was significantly higher in patients with high-AFP-secreting than in patients with low-AFP-secreting HCC group, 28% vs. 6% respectively (P < 0.001) (Figure 4A). We performed logistic regression analysis to evaluate the association of pre-LT AFP ≥ 20 with tumor recurrence. The odds ratio (OR) of tumor recurrence comparing patients with pre-LT AFP > 20 to patients with AFP < 20 was 5.1 (95% Confidence interval (CI) 1.7 - 15, P = 0.003). However, when adjusted for other variables including UCSF, vascular invasion, and tumor differentiation, the association of AFP ≥ 20 and HCC recurrence was not statistically significant (P = 0.38, 0.25, 0.08, and 0.66 respectively). Five patients had a lesion on explant that was mixed HCC/cholangiocarcinoma. However, none of these patients had tumor recurrence following LT.
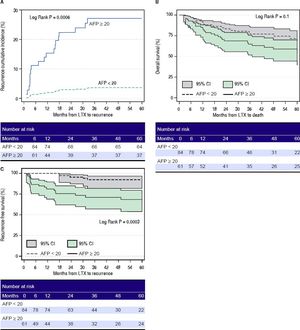
A.Cumulative incidence of hepatocellular carcinoma recurrence after liver transplant in patients with AFP ≥ 20 ng/mL (solid line) vs. patients with AFP < 20 ng/mL (dotted line). B. Overall survival after liver transplant in patients with AFP ≥ 20 ng/mL (solid line with dark grey box indicating 95% confidence interval) vs. patients with AFP < 20 ng/mL (dotted line with light grey box indicating 95%, confidence interval). C. Recurrence-free survival after liver transplant in patients with AFP ≥ 20 ng/mL (solid line with dark grey box indicating 95% confidence interval) vs. patients with AFP < 20 ng/mL (dotted line with light grey box indicating 95% confidence interval). AFP: alpha-fetoprotein. LTX: liver transplant. Cl: confidence interval.
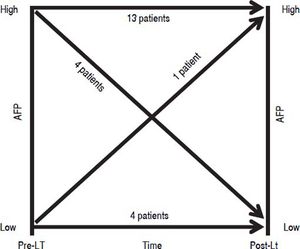
Out of the 22 patients who had recurrence 5 (22.7%) had AFP < 20 ng/mL prior to transplant and 17 (77.3%) had AFP ≥ 20 ng/mL (Figure 5). Among the five patients with low AFP pre-LT, four continued to have low AFP levels while one patient’s AFP increased to 1,591 ng/mL post-LT. On the other hand, among the 17 patients with pre-LT AFP ≥ 20 ng/mL, 13 had worsening AFP following LT, but unexpectedly 4 patients dropped their AFP levels below 20 ng/mL post LT (Figure 5).
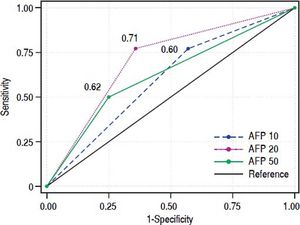
Accuracy of AFP for prediction of HCC recurrenceWe then evaluated the accuracy of AFP as the predictor of HCC recurrence following LT. The sensitivity, specificity, positive predictive value, negative predictive value of AFP, as predictor of HCC recurrence post-LT, based on various cutoff values of AFP are summarized in Figure 2B. As the cutoff value of AFP increased, the sensitivity decreased and specificity increased. The overall AUC of AFP as the predictor of HCC recurrence following LT was 0.71 (Figure 2C). We then compared the AUCs for three different AFP cutoffs, 10, 20, and 50 ng/mL (Table 3 and Figure 6). The AUC for AFP of 20 ng/mL was statistically better than the AUC for AFP of10 ng/m (P < 0.001), but was not statistically different than the AUC for AFP of50 ng/mL (P = 0.1).
We evaluated the differences between patients who had tumor recurrence following LT compared to patients without recurrence. There was no difference between the two groups in terms of baseline characteristics including age, sex, ethnicity, and etiology of liver disease (Table 4). Patients with HCC recurrence had a longer median time from listing to LT compared to non-recurrence, however this difference did not reach statistical significance, 267 vs. 131 days respectively (p = 0.065). Patients with HCC recurrence had higher serum AFP levels compared to the non-recurrence group both before and after liver transplant, median 41 vs. 11.9 pre-LT (P = 0.002) and 10.9 vs. 3.0 post-LT (p < 0.001), respectively. The biological MELD score was comparable between the two groups (Table 4).
Clinical, laboratory, and pathologic differences between patients with and without tumor recurrence
| Variable | No (n = 123) | Yes (n = 22) | P value |
|---|---|---|---|
| Clinical features: | |||
| Male sex, n (%) | 91 (74%) | 19 (86.4%) | 0.28 |
| Age (years) | 59 (55-63) | 58 (55-64) | 0.94 |
| Ethnicity, n (%) | 0.93 | ||
| White | 66 (53.7%) | 13 (59.1%) | |
| African American | 38 (30.9%) | 7 (31.8%) | |
| Asian | 6 (4.9%) | 2 (9.1%) | |
| Hispanic | 4 (3.3%) | 0 (0%) | |
| Other | 9 (7.2%) | 0 (0%) | |
| Etiology of liver disease, n (%) | 0.22 | ||
| HCV | 82 (66.7%) | 12 (54.6%) | |
| HCV/ETOH | 6 (4.9%) | 4 (18.2%) | |
| ETOH | 7 (5.8%) | 1 (4.5%) | |
| HBV | 11 (8.9%) | 3 (13.7%) | |
| NASH | 4 (3.3%) | 1 (4.5%) | |
| Other | 13 (10.6%) | 1 (4.5%) | |
| Days from listing to LT | 131 (48-283) | 267 (134-387) | 0.065 |
| Laboratory: | |||
| Pre-LT AFP (ng/mL) | 11.9 (5.2-45.1) | 41.0 (22.0-323.0) | 0.002 |
| Post-LT AFP (ng/mL) | 3.0 (2.0-4.3) | 10.9 (4.1-104.0) | < 0.001 |
| MELD | 10 (8-15) | 11 (7-17) | 0.99 |
| WBC (109/L) | 5.1 (3.7-6.0) | 5.7 (4.3-7.4) | 0.055 |
| Hgb (g/dL) | 12.0 (10.5-13.9) | 13.4 (10.6-14.4) | 0.39 |
| MCV (fL) | 94.0 (89.7-98.2) | 89.7 (86.4-94.9) | 0.015 |
| PLT (103/microL) | 84 (53-111) | 111 (62-139) | 0.071 |
| BUN (mg/dL) | 15 (11-18) | 14 (11-18) | 0.64 |
| Creatinine (mg/dL) | 0.9 (0.7-1.0) | 1.0 (0.8-1.1) | 0.13 |
| TP (g/dL) | 6.9 (6.2-7.4) | 7.4 (6.5-7.6) | 0.029 |
| Alb (g/dL) | 3.3 (2.8-3.8) | 3.5 (3.1-4.2) | 0.11 |
| ALP (U/L) | 128 (98-166) | 127 (97-164) | 0.90 |
| AST (U/L) | 65 (44-110) | 51 (35-82) | 0.096 |
| ALT (U/L) | 41 (28-77) | 36 (22-61) | 0.17 |
| AST:ALT ratio | 1.5 (1.1-1.8) | 1.5 (1.2-2.2) | 0.52 |
| T.Bili (mg/dL) | 1.3 (0.8-2.4) | 1.5 (0.6-3.6) | 0.66 |
| PT (sec) | 12.6 (11.6-14.6) | 12.5 (11.3-15.1) | 0.53 |
| INR | 1.2 (1.1-1.4) | 1.3 (1.1-1.5) | 0.85 |
| Explant Pathology: | |||
| Number of lesions, n (%) | 0.002 | ||
| 1 | 63 (51.2%) | 9 (40.9%) | |
| 2 | 30 (24.4%) | 2 (9.1%) | |
| 3 | 18 (14.6%) | 3 (13.6%) | |
| 4 | 3 (2.4%) | 0 (0%) | |
| 5 | 3 (2.4%) | 3 (13.6%) | |
| 6 | 4 (3.3%) | 0 (0%) | |
| > 6 | 2 (1.7%) | 5 (22.8%) | |
| Largest lesion (cm) | 2.5 (2.0-3.5) | 3.2 (2.0-6.0) | 0.13 |
| Total tumor size (cm)* | 3.5 (2.3-5.2) | 7.5 (2.7-16.9) | 0.003 |
| Tumor location, n (%) | 0.52 | ||
| Right lobe | 72 (59.0%) | 12 (54.5%) | |
| Left lobe | 15 (12.3%) | 1 (4.6%) | |
| Multi-lobar | 33 (27.0%) | 9 (40.9%) | |
| Caudate lobe | 2 (1.7%) | 0 (0%) | |
| Unknown | 1 (0.01%) | 0 (0%) | |
| Tumor differentiation, n (%)** | < 0.001 | ||
| Well | 19 (15.4%) | 0 (0%) | |
| Moderate | 77 (62.6%) | 12 (54.9%) | |
| Poor | 6 (4.9%) | 9 (40.3%) | |
| Unknown | 21 (17.1%) | 1 (4.8%) | |
| Microvascular invasion, n (%)*** | < 0.001 | ||
| Yes | 23 (18.6%) | 16 (72.8%) | |
| No | 85 (69.2%) | 5 (22.7%) | |
| Bile duct | 0 (0%) | 1 (4.5%) | |
| Unknown | 15 (12.2%) | 0 (0%) | |
| Total number of loco-regional therapies, n (%) | 0.19 | ||
| 0 | 45 (36.6%) | 7 (31.8%) | |
| 1 | 58 (47.2%) | 8 (36.4%) | |
| 2 | 16 (13.0%) | 5 (22.7%) | |
| 3 | 2 (1.6%) | 2 (9.1%) | |
| 4 | 2 (1.6%) | 0 (0%) | |
| Patients with viable tumor, n (%) | 0.47 | ||
| Yes | 108 (87.8%) | 21 (95.5%) | |
| No | 15 (12.2%) | 1 (4.5%) | |
| Within Milan, n (%) | 0.005 | ||
| Yes | 104 (84.6%) | 13 (59.1%) | |
| No | 19 (15.4%) | 9 (40.9%) | |
| Downstaged to Milan, n (%) | 13 (10.6%) | 4 (18.2%) | 0.29 |
| Within UCSF, n (%) | < 0.001 | ||
| Yes | 108 (87.8%) | 13 (59.1%) | |
| No | 15 (12.2%) | 9 (40.9%) | |
| Downstaged to UCSF, n (%) | 3 (2.4%) | 3 (13.6%) | 0.045 |
Quantitative data are expressed as median (25%-75% Interquartile ranges (IQRs)). Categorical variables are reported as percentages. P values for statistical significance were calculated using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. AFP: alpha-fetoprotein. HCV: hepatitis C virus. ETOH: alcoholic liver disease. HBV: hepattis B virus. NASH: non-alcoholic steatohepatitis. LT: liver transplant. MELD: model for end stage liver disease score. WBC: white blood cell count. Hgb: hemoglobin. MCV: mean corpuscular volume. PLT: platelet count. BUN: blood urea nitrogen. TP: total protein. Alb: albumin. ALP: alkaline phosphatase. AST: aspartate aminotransferase. ALT: alanine aminotransferase. T.Bili: total bilirubin. PT: prothrombin time. INR: international normalized ratio. UCSF: University of California San Francisco.
Overall 40.9% of the patient who had HCC recurrence were outside of Milan and UCSF criteria. Of the patients who had HCC recurrence, 40.9% had poor differentiation on explant compared to 4.9% of patients without HCC recurrence (P = < 0.001). None of the 15 patients who had well differentiated tumors on explant had HCC recurrence following LT. The rate of MVI in patients with HCC recurrence was 72.8% compared to 18.6% in patients without HCC recurrence, p < 0.001 (Table 4).
Of the 39 patients who had MVI on explant, 59% did not develop HCC recurrence following LT compared to 41% who did develop recurrence. On the other hand, out of 90 patients who did not have MVI on pathology, only 5% developed HCC recurrence following LT. Total of 15 patients had poor differentiation on explant. Out of these 15 patients, 60% had tumor recurrence following LT vs. 40% with no recurrence (Table 4). However, the status of MVI and differentiation could not be evaluated in 15 and 22 patients respectively. Because of prior locoregional therapy effect and resulted tumor necrosis, there was not enough viable tumor in the explant tissue for the pathologist to assess for MVI and differentiation in this subgroup of patients.
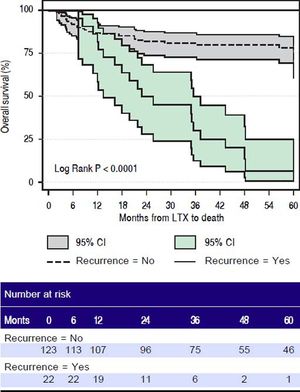
SurvivalMedian follow up of patients in our cohort was 1,231 days (range 63 -4,287 days). Overall 48 out of 145 (33.1%) patients died in our series. The cumulative recurrence incidence was significantly higher in patients with high-AFP-secreting group compared to low-AFP-secreting HCC group (P < 0.0006) (Figure 6A). Patients in the high-AFP-secreting group had a trend towards lower survival at 3 and 5 years compared to patients in the low-AFP-secreting group. The overall 6-month, 1-year, 3-year, and 5-year survival rates for patients in the low-AFP-secreting group vs. the high-AFP-secreting group were 93%, 88.1%, 78.7%, 71.4% vs. 93%, 85.2%, 65%, 58.7% respectively, (p = 0.1)(Figure 6B). The 6-month, 1-year, 3-year, and 5-year recurrence-free survival for patients in the low-AFP-secreting group was significantly higher compared to high-AFP-secreting group 100%, 100%, 92%, 92% vs. 87%, 81.3%, 71.3%, 68.5%, respectively (p = 0.0003) )(Figure 6C). The overall 6-month, 1-year, 3-year, and 5-year survival for patients in the HCC non-recurrence group was significantly higher than HCC recurrence groups 91.9%, 87%, 81%, 78.3% vs. 100%, 86.4%, 30%, 6.7% respectively (p < 0.0001) (Figure 7).
Survival after liver transplant in patients who had recurrence of hepatocellular carcinoma (solid line with dark grey box indicating 95% confidence interval) versus patients who had no recurrence of hepatocellular carcinoma (dotted line with light grey box indicating 95% confidence interval). LTX: fiver transplant. Ci: confidence interval.
In the current study, we aimed to assess the clinical, pathological, and outcome differences based on tumor biology between two groups of patients categorized into high-AFP-secreting and low-AFP-secreting HCCs, applying serum AFP cut-off of 20 ng/mL. AFP is a glycoprotein with 591 amino acids.22 It is encoded by the AFP gene on the long arm of chromosome 4.23 AFP is the most abundant protein of the fetus and is produced by the yolk sac and liver during fetal development.24 AFP has been recognized as a marker for diagnosis of HCC;25 however, it has several limitations: 1) AFP can be elevated in other malignancies such as testicular cancer; 2) AFP can rise in chronic hepatitis and can fluctuate with underlying inflammation; 3) AFP is a marker of liver regeneration and can rise in conditions associated with enhanced liver regeneration; and 4) AFP can be normal in up to 30% of patients with HCC.26–31 Our findings are consistent with prior studies, showing that a proportion of HCC tumors do not produce AFP.32 In addition, the cut-off value for AFP is inconsistent between various studies.(8) In our series, when we used a cut-off of 10 ng/mL, 40% of patients had normal AFP levels vs. 60% with abnormal levels. When we used a cut-off of 20 ng/mL, 58% had normal AFP levels vs. 42%.
These findings raise the question about the origin and prognosis of HCC based on the tumor AFP status. Recent genomic studies of patients with HCC have identified two subclasses, proliferative and non-proliferative, based on common molecular features.33 The proliferative group is characterized by a worse prognosis and higher AFP levels as compared to the non-proliferative group.33 Additionally, HCCs that originate from hepatic stem cell-like cells or hepatocytic progenitor-like cells are characterized by high AFP as compared to mature hepatocyte-like HCCs which have low AFP.34,35 There are two proposed hypotheses that the origin of the HCCs that secrete high levels of AFP, which is a progenitor cell marker, is either from mature hepatocytes during malignant transformation (dedifferentiation hypothesis) or from progenitor cells that are differentiating toward hepatocytes lineage (maturation-arrest hypothesis).36 Additionally, it has been hypothesized that the evolution of combined hepatocellular/cholangiocarcinoma tumors from common hepatic progenitor cells may account for the biphenotypic features of these tumors.37 In our study, five patients had a lesion on explant that was reported as a biphenotypic HCC/cholangiocarcinoma lesion. Although there have been reports of worse outcomes in patients with HCC/cholangiocarcinoma, none of the five patients in our series had tumor recurrence following LT.38
In the current study, we categorized the patients based on their tumor biology on the basis of AFP cutoff of 20 ng/mL and demonstrated that patients with high-AFP-secreting tumors (AFP ≥ 20 ng/mL) had larger tumor volume (larger lesions) and more aggressive HCCs (presence of microvascular invasion and poor differentiation) as compared to patients who had low-AFP-secreting tumors (AFP < 20 ng/mL). In addition, the high-AFP-secreting group tumors had a higher rate of overall tumor recurrence compared to the low-AFP secreting group. These findings are consistent with other recent studies. In a study of 665 patients by Agopian et al., patients who underwent liver transplant were categorized based on the serum AFP levels of 10 ng/mL as non-AFP producing HCCs and AFP producing HCCs.32 They showed that patients with non-AFP producing tumors compared to patients with AFP-producing tumors had fewer lesions on pathology (25% vs. 35% with > 2 lesions, P = 0.03), smaller pathologic cumulative diameter (4.2 vs. 5 cm, P = 0.02), fewer microvascular invasion (17% vs. 22%) and macrovascular invasion (2% vs. 9%) (P < 0.001), and fewer poorly differentiated tumors (15% vs. 28%, P < 0.001).32 In another study of 108 patients with HCC who underwent liver resection, the patients with AFP ≤ 20 ng/mL had significantly higher cell differentiation and lower microvascular invasion rates compared to patients with higher AFP > 20 ng/mL group.39 In a study of two cohorts of patients by Yamashita, et al., patients with AFP positive tumors had more advanced TNM stage and portal vein invasion compared to AFP negative HCCs.40
In terms of survival between patients with high-AFP-secreting versus low-AFP-secreting tumors, we did not find any statistical difference. Various groups have previously shown that patients with higher AFP levels have worse outcomes post LT as shown by higher tumor recurrence and lower survival rates. In the study of 211 patients by Hameed, et al. pre-LT serum AFP > 1000 ng/mL was a strong predictor of HCC recurrence following LT.41 The 1 and 5 year rates of survival without HCC recurrence were 90% and 52.7%, respectively, for patients with a pre LT AFP > 1000 ng/mL and 95% and 80.3%, respectively, for patients with an AFP level < 1000 ng/mL (p = 0.026).41 Agopian, et al. showed that patients with non-AFP producing tumors had a significantly superior recurrence-free survival at 1,3,and 5 years (88%, 74%, and 67% vs. 76%, 59% and 51% respectively, p = 0.002) and lower 5-year recurrence rates (8.8% vs. 22%, p < 0.01) than patients with non AFP-producing tumors.32 In a study of more than ten thousand patients by Berry, et al., patients with serum AFP level < 15 ng/mL had no excess post-transplant mortality. However, patients with a serum AFP level of 16 to 65 ng/ mL, patients with a serum AFP level of 66 to 320 ng/mL, and patients with a serum AFP level > 320 ng/mL had progressively worse post-transplant mortality.18 The lack of statistically significant difference in survival in our study may be explained by our small sample size and the retrospective nature of our study.
HCC recurrence following LT is an unfortunate event with limited therapeutic options and is associated with poor prognosis.42 In our study, the overall 6-month, 1-year, 3-year, and 5-year survival for patients with HCC recurrence were significantly lower compared to non-recurrence group. Patients with HCC recurrence had higher serum AFP levels, larger tumor volume, higher rates of MVI, worse tumor differentiation, and higher likelihood of being outside of Milan and UCSF criteria compared to patients that did not develop HCC recurrence. These findings are consistent with prior studies by other groups.43
In summary, recent studies have shown that the MC are suboptimal in predicting tumor recurrence, suggesting that assessing tumor recurrence following liver transplant solely based on tumor size and number is overly simplistic.44 This implies that other factors such as tumor biology play a significant role in predicting tumor recurrence post-LT.45,46 In this study, we defined the clinical and pathological differences of HCC patients undergoing LT, based on their tumor biology: high-AFP-secreting (AFP ≥ 20 ng/mL) and low-AFP-secreting (AFP < 20 ng/mL) tumors. We show that patients with high-AFP-secreting tumors have larger lesions; more aggressive tumors- higher rates of MVI and poor differentiation; and higher tumor recurrence compared to patients with low-AFP-secreting group. However, AFP is a suboptimal predictor of tumor recurrence following liver transplant in HCC patients. Our study is limited by small sample size and its retrospective nature. In addition, we did not include data regarding active versus cured hepatitis (eg. hepatitis C). It is known that AFP can increase in conditions that can enhance liver regeneration, such as viral hepatitis, which can affect the test results such. We believe that further understanding of the biology and origin of the HCC tumors, eg. AFP-secreting vs. non-secreting or progenitor vs. non-progenitor, are essential to precisely differentiate diverse classes of tumors. This might lead to the discovery of novel biomarkers that can potentially enhance patient selection and prediction of clinical outcomes in the field of LT.
Abbreviations- •
AFP: alpha feto protein.
- •
HCC: hepatocellular carcinoma.
- •
IQR: interquartile ranges.
- •
LT: liver transplantation.
- •
MC: Milan criteria.
- •
MELD: model for end-stage liver disease.
- •
MVI: micro-vascular invasion.
- •
UCSF: University of California, San Francisco.
The authors declares that there is no conflict of interest regarding the publication of this article.
DisclosuresNone.
FundingNational Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079
AcknowledgementWe would like to acknowledge partial support for the statistical analysis from the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
The authors express appreciation to Carol Thompson, MS MBA, Johns Hopkins Biostatistics Center, Johns Hopkins Bloomberg School of Public Health, for her assistance with the biostatical analysis.
Authors ContributionsAG (study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, drafting of the manuscript); MM (study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, drafting of the manuscript); JGW (analysis and interpretation of data, statistical analysis, drafting of the manuscript); AK (Study concept and design, drafting of the manuscript); RAA (acquisition of data, analysis and interpretation of data); KS (acquisition of data, analysis and interpretation of data); CG (analysis and interpretation of data); MG (analysis and interpretation of data, statistical analysis); SO (acquisition of data); AMC (acquisition of data); BP (acquisition of data); BS (study concept and design, acquisition of data, drafting of the manuscript, analysis and interpretation of data, statistical analysis, critical revision of the manuscript for important intellectual content, study supervision).