Human immunodeficiency virus (HIV) and hepatitis C virus (HCV) co-infection generates sustained inflammation with increased reactive oxygen species production. The pathogenic impact of systemic oxidative stress is known to influence drug treatment and follow-up. The aim of this case–control study was to compare the redox status in HCV–HIV co-infected with respect to HIV-infected individuals and to explore the relation between redox and HIV follow-up variables.
Patients or materials and methodsBlood samples were drawn from 330 individuals divided into three groups: HIV, HCV–HIV and presumable healthy subjects. Redox, hematological, hemochemical, immunologic and virological indexes were determined.
ResultsBoth HIV groups had significant differences in global indexes of damage and antioxidant status (p<0.05) with respect to the supposedly healthy individual group. HCV–HIV group showed a significantly higher damage (total hydroperoxide and advanced oxidation protein products) compared to the control and HIV groups (p<0.05). The overall modification of the redox indexes showed that 72% of individuals with simultaneous detrimental differences were related to HCV–HIV condition.
ConclusionsThese results corroborate that oxidative stress occurs in the HIV condition and also during HCV–HIV co-infection, with different molecular changes of follow-up indexes. Redox indexes diagnosis should be considered in early diagnosis and treatment of HCV–HIV co-infection.
Hepatitis C virus (HCV) infection is an important health problem worldwide because a considerable number of infected patients progress to chronic liver diseases conducing to death [1]. Oxidative stress (OS) caused by chronic inflammation has emerged as a major player in liver diseases of different etiologies, including hepatitis C [2,3]. OS occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the antioxidant defense mechanisms affecting redox circuits and modulating transcription factors or not, influencing cellular survival, adaptation or death response [4]. OS perturbs lipid peroxidation, thereby contributing to the development of steatosis [5].
Liver diseases due to chronic HCV infection are a major risk of morbidity and mortality among HIV-infected patients [6]. Competing risks, such as exposure to liver carcinogens, hepatotoxic therapies, including antiretrovirals (ARV) and more advanced HIV immunosuppression may put HIV–HCV co-infected patients at higher risk of adverse outcomes compared to HIV or HCV-infected patients [7].
HIV infection modifies the natural course of HCV infection in several ways. HCV RNA concentrations are increased in HIV-infected patients [8]. Liver disease progresses more rapidly in HIV–HCV co-infected patients than in patients infected with HCV [9,10]. However, the mechanisms by which HIV infection increase the risk of liver disease are poorly understood and are probably multi-factorial. Both HIV and HCV-monoinfections have been recognized as conditions that elevate OS, which in turn contributes to liver fibrosis [11].
Infection with HIV is also characterized by depletion of antioxidants [12,13]. Also, OS induces the production of several inflammatory cytokines and promotes lymphocyte apoptosis and T cell dysfunction, therefore contributing to increased viral replication and progression of immunodeficiency in patients dually infected with HIV and HCV [14].
OS generated in hepatocytes is one of the important factors that stimulates the hepatic stellate cell proliferation and accumulation of collagen, initiating and facilitating the fibrogenic process [5]. Thus, in addition to the immunosuppression and antioxidant deficiencies caused by HIV and HCV and the elevated OS observed in HIV/HCV coinfection may contribute to a more rapid progression of liver fibrosis by stimulating HCV replication and increasing production of ROS in hepatocytes. OS, exacerbated by immunosuppression, concomitant exposure to viral infections, and depletion of antioxidants, causes hepatic cell damage [15].
The administration of antioxidants appears to be effective even in patients who have failed to respond to previous anti-HCV therapy. While the use of antioxidants may not eliminate the virus, it may reduce hepatic inflammation and fibrosis and slow disease progression. Optimal therapy with a spectrum of antioxidants may slow progression of liver disease, while interferon alpha and ribavirin treatment ameliorate HCV replication [16,17]. However, there is limited information in the literature about OS and antioxidant status in HIV-HCVcoinfection. Considering this background, the aim of the study was to assess the redox status in HCV-HIV co-infected Cuban patients and compare them to HIV positive patients and supposedly health volunteers. In addition follow-up clinical biomarkers were evaluated. All data were statistical analyzed and the relation between these variables was explored.
2Experimental procedure2.1Study design, standard protocol approvals and patient consentsA case–control study was designed enrolling non-HIV and HIV-AIDS positive individuals. All the patients were selected from the out-patients clinic at the Institute “Pedro Kourí” (IPK) Hospital for HIV. They all gave written informed consent to take part in the study after a verbal and written explanation of the methods and risks involved were given. The work was developed by a multidisciplinary group, including clinical experts in HIV/AIDS management. Procedures were previously reviewed and approved by the Institute “Pedro Kourí” Committee for Research on Human Subjects considering one year for inclusion. The study is in accordance with the principle of the Declaration of Helsinki concerning the Ethical Principles for Medical Research Involving Human Subjects. The protocol was also approved by Determinant program of Cuban Ministry of Health (Code 151068).
2.2PatientsNon-probabilistic convenient sampling was used in accordance with the assistance of patients to the specialized consult in a tertiary Hospital. The inclusion criteria for HCV-HIV were HIV-1 antibodies and a chronic HCV infection confirmed by Western blotting and plasma HCV-RNA assays. The exclusion criteria were as follows: (1) smokers, (2) history of drug use (including vitamins, iron, and antibiotics), (3) blood transfusion history and no recent bleeding, (4) not pregnant and lactating, (5) not menstruating during blood acquisition, (6) hepatitis B co-infection, and (7) patients with others hepatic pathologies. Three hundred thirty subjects ranging from 30 to 50 years of age were enrolled sequentially. The individuals were divided in three groups: 110 supposedly healthy individual, 110 HIV mono-infected patients and 110 HCV-HIV co-infected patients. All HIV subjects were assessed at the clinical visit. Anthropometry and laboratory tests were performed.
Patients underwent a screening, which included the evaluation of their medical records, diet, and supplemental intake history, anthropometrics data (weight, height), and review of clinical lab results. Demographic and age data were processed by SIDATRAT (software package 2008). Subjects were classified according to gender, age, ethnicity, viral load and CD4+ T lymphocyte subset count.
2.3TreatmentsThe ARV regimen consisted of a triple-drug combination allocated free, including two nucleoside reverse transcriptase inhibitors (IRT) and one protease inhibitors (PI), according to current guidelines were prescribed. The ARV drugs used in the different combinations were prescribed daily at the following doses: RTI zidovudine 600mg, lamivudine 300mg, didanosine 400mg, PI indinavir 2400mg, ritonavir 1200mg, saquinavir 2400mg, nelfinavir 2250mg. Patients also were oriented to use anti-hepatitis C drugs (pegylated interferon alfa plus ribavirin) according to the ARV regimen that was established.
2.4Flow cytometry analysisA study of T lymphocytes subsets CD3+/CD4+ in total blood was carried out. For each T lymphocyte, subsets TM, CD3, and CD4 were used. These analyses were performed on a Cyflow Space Cytometer (PARTEC GmbH, Münster, Alemania) by FloMax 2014, Versión 2.9 program.
2.5HIV-RNA plasma viremia (viral load)Viral load was determined with the Biomerieux polymerase chain reaction (PCR-NASBA) ultrasensitive assay with the lower limit of quantification of 50IU.
2.6Oxidative stress parametersVenous blood samples were taken from each fasted patient between 8.00 and 10.00 in the morning after informed consent was signed. Blood samples were collected by venipuncture into heparin-treated tubes and centrifuged to obtain serum.
For the SOD and CAT assays, hemoglobin was extracted from the hemolysate. For the rest of the analyse, 3mL of serum were employed. Serum samples were frozen at −70°C and protected from light exposure until analyses were carried out.
All redox parameters were determined by spectrophotometric methods using an Ultrospect Plus Spectro-photometer from Pharmacia LKB.
2.7Glutathione concentrationSerum reduced glutathione (GSH) was determined spectrophotometrically after the reaction with 5,5′-Dithiobis (2-nitrobenzoic acid) [18]. All of the non-protein sulfhydryl groups are in the form of reduced glutathione. DTNB is a disulfide chromogen that is readily reduced by sulfhydryl compounds to an intensely yellow compound. The observance of the reduced chromogen is measured at 412nm and is directly proportional to the GSH concentration. GSH (Sigma, St. Louis, MO, USA) was used to generate standard curves.
2.8Malondialdehyde concentrationMalondialdehyde (MDA) concentrations were analyzed with the LPO-586 kit obtained from Calbiochem (La Jolla, CA, USA). In this assay, stable chromophore production after 40min of incubation at 45°C is measured at a wavelength of 586nm by Pharmacia Spectrophotometer. To ensure that no lipid oxidation occurs during the assay, BHT [0.01% (v/v) of a 2% stock solution in ethanol] and EDTA (1mM final concentration) were added to the sample prior to assay develop. Freshly prepared solutions of malondialdehyde bis [dimethyl acetal] (Sigma, St. Louis, MO, USA) assayed under identical conditions were used as reference standards. Concentrations of MDA in serum samples were calculated using the corresponding standard curve and values were expressed as nmolg−1 Hb [19].
2.9Peroxidation potential (PP)For the determination of the susceptibility to lipid peroxidation, serum samples were incubated with a solution of cupric sulfate (final concentration of 2mM) at 37°C for 24h. Then, malondialdehyde (MDA) concentration was determined at 587nm using the method described previously. The PP was calculated by subtracting the MDA concentration at time 0 from the one obtained at 24h [20,21].
2.10Total hydroperoxide (HPO)HPO was measured by Bioxytech H2O2-560 kitCat.21024 (Oxis International Inc., Portland, USA). The assay is based on the oxidation of ferrous ions to ferric ions by hydroperoxides under acidic conditions. In this assay peroxide first reacts with sorbitol (which provides sensitivity enhancement), converting it to a peroxyl radical, which in turn initiates Fe2+ oxidation to Fe3+. Ferric ions bind with the indicator dye xylenol orange (3,3′-bis(N,N-di(carboxymethyl)-aminomethyl)-o-cresolsulfone-phatein, sodium salt) to form a stable colored complex which can be measured at 560nm [22].
2.11Superoxide dismutase (SOD)SOD activity was measured by the method suggested by Marklund. This method utilizes the inhibition of auto-oxidation of pyrogallol by SOD [23]. In this method the auto-oxidation of pyrogallol was investigated in the presence of EDTA at pH 7.9 the reaction is inhibited to 99% by superoxide dismutase. One unit of SOD activity is defined as the amount of the enzyme required to inhibit the rate of pyrogallol auto-oxidation by 50%.
2.12Catalase (CAT)CAT activity was measured according to the method of Clairbone [24]. The initial absorbance decrease rate at 240nm was monitored at 30°C. One unit of this enzyme is defined as the activity to consume 1μmol of hydrogen peroxide per minute. Using a molar extinction coefficient of 43.6M−1cm−1, the rate of the first 30s was used to calculate the activity. Catalase activity was expressed as Umg−1 Hb.
2.13Advanced oxidation protein products (AOPP)Serum AOPP was measured according to the methods of Witko-Sarsat et al. [25]. Chloramine-T solution that absorbs at 340nm in the presence of potassium iodide was used to generate calibration curves. The absorbance of the reaction was read at 340nm against a blank containing phosphate-buffered saline. The values were expressed in chloramine T equivalents and corrected by serum albumin concentrations.
2.14Biochemical indexesBlood parameters such as hematocrit, hemoglobin, and erythrocyte sedimentation rate (ESR) were screened by Hematological counter MICROS 60. Others such as triglycerides, creatinine, cholesterol, glucose, uric acid, urea, albumin, alkaline phosphatase (ALP), Gamma-Glutamyl transferase (GGT), aspartate (ASAT) and alanine aminotransferase (ALAT) activity were performed by standard procedures in HITACHI analyzer 912, all in a specialized laboratory of IPK Hospital.
2.15Statistical analysesFor descriptive statistics of continuous variables, means and standard deviations were calculated, whereas categorical variables were expressed as proportions. The normality of variables was evaluated by the Kolmogorov–Smirnov test. Comparisons between groups were assessed using Kruskal–Wallis test followed by a post hoc Dunn's Multiple Comparison Test. Pearson correlation coefficient was used to determine the relationship among the different parameters combining redox and follow-up indexes. Statistical significance was defined as p<0.05. The SPSS software package version 20 and GraphPad Prism were used for all statistical analyses.
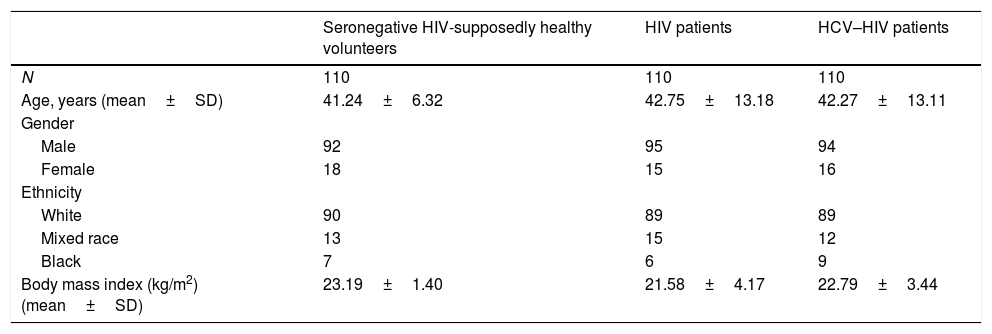
3ResultsThe demographic characteristics of the 330 subjects according to each study group are showed in Table 1. There were no statistical significant differences between the groups according to demographics such as age, gender, body mass index (BMI), ethnicity and number of patients (p>0.05). A major percentage of patients in the three groups were older than 35 years, skin color white and were on normal-weight.
Age, gender, ethnicity and body mass index of participants attended in IPK.
| Seronegative HIV-supposedly healthy volunteers | HIV patients | HCV–HIV patients | |
|---|---|---|---|
| N | 110 | 110 | 110 |
| Age, years (mean±SD) | 41.24±6.32 | 42.75±13.18 | 42.27±13.11 |
| Gender | |||
| Male | 92 | 95 | 94 |
| Female | 18 | 15 | 16 |
| Ethnicity | |||
| White | 90 | 89 | 89 |
| Mixed race | 13 | 15 | 12 |
| Black | 7 | 6 | 9 |
| Body mass index (kg/m2) (mean±SD) | 23.19±1.40 | 21.58±4.17 | 22.79±3.44 |
SD: standard deviation.
Note: No significant differences were detected in comparison between variables for the different groups (p>0.05).
At the time of the study, 41/110 (37%) co-infected patients had more than 10 years of HIV diagnosis, 44/110 (40%) between 5 and 10 years of HIV diagnosis and 25/110 (23%) had less than 5 years of HIV diagnosis. The co-infected patients were diagnosed with HCV infection one year before the study. All subjects were seronegative for other hepatitis viruses markers (including hepatitis A, C, D and E virus) and other co-infections. Also, the subjects did not have other comorbidities/complications (Diabetes mellitus, Hypertension, Rheumatoid arthritis, Asthma, Chronic obstructive pulmonary disease, etc.). It was not informed concomitant drugs (including alcohol) or antioxidant supplementation used at the moment of the study. All patients reported taking medications for the co-infection according to the doctor's prescribed indication.
The mean value of all biochemical, redox indexes and HIV progression markers evaluated for control and HIV groups are shown in Tables 2 and 3.
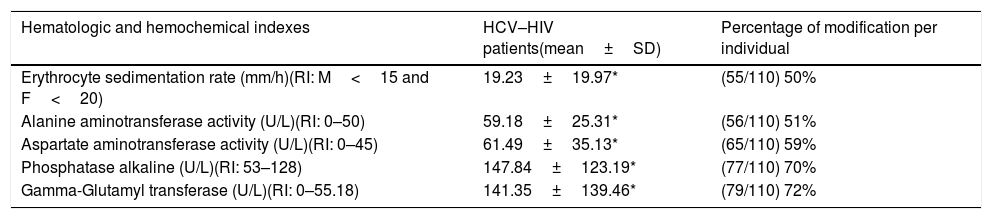
Hematologic and hemochemical indexes data of HCV–HIV patients group.
| Hematologic and hemochemical indexes | HCV–HIV patients(mean±SD) | Percentage of modification per individual |
|---|---|---|
| Erythrocyte sedimentation rate (mm/h)(RI: M<15 and F<20) | 19.23±19.97* | (55/110) 50% |
| Alanine aminotransferase activity (U/L)(RI: 0–50) | 59.18±25.31* | (56/110) 51% |
| Aspartate aminotransferase activity (U/L)(RI: 0–45) | 61.49±35.13* | (65/110) 59% |
| Phosphatase alkaline (U/L)(RI: 53–128) | 147.84±123.19* | (77/110) 70% |
| Gamma-Glutamyl transferase (U/L)(RI: 0–55.18) | 141.35±139.46* | (79/110) 72% |
SD: standard deviation, RI: reference interval, M: masculine, F: feminine.
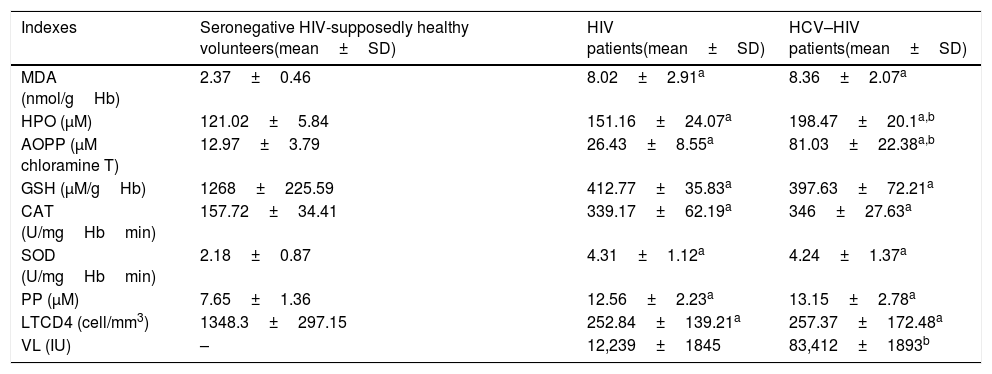
Redox indexes and HIV progression markers data of the different studied groups.
| Indexes | Seronegative HIV-supposedly healthy volunteers(mean±SD) | HIV patients(mean±SD) | HCV–HIV patients(mean±SD) |
|---|---|---|---|
| MDA (nmol/gHb) | 2.37±0.46 | 8.02±2.91a | 8.36±2.07a |
| HPO (μM) | 121.02±5.84 | 151.16±24.07a | 198.47±20.1a,b |
| AOPP (μM chloramine T) | 12.97±3.79 | 26.43±8.55a | 81.03±22.38a,b |
| GSH (μM/gHb) | 1268±225.59 | 412.77±35.83a | 397.63±72.21a |
| CAT (U/mgHbmin) | 157.72±34.41 | 339.17±62.19a | 346±27.63a |
| SOD (U/mgHbmin) | 2.18±0.87 | 4.31±1.12a | 4.24±1.37a |
| PP (μM) | 7.65±1.36 | 12.56±2.23a | 13.15±2.78a |
| LTCD4 (cell/mm3) | 1348.3±297.15 | 252.84±139.21a | 257.37±172.48a |
| VL (IU) | – | 12,239±1845 | 83,412±1893b |
SD: standard deviation, PP: peroxidation potential, CAT: catalase, SOD: superoxide dismutase, HPO: hydroperoxide, MDA: malondialdehyde, GSH: glutathione, AOPP: advanced oxidation protein’ product, LTCD4: T CD4+ lymphocyte absolute count, VL: viral load
Different letter represents significant differences (p<0.05):
Hemoglobin media values and, hematocrit, platelet, percentage of leucocytes, neutrophils, lymphocytes and monocytes from HCV-HIV group remained within the physiological reference intervals. The same trend was found in creatinine, albumin, uric acid, urea, glucose, cholesterol and triglycerides indexes values.
The ESR’ mean values of HCV-HIV patients was out of the interval considered as physiological-reference, above the maximum value of the interval. The same characteristic was found with the mean value of AST, ALT, GGT and alkaline phosphatase in the HCV–HIV group (Table 2).
MDA is the stable end product of the oxidative degradation of polyunsaturated fatty acids. This makes MDA a marker of lipid peroxidation. AOPP is generated by chloramine oxidants from the active neutrophil myeloperoxidase enzyme (predominantly hypochlorase, acid and chloramines) activated during inflammation. MDA, AOPP and HPO serum concentrations were significantly higher (p<0.05) in HIV and HCV-HIV groups with respect to supposedly healthy individual group. For AOPP and HPO significant differences were found between HIV and HCV-HIV groups.
GSH redox cycle acts as a direct endogenous scavenger of hydroxyl radicals, involved in the detoxification and metabolism of a number of substances in the liver. GSH reduction modify related functions such as reducing capacity, protein biosynthesis, immune function, accumulations of lipid peroxidation products and detoxification capacity leading to the accumulation of hepatotoxic metabolites and to liver damage. Serum GSH levels were significantly lower (p<0.05) in HIV and HCV-HIV individuals compared to the supposedly healthy individuals` value without differences between HIV groups (p>0.05).
ROS and their metabolites are processed from the cell by enzymatic systems including SOD and CAT. The activity of the erythrocyte antioxidant enzyme SOD and CAT was significantly higher in HIV and HCV–HIV groups with respect to the supposedly healthy individual value (p<0.05) without differences between HIV groups (p>0.05) (Table 3).
PP is a global index that estimates serum antioxidant capacity and its susceptibility to lipid peroxidation. HIV and HCV-HIV patients had a significantly higher PP value, suggesting reduced lipid-serum antioxidant capacity with respect to supposedly healthy individuals (p<0.05). The PP value in the HCV–HIV group was not significant different with respect to the HIV group (p>0.05) (Table 3).
The progression markers of HIV/AIDS are lymphocyte T-CD4 count and viral load. The CD4 count was significantly lower (p<0.05) in HIV groups with respect to supposedly healthy individuals. No significant differences (p>0.05) were found between HIV groups with respect to CD4 count. HIV viral load was significantly higher (p<0.05) in HCV-HIV patients compare to HIV patients.
Correlation analysis between redox and other variables was done without any significant relation among studied indexes (p>0.05).
Simultaneous analyses of redox status identified 79 patients with HPO and AOPP alterations, which represents 72% of the HCV–HIV group.
4DiscussionViral infections have been reported to cause diverse pathophysiological outcomes that lead to hematologic and biochemical alterations. The ESR values found in the present study in the co-infected patients were high with respect to the reference interval. ESR is often raised in infections and inflammatory conditions like HIV and HCV. The increase in ESR in these conditions is attributed to increased production of acute phase proteins and the release of proteins by the causative organism into the circulation. Hence, ESR can be used as sensitive index of plasma protein changes due to inflammation or tissue damage in HIV and HCV infections. Otherwise the obtained values do not reach 50, which represent pathologic value. So that means that the results do not have clinical resound according to the unspecific test [26].
Liver enzymes alterations are associated to liver damage. In this study, ALT and AST levels of coinfected patients were outside the normal reference interval. HCV infection is one of the leading causes of liver cirrhosis and hepatocellular carcinoma, and it often requires liver transplantation [27]. In chronic hepatocellular injury including viral liver disease, ALT is more commonly elevated than AST; however, as fibrosis progresses, ALT activities typically decline, and the ratio of AST to ALT gradually increases, so that by the time cirrhosis is present, AST is often higher than ALT due to its reduction [28]. The half-life of mitochondrial AST released into circulation by progressive damage to mitochondria is the longest. Also ARV therapy often causes liver and mitochondrial toxicity even though the level of toxicity may be different for each ARV [29].
Increase of serum GGT levels in chronic HCV infection and HIV infection have been reported [30]. GGT is a cell-surface protein contributing to the extracellular catabolism of GSH. The enzyme is produced in many tissues, but most GGT in serum is derived from the liver [31]. The mechanisms whereby elevated GGT is related to hepatic steatosis have not been determined, but a higher GGT production could be secondary to a low-grade hepatic inflammation induced by hepatic steatosis [32]. The concept of serum GGT as primarily either an antioxidant or a pro-oxidant marker presents a challenge in understanding the GGT and disease relationships. GGT enhances the availability of cysteine to promote intracellular GSH resynthesis, thereby counteracting OS. GGT may also be proinflammatory, because it mediates recycling of the glutathione-containing inflammatory mediator leukotriene C4 into leukotriene D4 [33]. Additionally, GGT-activity can give rise to redox reactions, due to the interplay of reactive thiol metabolites of GSH (cysteinyl-glycine in the first place) with transition metal ions. When GGT levels are elevated, damage to red blood cell membranes can occur causing the release of these potentially toxic transition metals, which can further result in chain, prooxidant reactions. OS contribution evaluated as GGT increased activity could be related to modulation of transcription factors which in term influence on viral gene expression [30].
ALP has been used as general indicator of the maintenance and severity of tissue damage. ALP is an enzyme that is naturally found in biological tissues and fluids. The elevated serum levels of this enzyme are suggestive of increased level of inflammatory mediators resulting in increased oxidative stress. ALP activity modification could be related to redox altered state with consequent activation or deactivation of different biomolecules by phosphorylation [33].
Persons co-infected with HIV and HCV have an increased risk of AIDS and AIDS-related death compared to HIV mono-infected individuals [34]. Results of studies looking at the effect of HCV co-infection on HIV VL are diverse. Some observational studies reported higher HIV VL in co-infected persons [35] as observed in the present study, whereas others found no effect on HIV VL [36].
OS has been found to occur in various viral infections that may enhance viral replication. Many host mechanisms have been shown or are suspected to contribute to the pathogenesis of viral infections, such as ROS and cytokines [4,37]. OS could be related to both, viral replication and also implicated on cell apoptosis in HIV infection. ROS could modulate and activate nuclear transcription factors, which ultimately lead to HIV gene expressions, and concomitant to HIV-related opportunistic infections or malignancies [38,39].
It has been previously shown that the HIV-infected populations have significantly lower antioxidant concentrations than non-HIV individuals [12]. Similar alteration occurred in patient with ARV treatment and diverse clinical conditions [40]. Altered redox metabolism may contribute to amplify cellular damage resulting from the generation of oxidized products, some of which are chemically reactive that leads to covalently modification of critical macromolecules; thus altering the communication between cells [37].
OS generated in hepatocytes is one of the important factors that stimulates the hepatic stellate cell proliferation and accumulation of collagen, initiating and facilitating the fibrogenic process [41]. Thus, in addition to the immunosuppression and antioxidant deficiencies caused by HIV and HCV, the elevated OS observed in HIV/HCV coinfection may contribute to a more rapid progression of liver fibrosis by stimulating HCV replication and increasing production of ROS in hepatocytes [15].
HIV-HCVcoinfection is a condition characterized by immunosuppression due to HIV infection and concomitant exposure to HCV, is also accompanied by significantly lower antioxidant plasma levels that are significantly lower than those found either in HIV- or HCV-monoinfections [11]. Abnormally high levels of pro-oxidant species as a consequence of chronic immune system activation by HIV infections could lead to a decline of antioxidants defense molecules and cumulative damage of cellular components generating augmented lipid peroxidation products and oxidized proteins [38,42]. Almost redox implicated enzymes and molecules are physiologically endogenous generated and are involved in detoxification and general metabolism [4]. Coinfection with HIV is associated with more rapid evolution of hepatitis C virus (HCV)–associated liver disease despite ARV therapy, possibly due to redox immune dysregulation [15].
In the present study, the reliable redox markers altered in HIV-HCV coinfection with respect to HIV condition were HPO and AOPP. Peroxide and superoxide have the ability to generate others reactive species by interacting with free transition metals producing oxidative tension in the environment [4]. Oxidative molecules modifications contribute to lipid accumulation in the liver (steatosis), where it plays a major role in terms of necroinflammation and hepatic cell necrosis [5]. AOPP shows the oxidation-mediated protein damage and plays a role as an inflammatory mediator. Oxidative modified proteins, AOPP, are long-term indicators of OS, which possess pro-oxidant activity inducing lipid peroxidation and increasing pro-inflammatory and adhesive molecules as well as cytokines [43,44]. In addition to increased formation, decreased removal/detoxification of AOPP may contribute to OS. There is increasing evidence that the liver plays important roles in the elimination of AOPP [45].
The clinical outcome of HIV infection contribute to exacerbate oxidative metabolism adding risk of molecular damage and also improving diverse virus replication or/and accruing poly pathology condition [46]. These findings could be explained in part by several mechanisms such as low intake of antioxidant or their precursors and mal absorption. Also ARV treatment has additional impact on pre-existing OS related to HIV condition [41].
Direct relation explored by correlation between the biochemical, redox and other indexes was not found. It could mean that the chemical relation among the studied metabolites could be influenced by reactions and products in the cell and organism with diverse impact on fluids. This requires a deep evaluation or analyses by other correlation methods in order to establish the nexus. No previous articles reporting this relation were found.
Considering previous elements, some authors have been suggested to evaluate through redox indexes the use of antioxidants as agents that might reduce the incidence of OS as consequences of infection or treatment [17,47].
Taking into account that causes of polypathologies are complex and multifaceted, the recognition of molecular and cellular concert involved are crucial. A causal relationship between some elements such as oxidative macromolecules modifications, immunological status and viral load has emerged but the mechanism by which these molecular and biochemicals events occur remain to be established. The OS evaluations will therefore become potential useful to characterize infection, antiviral combinations effects, as well as alternative therapies for counteracts oxidative damage [37].
Therefore, more deep and detailed research must be conducted to better understand the redox molecular mechanism and specific pathways involved in HIV/HVC co-infection. The growing interest shown by redox metabolism researchers should provide answers for many of these unsolved questions.
The present study contributes to evidences that OS evaluated in blood by several parameters could increase during HCV-HIV coinfection. It is suggested that cumulative damage reported had direct impact on functional efficiency of cell and tissues. Metabolic abnormalities as altered redox indexes remain an important part of complications in HIV infection and comorbidities.
Altered redox status on HCV-HIV coinfection could play a causal role in the progression of both pathologies by promoting damage to cell structure and functions and also redox driven process are stimulated modulating different stages of inflammation.
Redox indexes determination should be considering in early diagnosis and treatment of HCV–HIV co-infection, which would be worthwhile to conduct a more comprehensive study and manage of patients.
The recent development of antiviral agents that act directly on viral replication (direct-acting antivirals [DAA]) are grouped in four classes: nonstructural proteins 3/4A (NS3/4A) protease inhibitors (PIs): Glecaprevir, Paritaprevir, Voxilaprevir NS5B nucleoside polymerase inhibitors (NPIs): sofosbuvir, NS5B non-nucleoside polymerase inhibitors (NNPIs): Daclatasvir, Elbasvir, Ledipasvir, Ombitasvir, Velpatasvir, Pibrentasvir and NS5A inhibitors: Dasabuvir. Interesting there are some publications that suggest an increasing risk for hepatocellular carcinoma HCC in patients treated with DAA [48]. But other publications did not support this finding [49]. Increased risk of HCC and oxidative stress remains to be studied and continued long-term observational studies will be needed.AbbreviationsHIV human immunodeficiency virus hepatitis C virus oxidative stress reactive oxygen species antiretrovirals nucleoside reverse transcriptase inhibitors protease inhibitors glutathione malondialdehyde peroxidation potential total hydroperoxide superoxidedismutase catalase advanced oxidation protein products erythrocyte sedimentation rate alkaline phosphatase Gamma-Glutamyl transferase aspartate aminotransferase alanine aminotransferase
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors gratefully thank to healthy volunteers and persons with HIV infection who enthusiastically participate in the study.
This work was partially supported by the Ministry of Public Health, Republic of Cuba (Project No. 151068).