Paracoccidioidomycosis is a systemic granulomatous disease caused by the dimorphic fungus Paracoccidioides brasiliensis and is restricted to Latin America. It normally affects lungs, skin and lymph nodes. Abdominal organs are usually not involved. In rare cases paracoccidioidomycosis may simulate neoplasm. Herein we describe our experience with four cases of paracoccidioidomycosis mimicking cholangiocarcinoma. To the best of our knowledge, this is the largest case series on this subject produced in English. Paracoccidioidomycosis must be considered as a differential diagnosis of cholangiocarcinoma, especially in individuals who come from endemic areas.
Paracoccidioidomycosis (PCM) is one of the most important deep mycoses in Latin America1 and was originally described by Adolfo Lutz in 1908.2 The etiologic agent is the dimorphic fungus Paracoccidioides brasiliensis.1 It presents itself in two major forms: subacute (3-5% of cases), affecting mostly children and young adults, frequently compromising the reticuloendothelial system,1,3 and chronic (90% of cases), involving predominantly adult males, normally affecting lungs, lymph nodes and skin.1,3 Identification of the fungus in the affected tissue (paracoccidioidal granuloma) or culture of exudate establishes diagnosis.1 Herein we describe four cases of PCM simulating cholangiocarcinoma.
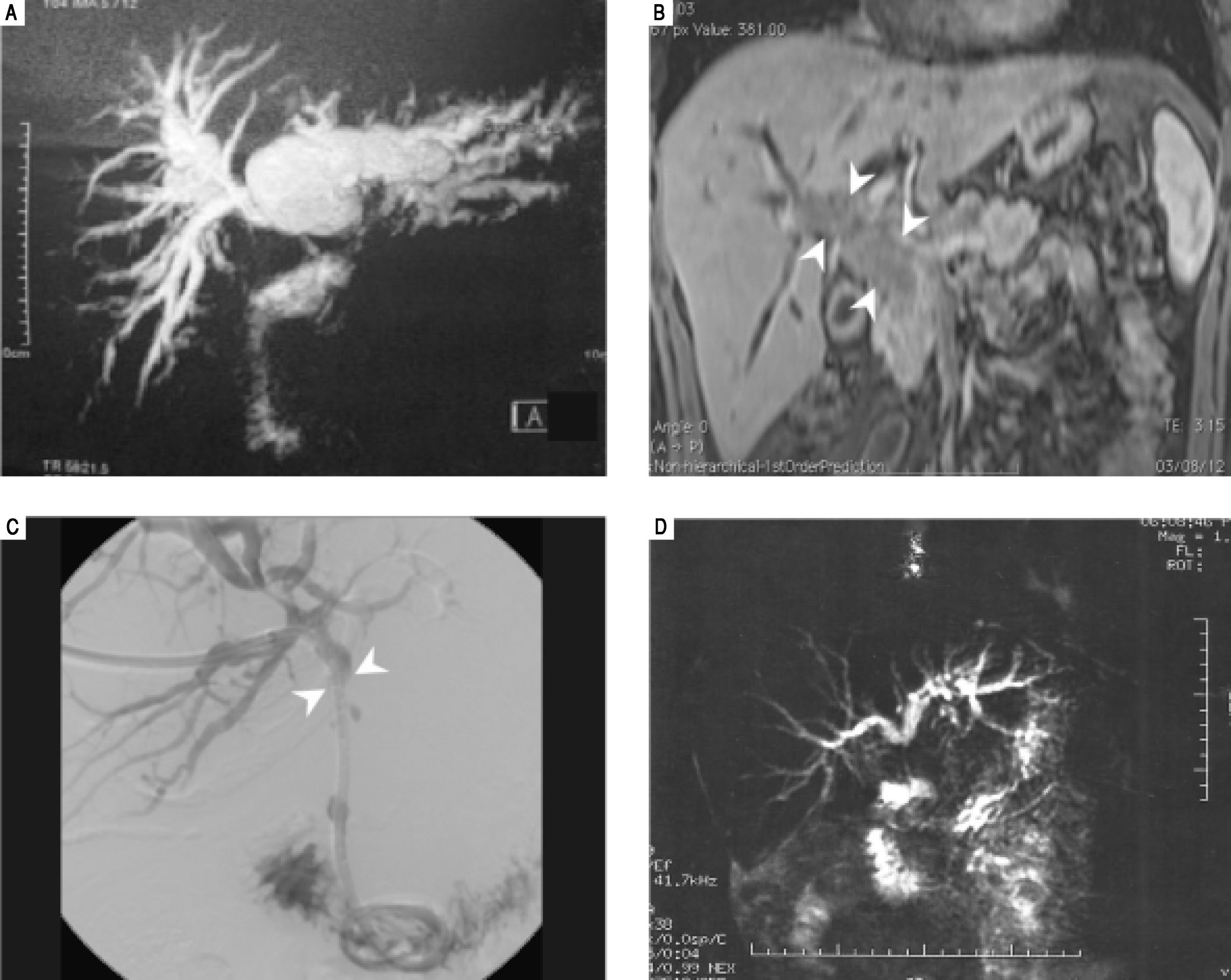
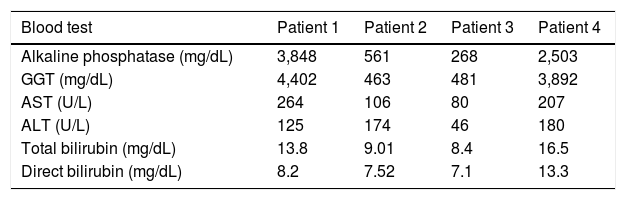
Case ReportPatient 1A 53-year-old man, from the rural area of Rio de Janeiro, Brazil, was admitted with a three-month history of jaundice, pruritus, acholic stools, choluria and weight loss of 10 kg. He denied fever. Physical examination revealed a painful and palpable left liver lobe in the epigastrium. No peripheral lymphadenopathy was detected. Anemia and elevated liver enzymes and bilirubin were present (Table 1). Tumor markers (CEA and CA19-9) were normal. Thorax X-ray was normal. Abdominal ultrasonography showed hepatomegaly, intrahepatic left bile duct dilatation without perihilar linphadenomegaly and an empty gallbladder. MRI confirmed left bile duct dilatation without extrinsic compression (Figure 1A).
Admission patients’ blood tests results.
| Blood test | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Alkaline phosphatase (mg/dL) | 3,848 | 561 | 268 | 2,503 |
| GGT (mg/dL) | 4,402 | 463 | 481 | 3,892 |
| AST (U/L) | 264 | 106 | 80 | 207 |
| ALT (U/L) | 125 | 174 | 46 | 180 |
| Total bilirubin (mg/dL) | 13.8 | 9.01 | 8.4 | 16.5 |
| Direct bilirubin (mg/dL) | 8.2 | 7.52 | 7.1 | 13.3 |
Patient 1: MR T2-weighted showing an important biliary tree dilatation, mostly in left duct. B. Patient 2: MRI image showing an infiltrative mass inside the hepatocholedocus (arrow heads), C. Patient 3: Percutaneous cholangiography during biliary drain placement. Main duct blockage is evidenced (arrow heads). D. Patient 4: MR T2-weighted showing obstruction of common bile duct with upstream dilatation.
Two years earlier, he had been diagnosed with ganglionar PCM located in the cervical region and has been being treated since with itraconazole 200 mg/day. No history of other diseases or previous surgery was known.
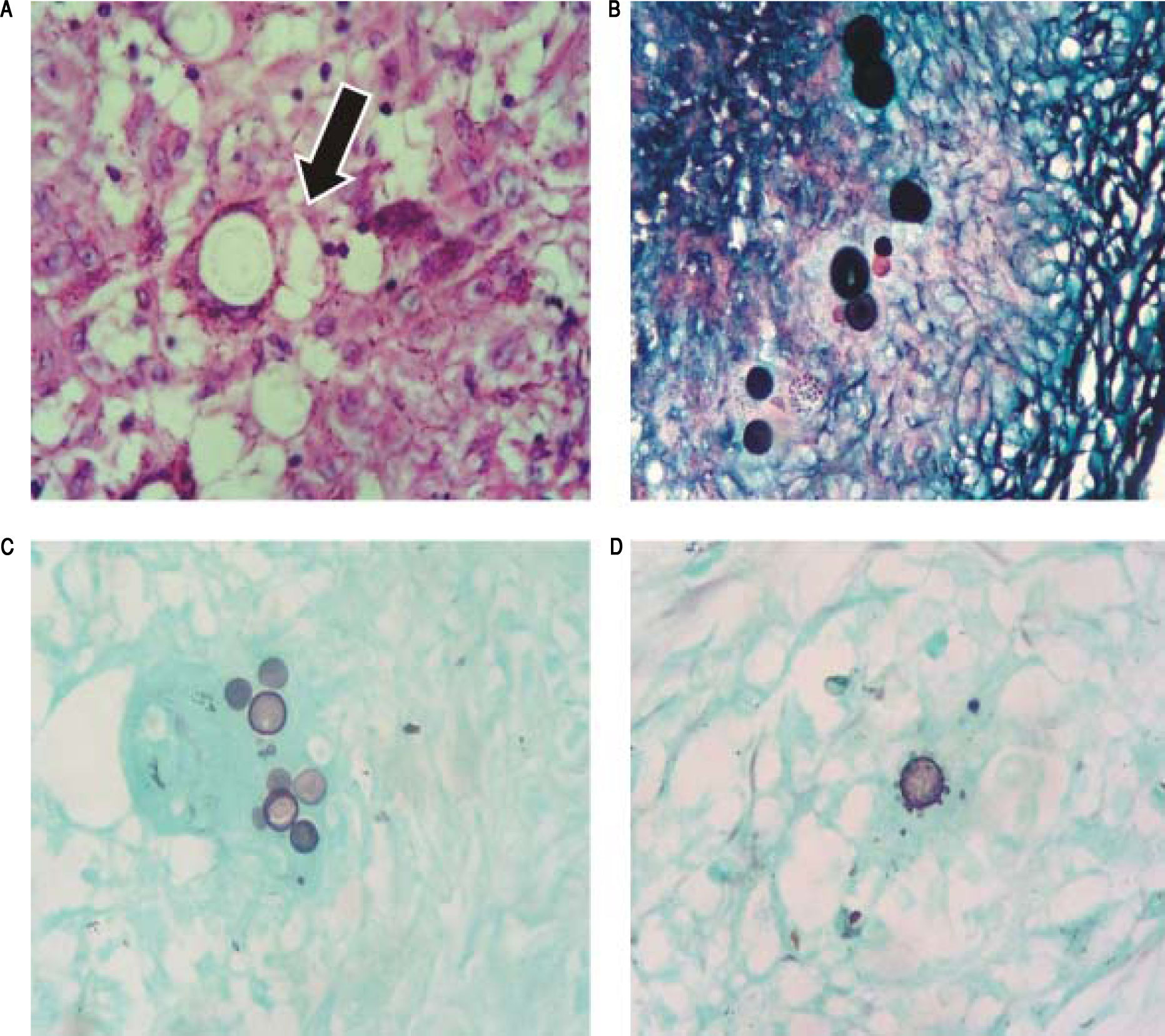
An early stage intrahepatic cholangiocarcinoma was suspected and the patient was referred to surgery. Left hepatectomy with common bile duct resection and Roux-en-Y biliary-enteroanastomosis reconstruction was performed. He was discharged without complications after seven days. Histopathological evaluation revealed areas of cavitation and biliary abscesses in the liver, in which fungal elements compatible with P. brasiliensis were present (Figure 2A). After diagnosis of biliary PCM, treatment with itraconazole was resumed. One year after surgery, he developed a new episode of ganglionar PCM in the inguinal region. He is doing well, six years after hepatectomy.
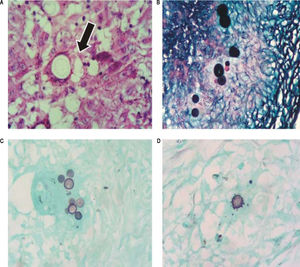
Patient 1: area of granuloma containing fungal spherical elements (arrow) compatible with P. brasiliensis, H&E stain x400. B. Patient 2: fungal structures with budding affecting lymph node, Grocott’s methenamine silver stain x400. C and D. Patient 3 and 4: fungal structure with characteristic “Mickey Mouse” and “steering wheel” budding, respectively, Grocott’s methenamine silver stain x400.
A 22-year-old man, from Rio de Janeiro was transferred from a primary hospital with one-month history of jaundice associated with diarrhea and a substantial weight loss of 20 kg. He denied fever or abdominal pain. No peripheral lymphadenopathy was found. No morbidities were known. No history of rural work or previous fungal disease was reported. Laboratory exams confirmed anemia and elevation of serum bilirubin as well as liver enzymes (Table 1). Serology for hepatitis and HIV were negative. MRI showed an infiltrative mass inside the hepatocholedocus, with restricted diffusion, obstructing primary and secondary biliary ducts, especially in the right side (Figure 1B), suggestive of extra-hepatic cholangiocarcinoma.
The patient was submitted to laparotomy. The liver had some whitish lesions suggestive of secondary implants. A large quantity of lymph nodes was observed in the hepatic hilum, hepatic artery and para-aortic. A frozen section biopsy of the liver and para-aortic lymph node did not show malign neoplasia. Transcystic cholangiography showed obstruction of the passage of contrast through the distal bile duct. The illness was considered to be at an advanced stage, and surgical team decided to perform cholecystectomy, internal/external drainage of the biliary tract, lymph node excision, and biopsies. After surgery, he developed biliary fistula and pancreatitis and responded well to clinical therapy. Microscopic analysis revealed granulomatous chronic inflammatory process affecting the gallbladder, liver and lymph nodes, associated to numerous giant multinucleated cells and fungal structures compatible with P. brasiliensis (Figure 2B). After 30 days of intravenous amphotericin B, the patient left the hospital in a generally good state, taking itraconazole orally. The biliary drain was removed after one year and, five years after surgery, the patient is doing well.
Patient 3A 53-year-old woman was transferred from a primary hospital from the rural area of Rio de Janeiro with a three-month history ofjaundice, pruritus, acholia, choluria and weight loss of 13 kg. At the primary hospital, she was submitted to percutaneous transhepatic cholangiography, which showed intrahepatic biliary dilatation and main bile duct blockage. An internal-external biliary drain was placed at the same time (Figure 1C). Admission blood tests showed anemia and elevated serum bilirubin and canalicular enzymes (Table 1). CT scan confirmed biliary dilatation above hepatic confluence and identified gallbladder with thick and infiltrated wall, compatible with cholecystitis, as well as para-aortic and celiac trunk lym-phadenomegaly. The patient was considered to have advanced illness and treatment was limited to antibiotic therapy for cholecystitis, once biliary tract was already drained. After 9 days, she presented abdominal sepsis and underwent emergency laparotomy. During surgery, choledocoduodenal fistula, cholecystitis and inflammatory pseudotumoral involvement of duodenum were identified. The emergency team opted to perform cholecystectomy, Billroth II distal gastrectomy, and mesenteric lymph node biopsy. After surgery, the patient experienced cholangitis, but responded well to antibiotics. Histopathological evaluation revealed granulomatous chronic inflammatory process with round fungal structures, compatible with P. brasiliensis affecting the gallbladder, gallstones, mesenteric lymph node, and stomach wall (Figure 2C). The patient received amphotericin B for 30 days and was discharged 47 days after surgery taking itraconazole orally. The biliary drain was detached one year after surgery and patient is well and asymptomatic, 38 months after surgery.
Patient 4A 48-year-old woman from Rio de Janeiro was admitted with a two-month history of abdominal pain, cholestatic jaundice and weight loss of 12 kg. She had arterial hypertension, which was under control, and had undergone a hysterectomy 10 years before, due to myomatosis. No peripheral lymphadenopathy was found. Laboratory tests confirm cholestasis (Table 1). MRI showed obstruction of main bile duct next to the confluence (Figure 1D). The patient was submitted to percutaneous biliary drainage and posteriorly referred to surgery.
Bilateral subcostal laparotomy was performed. Hepatic pedicle had a thick tissue evocative of malignant infiltration. Lymphadenomegaly was present in the hepatic hilum, as well as celiac trunk and retropancreatic. An analysis of these lymph nodes revealed caseous material with frozen section negative for neoplasia. An inflammatory or infectious pathology was suspected. As the biliary tract was already drained, no other procedure was performed. Anatomopathological examination revealed granulomatous chronic inflammatory process with round fungal structures with budding, compatible with P. brasiliensis (Figure 2D). The postoperative period was uneventful. She received amphotericin B intravenously for 30 days and completed treatment with oral itraconazole. Biliary drain was removed after 10 months. The patient is in good shape, with mild canalicular enzymes elevation 32 months after surgery.
DiscussionPCM constitutes a serious public health problem because it is potentially quite disabling, affecting people during the economically productive years, predominantly male individuals (1 female : 10-15 male).1 It is considered to be the third leading cause of death from chronic infectious disease, in Brazil.1 About 80% of the cases reported occurred in Brazil and most of the remaining ones occurred in Venezuela, Colombia and Argentina.1 In Mexico, the endemic area is located in the South between the Gulf of Mexico and the Pacific Coast.4
The pathogen agent is the dimorphic fungus Paracoccidioides brasiliensis, which is present in nature in filaments containing infective propagules called conidia. Once inhaled, the propagules give rise to fungi yeast forms that will constitute their parasitic form in host tissues.1
The major risk factor for infection are professions or activities related to management of soil contaminated with the fungus, such as agricultural activities, earthmoving, soil preparation, gardening, and transport of plant goods. Contamination is primarily acquired in the first two-decade of life, but the presentation of clinical manifestations occurre more frequently in adults between 30 and 50 years, after reactivation of latent endogenous focus.1
Abdominal involvement in PCM is normally seen in the sub-acute form, with a wide variety of clinical manifestations, ranging from nausea, vomiting, ascites, jaundice, variable abdominal pain, hepatosplenomegaly, malabsorption syndrome, tumor mass, intestinal obstruction, peritonitis, and mesenteritis, to even acute perforative abdomen.3,5 Jaundice usually appears at a late stage, and it is associated with more severe disease.6 It is normally caused by extrinsecal compression of the common bile duct by lymph nodes.6 Other causes described are intraluminar granulomatous lesion of the common bile duct (intrinsic lesion), hepatic lesion caused by blastomycotic hepatitis and pancreatic PCM. 6-8
In the cases reported here, the presence ofjaundice associated with other symptoms, as well as imaging exams compatible with cholangiocarcinoma, led us to this misdiagnosis. Patient 1 had a segmental intrahepatic biliary obstruction, which was probably caused by portal granulomas, as described by Brito, et al.9 Patient 2 displayed a mass causing obstruction of choledocus, compatible with intraluminar granulomatous lesion of the common bile duct. Patients 3 and 4 were misdiagnosed with Bismuth type 1 and 2 Klatskin tumor respectively. In both cases, extrinsecal compression of the common bile duct by lymph nodes and hepatic hilum inflammatory infiltration caused by PCM infection were posteriorly considered the reasons for biliary obstruction.
Once the diagnostic hypothesis was cholangiocarcinoma, patients 1, 2 and 4 were submitted to intention-to-treat surgery. Patient 3 was considered to have advanced neoplasia, but underwent emergency surgery due to abdominal sepsis. Choledocoduodenal fistula was identified, which is a complication of PCM infection not yet described. In all cases, diagnosis of PCM was confirmed after anatomopathological examination of specimen. In patient 3, even the gallbladder and stomach were involved with P. brasiliensis.
After PCM was diagnosed, patients were investigated for fungal involvement of other organs, like lungs, skin, nasopharynx or lymph nodes and specific treatment was proposed. The usual therapy involves antifungal drugs, such as sulfanilamide derivatives, azole compounds and amphotericin B, which has been used in advanced cases.1 Patient 1 was already using itraconazole, due to a past history of ganglionar PCM, and treatment was resumed. Patients 2, 3 and 4 manifested the disease in a more advanced state and were initially treated with amphotericin B, which was changed to itraconazole after hospital discharge. Recommended dosage of itraconazole is 200 mg per day. Treatment can last up to 24 months and should not be interrupted.1 A cure for PCM patients may never be achieved because there is always a chance of later recurrence, as could be observed with patient 1.
ConclusionThe cases reported here are good examples of infectious disease mimicking a neoplasm. In the presence of obstructive jaundice and suspicion of cholangiocarcinoma, in Brazil and other endemic areas in Latin America, we should regard PCM as a differential diagnosis, particularly if the patient has a previous history of infection by Paracoccidioides brasiliensis.
Abbreviations- •
PCM: paracoccidioidomycosis.
The authors declares that there is no conflict of interest regarding the publication of this article.
Supportive FoundationsThere was no funding source.
DisclosurePatient 1 has been published previously as a case report.