Some of the evidence on whether antioxidant supplements are effective in treatment of liver diseases is contradictory. Here we perform a descriptive analysis of the available data in vivo and in vitro of the possible antiviral action and controversy of several antioxidant molecules against HCV.
Currently, several specific direct-acting antiviral agent (DAA) options are available to fight hepatitis C virus infection. Many efforts have been dedicated to the identification of new therapeutic strategies aimed at targeting virus–host cell interactions. A common mechanism for many RNA viruses that replicate in the cytoplasm is the alteration of the cell environment by deregulation of oxidative stress and antioxidant systems. Because antioxidants defend cells from damage caused by reactive oxygen species (ROS), they represent interesting molecules to fight infectious diseases. Based on the foregoing, the use of antioxidants in viral hepatitis infection as antiviral adjuvants generated much controversy some years ago, and yet to date, we still do not know if they could be beneficial to favor an adequate cellular response in the presence of specific antivirals. Here, we will provide some recent data that will allow us to have a better understanding about the relationship between HCV replication, new DAAs, oxidative stress and antioxidant compounds.
2HCV antiviral optionsWe remember in 2011 the birth of a new era in antivirals, when the first generation of DAAs against HCV was reported. Since then, we have learned a lot about the virology of HCV; however, we still have doubts about the basic mechanisms that the cell uses to counteract the viral attack. These mechanisms include modulation of apoptosis, oxidative stress, stress of the endoplasmic reticule, cell communication, and others. Several specific antiviral options are currently available to fight hepatitis C virus infection. Specific anti-HCV drugs include protease inhibitors (such as Simeprevir), NS5A inhibitors (such as daclatasvir and ledipasvir) and RNA polymerase inhibitors (such as Sofosbuvir) which jointly block the release of virions from infected cells [1].
3Oxidant/antioxidant defense network induced by virusIt is well known that oxidative stress is altered during viral infections. This can be caused by the decrease in antioxidant defenses and the increase in reactive oxygen species (ROS) production in different cell compartments [2]. HCV can modulate intracellular redox sensitive signaling pathways involved in several cell functions in order to promote viral replication and pathogenesis. In particular, several papers have reported that ROS and reactive nitrogen species (RNS) contribute to the development of HCV virus-induced pathogenesis in the liver. The emergence of strains resistant to HCV antiviral agents highlights the need to search for new drugs that act on new molecular targets [3]. An interesting approach should be targeting interactions between the virus and the host cell that could reduce viral replication and ameliorate cell functions. Physiological levels of ROS mediate cell signaling, while upregulated levels of ROS are involved in oxidative damage to cellular components and cell death [2]. Under this context, antioxidant enzymatic systems and natural compounds are triggered to mitigate or resolve this situation conforming an “antioxidant defense network”. In such a way, supplementation with antioxidants has been suggested to minimize the infection disease effects.
4Antioxidants to assist the antiviral approachAntioxidants represent interesting molecules that have been proposed for the treatment of several viral infections. In 2006, Friel et al. proposed the prophylactic use of nutritional supplement with antioxidants to counteract general pathogenic mechanisms underlying virus infection in humans [4]. Recently, Gonçalves et al. proposed orange juice as a dietary source of antioxidants for patients with hepatitis C under antiviral therapy. They demonstrated that orange juice was a convenient food in the diet of patients due to the increase in antioxidant capacity and decreased inflammation and cholesterol in blood serum, in addition to maintaining body mass, which protects against the harmful effects caused by C virus chronic hepatitis (CHC) [5]. Several authors, including our research group, have reported that natural and pharmacological compounds with antioxidant capacity significantly decrease the replication of several viruses [6–9]. Antioxidant therapy could be criticized because it involves a variety of unrelated drugs, rather than the specific or magic one used in today's pharmacotherapy. However, there is a scientific basis for this strategy as antiviral therapy.
It would be reasonable to increase the antioxidant capacity of the cell using exogenous compounds of synthetic origin or the diet, thus enhancing cell defenses against free radical formation. Natural antioxidants present in fruit, vegetables and marine animals, including vitamins C and E, astaxanthin, xanthophyll carotenoid, vegetal carotenoids, minocycline, arctigenin, fenofibrate, curcumin, and polyphenols (e.g. flavonoids), are currently considered to be beneficial [10]. It is reported that antioxidant enzyme levels (superoxide dismutase (SOD), catalase, and glutathione peroxidase) were increased in rats upon treatment with astaxanthin [11]. The antiviral activity of rosmarinic acid and luteolin is shown to be due to their high antioxidant, anti-inflammatory and neuroprotective potential. However, many controversies surround the effects of these compounds in experimental models, and their real benefits are still a matter of debate.
It is not known if different agents with antiviral activity can interfere with the modulation of the cellular redox state induced by HCV and decrease viral replication. Recent reports of antioxidant compounds highlight their antiviral activity against HCV and promote them as anti-HCV co-adjuvants that can improve treatment effectiveness, shorten the treatment period, and reduce the overall cost of therapy. In Table 1, relevant antioxidants proposed as anti-HCV agents are described. Clearly, this does not obviate the further search for drugs that specifically interfere with viral replication. This viewpoint describes the structure and mechanism of action of the main antioxidants studied as antiviral agents.
Relevant antioxidants proposed as anti-HCV agents.
| Antioxidant | Described function | Study model | Reported mechanism | Controversy | Chemical structure |
|---|---|---|---|---|---|
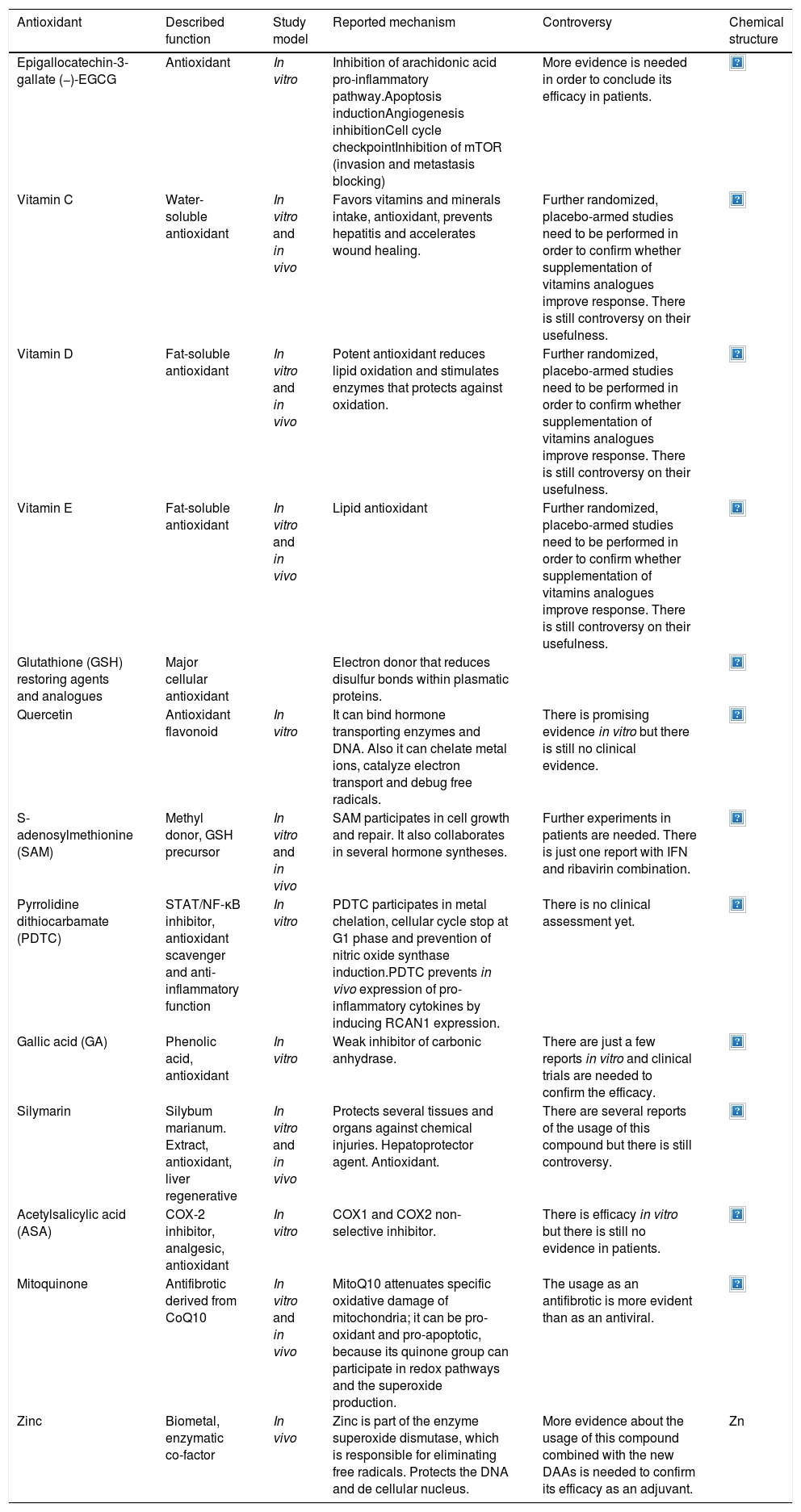
| Epigallocatechin-3-gallate (−)-EGCG | Antioxidant | In vitro | Inhibition of arachidonic acid pro-inflammatory pathway.Apoptosis inductionAngiogenesis inhibitionCell cycle checkpointInhibition of mTOR (invasion and metastasis blocking) | More evidence is needed in order to conclude its efficacy in patients. | |
| Vitamin C | Water-soluble antioxidant | In vitro and in vivo | Favors vitamins and minerals intake, antioxidant, prevents hepatitis and accelerates wound healing. | Further randomized, placebo-armed studies need to be performed in order to confirm whether supplementation of vitamins analogues improve response. There is still controversy on their usefulness. | |
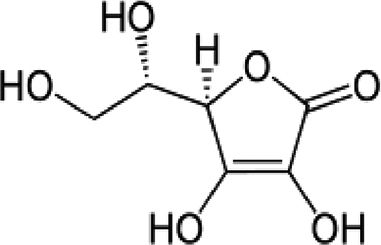
| Vitamin D | Fat-soluble antioxidant | In vitro and in vivo | Potent antioxidant reduces lipid oxidation and stimulates enzymes that protects against oxidation. | Further randomized, placebo-armed studies need to be performed in order to confirm whether supplementation of vitamins analogues improve response. There is still controversy on their usefulness. | |
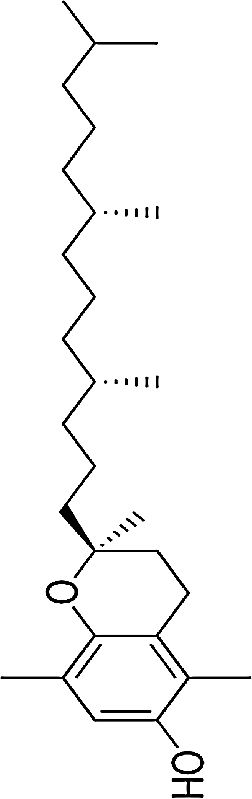
| Vitamin E | Fat-soluble antioxidant | In vitro and in vivo | Lipid antioxidant | Further randomized, placebo-armed studies need to be performed in order to confirm whether supplementation of vitamins analogues improve response. There is still controversy on their usefulness. | |
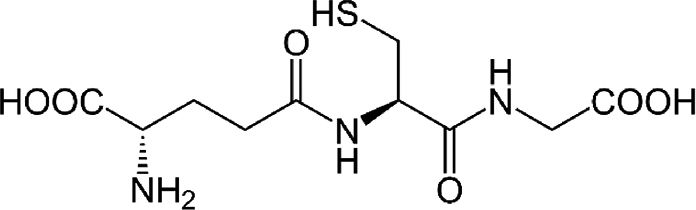
| Glutathione (GSH) restoring agents and analogues | Major cellular antioxidant | Electron donor that reduces disulfur bonds within plasmatic proteins. | |||
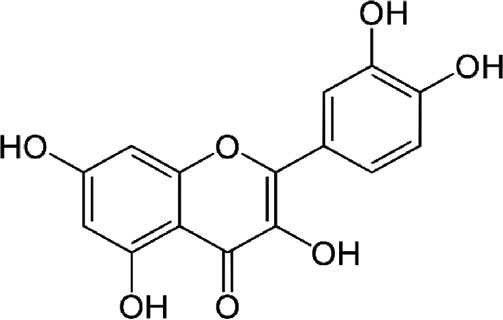
| Quercetin | Antioxidant flavonoid | In vitro | It can bind hormone transporting enzymes and DNA. Also it can chelate metal ions, catalyze electron transport and debug free radicals. | There is promising evidence in vitro but there is still no clinical evidence. | |
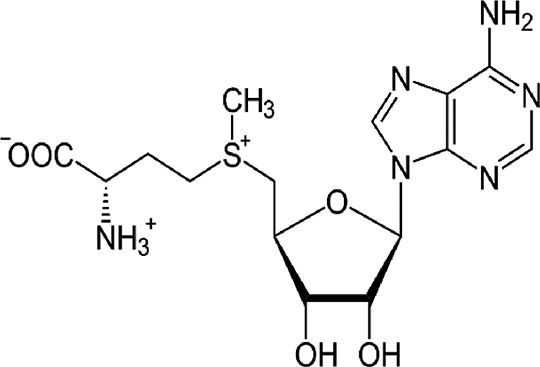
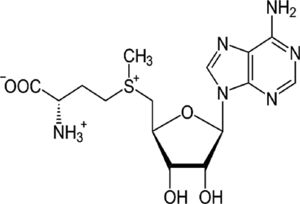
| S-adenosylmethionine (SAM) | Methyl donor, GSH precursor | In vitro and in vivo | SAM participates in cell growth and repair. It also collaborates in several hormone syntheses. | Further experiments in patients are needed. There is just one report with IFN and ribavirin combination. | |
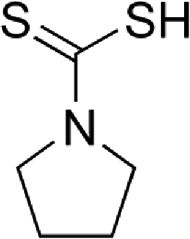
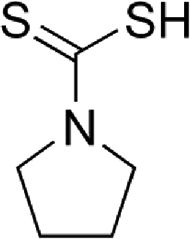
| Pyrrolidine dithiocarbamate (PDTC) | STAT/NF-κB inhibitor, antioxidant scavenger and anti-inflammatory function | In vitro | PDTC participates in metal chelation, cellular cycle stop at G1 phase and prevention of nitric oxide synthase induction.PDTC prevents in vivo expression of pro-inflammatory cytokines by inducing RCAN1 expression. | There is no clinical assessment yet. | |
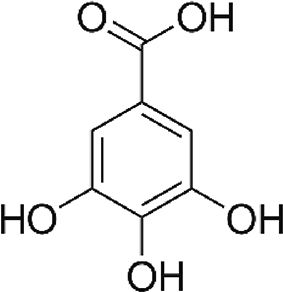
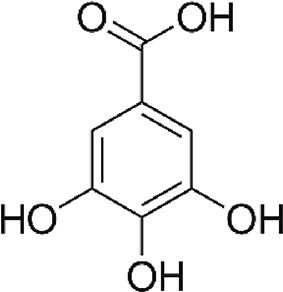
| Gallic acid (GA) | Phenolic acid, antioxidant | In vitro | Weak inhibitor of carbonic anhydrase. | There are just a few reports in vitro and clinical trials are needed to confirm the efficacy. | |
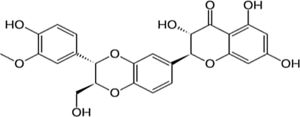
| Silymarin | Silybum marianum. Extract, antioxidant, liver regenerative | In vitro and in vivo | Protects several tissues and organs against chemical injuries. Hepatoprotector agent. Antioxidant. | There are several reports of the usage of this compound but there is still controversy. | |
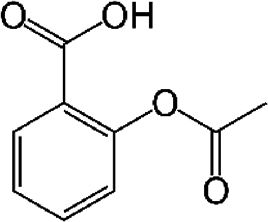
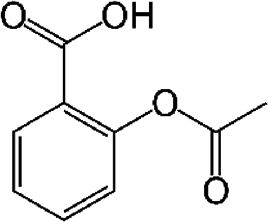
| Acetylsalicylic acid (ASA) | COX-2 inhibitor, analgesic, antioxidant | In vitro | COX1 and COX2 non-selective inhibitor. | There is efficacy in vitro but there is still no evidence in patients. | |
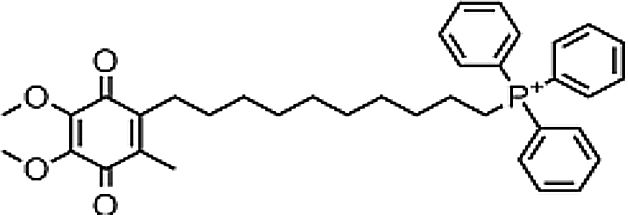
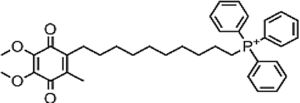
| Mitoquinone | Antifibrotic derived from CoQ10 | In vitro and in vivo | MitoQ10 attenuates specific oxidative damage of mitochondria; it can be pro-oxidant and pro-apoptotic, because its quinone group can participate in redox pathways and the superoxide production. | The usage as an antifibrotic is more evident than as an antiviral. | |
| Zinc | Biometal, enzymatic co-factor | In vivo | Zinc is part of the enzyme superoxide dismutase, which is responsible for eliminating free radicals. Protects the DNA and de cellular nucleus. | More evidence about the usage of this compound combined with the new DAAs is needed to confirm its efficacy as an adjuvant. | Zn |
This is the most abundant and bioactive catechin in green tea. It possesses antiviral activity because it can enhance in vitro synthesized HCV dsRNAs-induced innate immune responses in both HCV JFH-1-infected and -uninfected Huh7 cells. In cells treated with EGCG before HCV dsRNAs stimulation, the HCV dsRNAs induced the expression of IFN-λ1, RIG-I, TLR3 and several antiviral interferon sensitive genes (ISG15, MxA), thus inhibiting hepatitis C virus replication in hepatocytes [12]. The controversy remains regarding the mechanisms involved that are triggered by EGCG. There is no evidence about the modulation of ROS during these experiments and clinical studies are still missing.
4.2Vitamins C, D and EVitamin C protects cell components from free radical damage by reducing water-soluble radicals, scavenging lipid-peroxidation-derived radicals, or reducing tocopherol radicals to tocopherol. Currently, vitamin D deficiency is being investigated in CHC patients. A randomized double-blinded, placebo-controlled trial was performed to assess the dynamic changes in serum fibrogenic cytokines/enzymes in CHC patients with vitamin D deficiency after short-term supplementation with vitamin D [10]. Regarding vitamin E, the principal lipid soluble chain-breaking antioxidant in mitochondria, microsomes, and lipoproteins, it has been demonstrated that disease progression induced by partial hepatectomy is substantially attenuated by this vitamin [10,13,14]. There are several reports with conflicting or non-reproducible results using these vitamins; therefore, there is still controversy on the usefulness or causality correlation between supplementation and viral load.
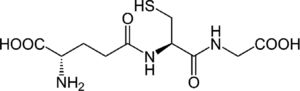
4.3Glutathione (GSH) restoring agents and analoguesIt has been shown that the replenishment of intracellular GSH obtained with the administration of GSH, a GSH derivative or a GSH precursor inhibited the replication of several viruses in vitro and in vivo[15]. We previously reported that S-adenosylmethionine increases the synthesis of GSH in Huh7 cells with and without non-structural HCV protein expression, and in the latter, replication decreases [7]. In fact, the oxidative environment established by GSH depletion during viral infection is needed to modify the expression of several antioxidant systems, thus disturbing the redox balance and favoring viral replication and maturation.
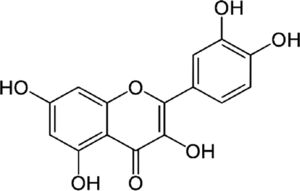
4.4QuercetinQuercetin is an antioxidant flavonoid. In vitro treatment of HCV infection with quercetin decreased intracellular viral accumulation and infectious particle production. It has been shown that quercetin inhibits viral protein production independently of viral genome replication. In conclusion, quercetin significantly decreases viral genome replication, the production of infectious HCV particles and the specific infectivity of the newly produced viral particles [16]. Additionally, dihydroquercetin has been shown to be beneficial as a hepatoprotective substance in the treatment of toxic hepatitis and liver fibrosis by enhancing antioxidant enzyme activity and decreasing the pro-oxidant effect. Nevertheless, clinical trial evidence is still missing.
4.5S-adenosylmethionine (SAM)Administration of SAM to non-responding patients infected with HCV, showed beneficial effects in combination with PEG-IFN and ribavirin. Our recent results indicate that there is a combination of actions regarding the molecular mechanisms of SAM on HCV replication. We have found that administration of SAM to HCV-expressing cells modulates the antioxidant defense systems at the transcriptional and translational level (SOD1, SOD2 and Thioredoxin 1). Also, we identified that the biosynthesis of GSH in the presence of SAM is increased in short periods of time. In addition, we found that MAT1/MAT2 turnover is switched in the presence of SAM. MAT1 is the enzyme responsible for the conversion of methionine to S-adenosylmethionine, and it is downregulated in hepatocarcinoma and liver diseases. But still we need more research to understand the role of SAM in the modulation of HCV.
4.6Pyrrolidine dithiocarbamate (PDTC)PDTC is a thiol-containing molecule that can function as anti-oxidant compound depending on the experimental conditions. The antioxidant properties of PDTC are attributed to its ability to scavenge radicals, to chelate ions, and to alter ROS metabolism. PDTC is also able to regulate antioxidant enzyme gene expression (MnSOD, heme oxygenase-1 and -glutamyl-cysteine synthetase [17]) and inhibit NF-κB. In addition, PDTC is able to suppress ROS accumulation induced by influenza virus infection [18]. There is in vitro evidence about the antiviral effect of PDTC against HCV [9]; however, further in vivo studies are needed.
4.7Gallic acid (GA)Gallic acid is a phenolic compound found in natural sources. It has several therapeutic health effects for many diseases including cancer, neurodegenerative diseases, diabetes, cardiovascular diseases and viral infectious diseases [19]. Our group has previously reported that GA downregulated NS5A-HCV protein expression (around 55%) and HCV-RNA levels (nearly 50%) compared with untreated cells. Additionally, GA treatment also decreased ROS levels in cells expressing HCV proteins [6]. These findings suggest the possibility that the antioxidant capacity of GA could contribute to the mechanism(s) involved in the downregulation of HCV replication in hepatoma cells; however, further experiments are needed to confirm these findings.
4.8SilymarinSilibinin is the main component of milk thistle (silymarin) and has strong antioxidant and antifibrotic properties. This natural compound inhibits radical formation, binds some radical species (scavenger), interferes with lipid peroxidation of membranes, and increases the intracellular content of scavengers. An intravenous administration of silibinin in nonresponders showed a decrease in the HCV-RNA level [20].
4.9Acetylsalicylic acid (ASA)It has been documented that sodium salicylate and ASA inhibit the replication of flaviviruses. In 2008, Trujillo Murillo et al. [8] reported that ASA exhibits anti-HCV properties because of its inhibitory effect on COX-2 expression, which is mediated in part by the activation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 (MEK1/2)/p38 MAPKs. These findings suggest the possibility that ASA could be an adjuvant in the treatment of HCV infection.
4.10MitoquinoneMitoquinone (MitQ) is a potent antioxidant that binds coenzyme Q10 (known as ubiquinone) to a triphenylphosphonium cation. The cation causes the attached antioxidant to accumulate several-hundred fold within mitochondria in vivo following oral administration, protecting the cell from oxidative damage. MitQ treatment demonstrated reduced serum aminotransferase in HCV infected patients. Thus, MitQ is outlining more as an antifibrotic agent than an antiviral agent [21].
4.11ZincPolaprezinc (a combination of zinc and l-carnosine), an antioxidant, has been studied as an adjuvant to IFN in the treatment of chronic HCV infection in many studies. There are a number of potential mechanisms of the beneficial effects of zinc including reduction of hepatic fibrosis, a decrease in ferritin, antioxidant activity, and improvement in hepatic encephalopathy with reduced levels of oxidative stress products such as malondialdehyde (MDA), 4-hydroxyalkenals (HAE), and 8-hydroxydeoxyguanine in plasma. Zinc has also been shown to negatively affect HCV replication justifying its use for treatment of HCV infection [22].
5New strategies for inhibiting ROS productionROS production is carried out in several cellular compartments, mainly ER and mitochondria. Several enzymes in the cells are able to produce ROS among which xanthine oxidase, cytochrome P450 oxidase, uncoupled nitric oxide synthase, NADPH (nicotinamide adenine dinucleotide phosphate) oxidases, and the mitochondrial electron transport chain are involved. However, only NADPH oxidases produce ROS as their primary and unique function. Antioxidants act in four different ways to fight viral infection: (1) they impair mechanisms involved in HCV replication, (2) improve liver enzyme levels, (3) protect against liver cell damage and (4) render interferon anti-viral therapy more effective. In fact, triple antioxidant therapies are on the rise, which include alpha-lipoic acid, silymarin and selenium in suppressing HCV-induced liver disease, when used together with vitamins C and E, and with a healthy diet and exercise.
6ControversyThe evidence on whether antioxidant supplements are effective in the treatment of liver diseases is contradictory. Based on the conducted randomized clinical trials, convincing evidence that beta-carotene, vitamin A, vitamin C, and vitamin E or their combinations are beneficial for the treatment of alcoholic, autoimmune, hepatitis B or hepatitis C virus liver diseases or liver cirrhosis could not be found, contrary to in vitro results. MitQ, on the other hand, has not been related with a decrease in viral load but with antifibrotic features. There are some antioxidants such as PDTC that have not been tested in HCV infected patients. It has a potent negative effect in HCV expression in vitro, but the data in vivo has still not been assessed. SAM was used as adjuvant treatment along with IFN and ribavirin in non-responding HCV patients in 2011; however, since then, and besides the reports in vitro, there is only one clinical study and this compound was not reported to be assessed in combination with the new DAAs. Nevertheless, all those agents still have promising features to fight CHC and more importantly as adjuvant therapy for the remaining fibrosis and cirrhosis.
All these data together suggest that antioxidants can effectively improve the response of HCV-infected patients, decreasing oxidative and nitrosative stress in liver injury, then ultimately improving inflammation and fibrosis progression. Some investigators think that although it is worth testing these antioxidant drugs in future clinical trials, they emphasize the need for investigation on the effects of different antioxidants on HCV replication before their use as adjunctive therapy.
In fact, HCV proteins, for their own replication, manipulate several intracellular signaling pathways; and some are finely regulated by the cellular redox state. The role of HCV-induced oxidative stress, cellular protein interplay, and molecular mechanisms involved remain undefined. With the current findings about the dual function of the balance of oxidative stress induced by the virus and the host cell and the future concepts available, it may be possible to establish new and more effective therapeutic targets for the treatment of HCV.
7Summary and conclusionsHCV infection is associated with oxidative/nitrosative stress. During the infection many damaging processes are triggered; lipid peroxidation, oxidative DNA damage, protein oxidation, and also a decrease in the antioxidant capacity of liver cells have been reported. Generation of oxidative stress by HCV infection is estimated to originate from mitochondrial dysfunction of hepatocytes. Whether the antioxidants may help to alleviate HCV infection would depend on the oxidative status within the cell or tissue, and also on the dosage and the type of antioxidant used. Controversy in the use of antioxidants during antiviral therapy could be related to the fact that an excess of some antioxidants within the cell is reported to be harmful or beneficial depending on the stage of infection. There are a lot of clinical studies in patients with different scenarios. In this case, a systematic review could be useful to compare results with all DAA-treated patients.