In newborns, dramatic changes occur in the blood and bone marrow during the first hours; there are rapid fluctuations in the quantities of leukocytes populations. In this work, we investigated leukocytes subsets counts in two types of blood samples (cord blood and capillary blood) extracted from healthy newborns.
MethodsBlood samples from Mexican neonates were collected by Instituto Nacional de Pediatría with written informed consent. For all samples we determined leukocytes populations; neutrophils, monocytes, total lymphocytes, and populations: T CD3+ cells, TCD4+ cells, T CD8+ cells, B CD19+ cells and NK CD16+56 cells by flow cytometry. We used the Mann–Whitney U test to compare leukocytes of cord and capillary blood; also to analyze the differences between gender and we obtained reference values of the cord and capillary blood in neonates.
ResultsWe observed higher absolute counts and frequencies of total lymphocyte in capillary blood compared with cord blood. In absolute numbers, the capillary blood showed significant differences in neutrophils, monocytes, lymphocytes, T CD3+ cells, T CD4+ cells, T CD8+ cells, B CD19+ cells, and NK cells; no significant differences were observed between genders.
DiscussionOur data contribute to newborn Mexican reference values for all these populations of leukocytes. We found that the dispersal range differs between the two types of blood, suggesting a different fate in the immune response. Immunophenotyping of the blood cell population to identify these cells is an essential tool in the diagnosis and follow-up of neonates with immunodeficiencies and other immune disorders.
The development of the human immune system begins early in the fetal period, but it is not completed at birth; post-natal maturation proceeds for months to years, even into adulthood. Neonates have little immunological memory and a developing immune system, which increases their vulnerability to infectious agents.1
Umbilical cord blood is the blood left over in the placenta and the umbilical cord after the birth of the baby. The cord blood is composed of all the elements found in whole blood; it contains red blood cells, white blood cells, plasma, platelets and is also rich in hematopoietic stem cells.2
The blood vessels are the part of the circulatory system, and microcirculation, that transports blood throughout the human body. Capillary blood sampling via a heel lance is the most common procedure performed in hospitalized neonates, capillary blood obtained by skin puncture is from a dynamic tissue fluidic system that contains circulating capillary blood, interstitial fluid and lymphatic fluid.3
Blood is a complex tissue consisting of a very specialized network of circulating immune cells. The compartments of first-line innate immune response and adaptative response are essential to protect from a wide variety of pathogens. Dysregulated immune response can lead to an increased susceptibility to infection, primary immunodeficiency, autoimmune diseases or cancer.4 Both genetic and non-genetic factors may contribute to variations in the number of human immune cells. It is also possible that different linages have different fluctuations in accordance with varied environmental and nutritional states.5 It is therefore important to have reference values for varied ethnic-geographic populations.
Immunophenotyping of blood lymphocyte subpopulations is an essential tool in the diagnosis and follow-up of children with immunodeficiencies, hematological diseases, and other disorders. Studies of leukocytes from preterm newborns have been limited, and there is little information available concerning the development and function of the innate immune system depending on the gestational age of newborns. Preterm infants, due to the immaturity of the immune system, are at a heightened risk of acquisition of recurrent bacterial infection during their first weeks of life, due to frequent exposure to the micro-organism, frequently, invasive procedures such as catheterization and intravascular or assisted ventilation need to be done in order to treat these infections.6,7
In this work, we investigated the counts of leukocytes subsets in two types of blood samples (cord blood and capillary blood) extracted from healthy newborns to establish reference ranges that may be helpful to the pediatricians to determine possible alterations in these cell subsets. To this, we have performed flow cytometric phenotyping to determine values reference and studied whether there are differences between genders.
Our data further show that the extent of dispersal between individuals varies widely for different blood leukocyte subsets.
MethodsStudy subjectsCord Blood was collected from full-term neonates from healthy mothers delivered by normal vaginal delivery. Neonates who had any maternal antenatal risk factors were excluded. Immediately after delivery, 1ml of cord blood was collected from the umbilical cord vein by venipuncture at the cut end of the cord attached to the placenta. Additionally, 1ml of capillary blood was collected by heel-prick. The blood samples were collected in tubes with anti-coagulated with ethylenediamine tetraacetic acid (EDTA).
All samples were processed and stained within three hours of the delivery and analyzed within 12h after staining. Total leukocytes counts were estimated using standard clinical hematology laboratory procedures.
This study was reviewed and approved by the institutional human ethics committees of the participating institutions: the ethics committee of Instituto Nacional de Pediatria no. 061/2010. Informed written consent was taken from mothers during the antenatal period itself. Eligible and willing participants (mother of neonates) were given detailed written and verbal information about the study in vernacular language and informed written consent was taken in the presence of a witness.
Lymphocyte population and subpopulation phenotypingTo assess the different leukocyte populations and subpopulations, we performed flow cytometric analysis of blood samples. Lymphocyte populations were enumerated in whole-blood samples and stained with the following mixtures of monoclonal antibodies (mAbs): anti-CD45-FITC/anti-CD14-PE, anti-CD3-FITC/anti-CD19-PE/anti-CD45-PerCP, anti-CD4-FITC/anti-CD8-PE/anti-CD3-PerCP, anti-CD3-FITC/anti-CD19-PE/anti-CD45-PerCP, and anti-CD3-FITC/anti-CD16+56PE/anti-CD45-PerCP. Also, ©1-FITC/©2-PE/anti-CD45-PerCP were used as isotype controls. All antibodies were purchased from BD Biosciences, San Diego, CA, USA. Samples were incubated for 20min at room temperature in the dark. After incubation, erythrocytes were lysed by suspending the cells in 500μl FACS lysing solution (BD Biosciences) for 10min. Cells were then washed with PBA (1% bovine serum albumin in PBS) and fixed using 1% formalin in PBS. A FACS Aria1 Flow cytometry (Becton Dickinson) was used to acquire 10,000 events and FlowJo v.10 (Tree Star) was used for the analysis of each leukocytes subpopulation evaluated.
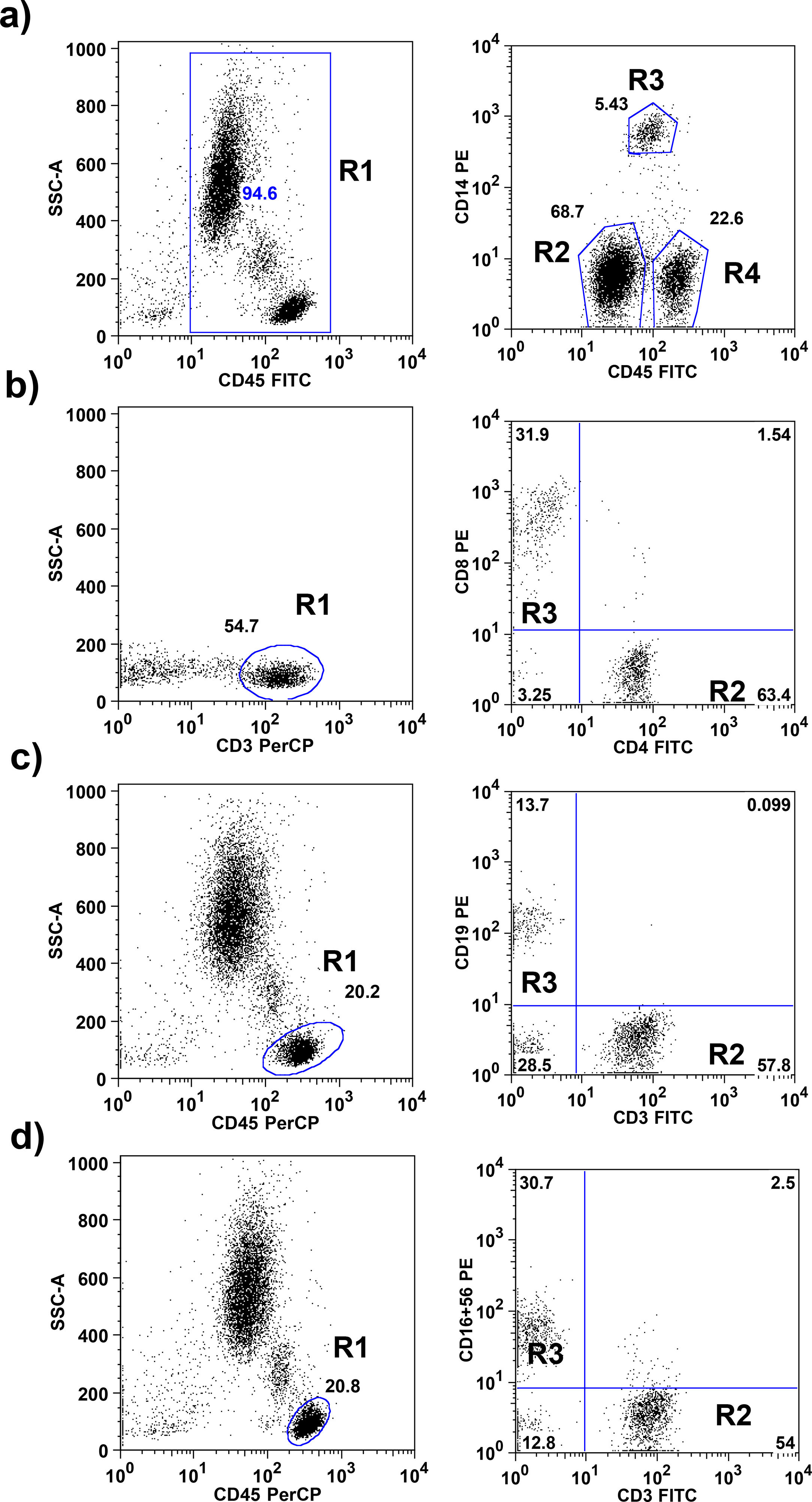
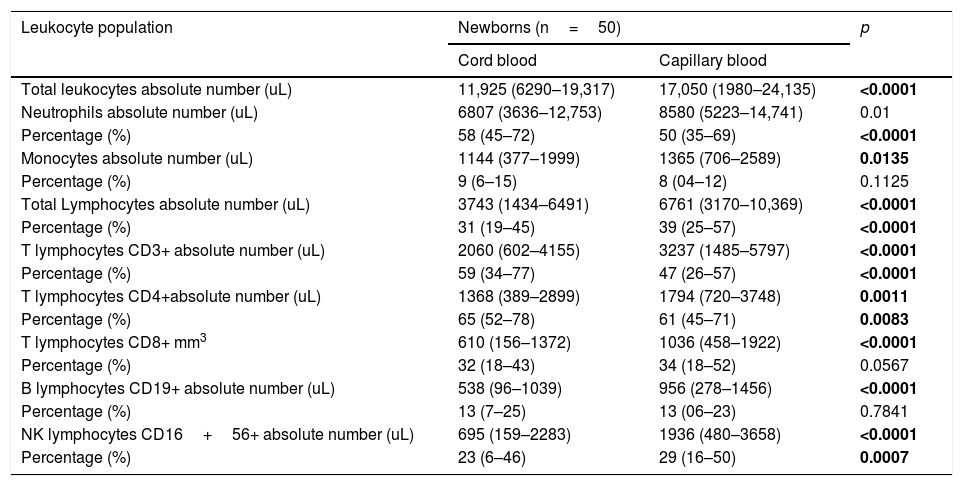
The gating strategies are explained in Fig. 1. Absolute numbers of cells were calculated by multiplying the relative proportion of particular cells populations with the absolute number of leukocytes obtained by an automatically analyzed differential white blood count obtained on the same days.
Flow cytometric gating strategies used for leukocyte subset identification. a) On CD45+ total leukocytes (R1) CD14 was used to distinguish neutrophils (R2), monocytes (R3) and lymphocytes (R4). b) The CD45+, CD3+ lymphocytes identified as T lymphocytes (R1; these cells subdivided in CD4+ T lymphocytes R2) and CD8+ T lymphocytes (R3). c) CD3+ T lymphocytes (R2) and CD19+ B lymphocytes (R3) were gated within the lymphocytes side-scatter and CD45+ region (R1). d) CD3+ T lymphocytes (R2) and CD16+ 56+ NK lymphocytes (R3) were gated within the lymphocytes side-scatter and CD45+ region (R1).
Statistical analyses were performed using GraphPad Prism, version 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Two-group comparisons were performed using the Mann–Whitney test. Stratified data were expressed as the mean value. The results were expressed as the median, and p-values <0.05 were considered to be significant. The normal reference values of cord and capillary blood were defined as 5–95 percentiles, and the median rank was shown at the same time.
ResultsCharacteristic of a healthy newbornA total of 50 pregnant women visiting the clinic met the inclusion criteria at the time of delivery and gave consent for the collection of the cord blood and capillary blood from their newborn. The study is part of a protocol approved by the local ethics committee of number 061/2010, which were developed according to the rules declared under the Declaration of Helsinki. The demographic data of the 50 neonates includes: gender 21 female and 28 male; average gestation age at birth in weeks was 39.7 with standard deviation SD (±0.87); average birth weight in Kg was 3211grams SD (±328.2) and average length in cm was 50.8 SD (±2.2).
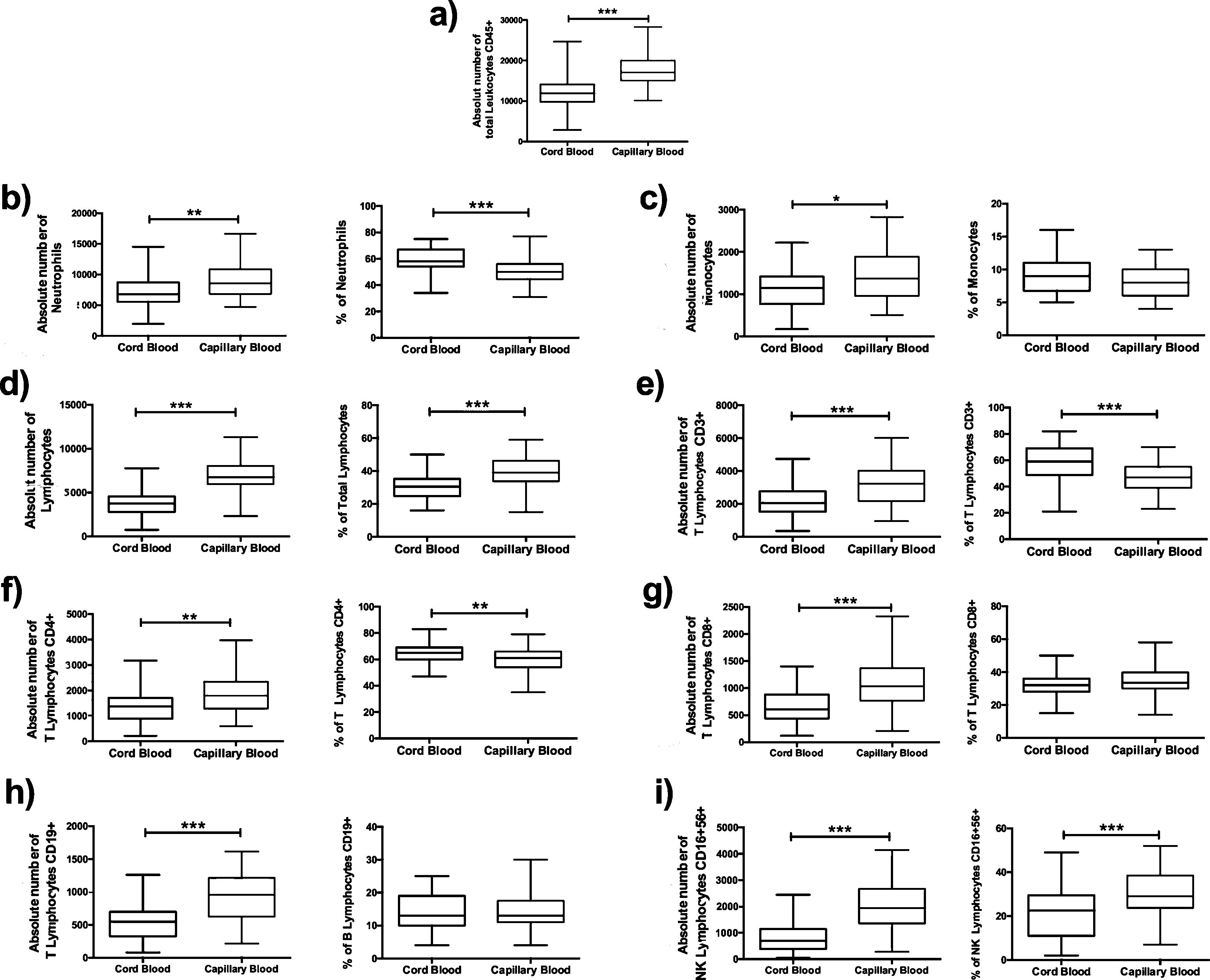
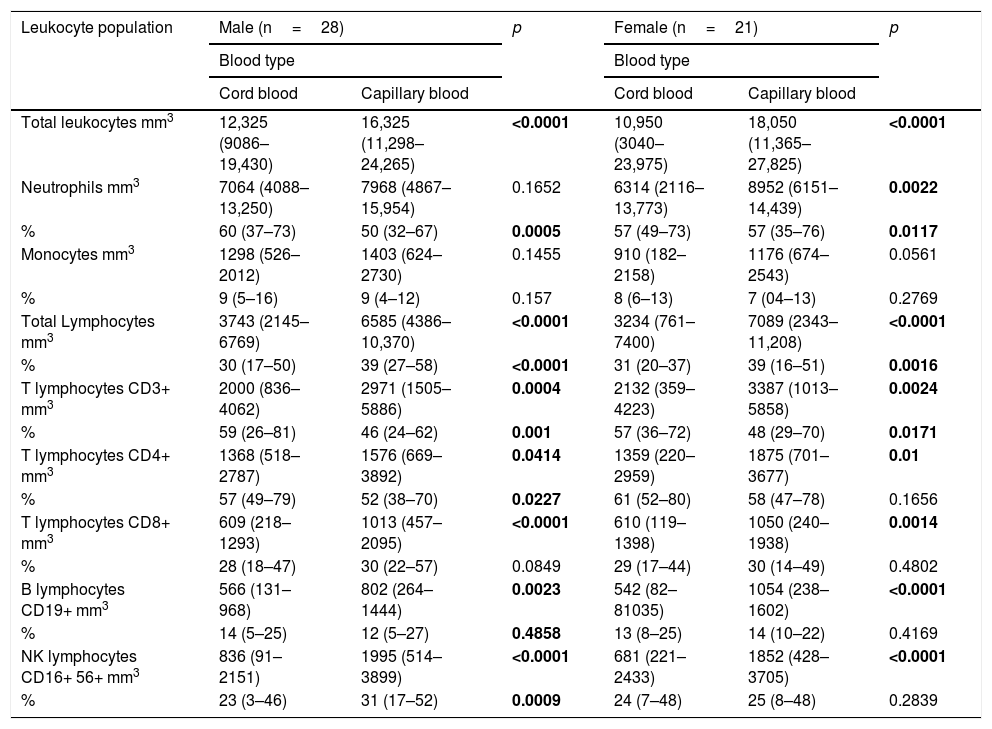
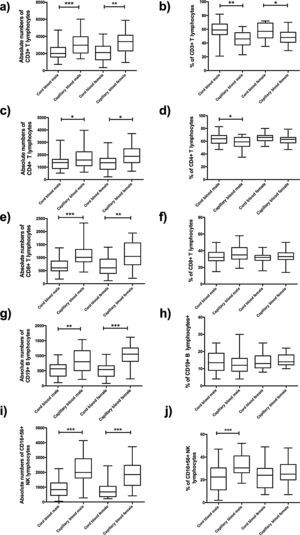
Comparison of cord blood and capillary blood in neonatal leukocyte subsets in absolute numbers and percentagesThe total leukocyte concentration in absolute numbers was higher in capillary blood (n=50) than in cord blood (n=50) (p=<0.0001), (Fig. 2a). Capillary blood showed higher absolute numbers in neutrophils (p= 0.01), monocytes (p= 0.0135), total lymphocytes (p=0.0001), T lymphocyte CD3+ (p=0.0001), T lymphocyte CD4+ (p=0.0001), T lymphocyte CD8+ (p=0.0001), B lymphocyte (p=0.0001) and NK lymphocyte CD16+56+ (p=0.0001) in comparison to cord blood (Fig. 2b–i). In the analysis of the subpopulations in cord blood and capillary blood, in percentages, significant differences were observed in neutrophils (p=<0.0001) (Fig. 2b), T lymphocyte CD3+ (p=0.0001) and T lymphocyte CD4+ (p=0.0083) (Fig. 2e and f, respectively), where values were higher for cord blood. Conversely, the higher percentage of capillary blood was for total lymphocyte (p=<0.0001) and NK lymphocyte CD16+56+ (p=0.0007) (Fig. 2d and i, respectively); while proportions of monocytes T lymphocytes CD8 and B lymphocyte CD19+ were similar between both types of samples (Fig. 2c, g, and h, respectively). In the T cell lineage, capillary blood and cord blood showed a similar average in CD4/CD8 ratio (2.2, 1.8 respectively; data not showed). A comparison between cord blood and capillary blood of leukocytes and their subsets, in absolute numbers or percentages and significant differences are given in Table 1.
Flow cytometric analysis of neutrophils, monocytes, total lymphocytes, T lymphocytes, B lymphocytes and NK lymphocytes in absolute numbers (cell/mm3) and percentages (%) from the cord and capillary blood of neonates (n=50). a) Total leukocytes; b) neutrophils, c) monocytes; d) total lymphocytes; e) CD3+ T lymphocytes; f) CD4+ T lymphocytes; g) CD8+ T lymphocytes, h) CD19+ B lymphocytes and i) CD16+ 56+NK lymphocytes. Differences between cord and capillary were compared using the Mann–Whitney U test. (*) significant, p=<0.05; (**) very significant, p=<0.001; and (***) highly significant, p=<0.0001.
Absolute numbers (μl of blood) and percentages (%) of total leukocytes and subpopulations of lymphocytes in cord blood and capillary blood of healthy newborns (n=50).
| Leukocyte population | Newborns (n=50) | p | |
|---|---|---|---|
| Cord blood | Capillary blood | ||
| Total leukocytes absolute number (uL) | 11,925 (6290–19,317) | 17,050 (1980–24,135) | <0.0001 |
| Neutrophils absolute number (uL) | 6807 (3636–12,753) | 8580 (5223–14,741) | 0.01 |
| Percentage (%) | 58 (45–72) | 50 (35–69) | <0.0001 |
| Monocytes absolute number (uL) | 1144 (377–1999) | 1365 (706–2589) | 0.0135 |
| Percentage (%) | 9 (6–15) | 8 (04–12) | 0.1125 |
| Total Lymphocytes absolute number (uL) | 3743 (1434–6491) | 6761 (3170–10,369) | <0.0001 |
| Percentage (%) | 31 (19–45) | 39 (25–57) | <0.0001 |
| T lymphocytes CD3+ absolute number (uL) | 2060 (602–4155) | 3237 (1485–5797) | <0.0001 |
| Percentage (%) | 59 (34–77) | 47 (26–57) | <0.0001 |
| T lymphocytes CD4+absolute number (uL) | 1368 (389–2899) | 1794 (720–3748) | 0.0011 |
| Percentage (%) | 65 (52–78) | 61 (45–71) | 0.0083 |
| T lymphocytes CD8+ mm3 | 610 (156–1372) | 1036 (458–1922) | <0.0001 |
| Percentage (%) | 32 (18–43) | 34 (18–52) | 0.0567 |
| B lymphocytes CD19+ absolute number (uL) | 538 (96–1039) | 956 (278–1456) | <0.0001 |
| Percentage (%) | 13 (7–25) | 13 (06–23) | 0.7841 |
| NK lymphocytes CD16+56+ absolute number (uL) | 695 (159–2283) | 1936 (480–3658) | <0.0001 |
| Percentage (%) | 23 (6–46) | 29 (16–50) | 0.0007 |
Values within parenthesis represent median (5th–95th percentile range); differences between cord and capillary were compared using the Mann–Whitney U test. p significant (bold font), p=<0.05; very significant, p=<0.01; and highly significant, p=<0.001. *Taking into account that 100% are T lymphocytes CD3.
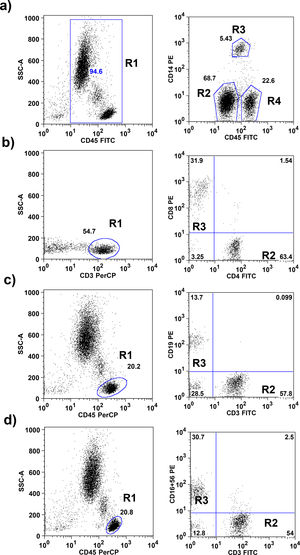
A comparison between cord blood and capillary blood of leukocyte and subsets of leukocytes in absolute numbers and percentages, divided into groups of males and females is shown in Table 2.
Absolute numbers (μl of blood) and percentages (%) of total leukocytes and subpopulations of lymphocytes in cord blood and capillary blood of male (n=28) and female (n=21) healthy newborns.
| Leukocyte population | Male (n=28) | p | Female (n=21) | p | ||
|---|---|---|---|---|---|---|
| Blood type | Blood type | |||||
| Cord blood | Capillary blood | Cord blood | Capillary blood | |||
| Total leukocytes mm3 | 12,325 (9086–19,430) | 16,325 (11,298–24,265) | <0.0001 | 10,950 (3040–23,975) | 18,050 (11,365–27,825) | <0.0001 |
| Neutrophils mm3 | 7064 (4088–13,250) | 7968 (4867–15,954) | 0.1652 | 6314 (2116–13,773) | 8952 (6151–14,439) | 0.0022 |
| % | 60 (37–73) | 50 (32–67) | 0.0005 | 57 (49–73) | 57 (35–76) | 0.0117 |
| Monocytes mm3 | 1298 (526–2012) | 1403 (624–2730) | 0.1455 | 910 (182–2158) | 1176 (674–2543) | 0.0561 |
| % | 9 (5–16) | 9 (4–12) | 0.157 | 8 (6–13) | 7 (04–13) | 0.2769 |
| Total Lymphocytes mm3 | 3743 (2145–6769) | 6585 (4386–10,370) | <0.0001 | 3234 (761–7400) | 7089 (2343–11,208) | <0.0001 |
| % | 30 (17–50) | 39 (27–58) | <0.0001 | 31 (20–37) | 39 (16–51) | 0.0016 |
| T lymphocytes CD3+ mm3 | 2000 (836–4062) | 2971 (1505–5886) | 0.0004 | 2132 (359–4223) | 3387 (1013–5858) | 0.0024 |
| % | 59 (26–81) | 46 (24–62) | 0.001 | 57 (36–72) | 48 (29–70) | 0.0171 |
| T lymphocytes CD4+ mm3 | 1368 (518–2787) | 1576 (669–3892) | 0.0414 | 1359 (220–2959) | 1875 (701–3677) | 0.01 |
| % | 57 (49–79) | 52 (38–70) | 0.0227 | 61 (52–80) | 58 (47–78) | 0.1656 |
| T lymphocytes CD8+ mm3 | 609 (218–1293) | 1013 (457–2095) | <0.0001 | 610 (119–1398) | 1050 (240–1938) | 0.0014 |
| % | 28 (18–47) | 30 (22–57) | 0.0849 | 29 (17–44) | 30 (14–49) | 0.4802 |
| B lymphocytes CD19+ mm3 | 566 (131–968) | 802 (264–1444) | 0.0023 | 542 (82–81035) | 1054 (238–1602) | <0.0001 |
| % | 14 (5–25) | 12 (5–27) | 0.4858 | 13 (8–25) | 14 (10–22) | 0.4169 |
| NK lymphocytes CD16+ 56+ mm3 | 836 (91–2151) | 1995 (514–3899) | <0.0001 | 681 (221–2433) | 1852 (428–3705) | <0.0001 |
| % | 23 (3–46) | 31 (17–52) | 0.0009 | 24 (7–48) | 25 (8–48) | 0.2839 |
Values within parenthesis represent median (5th–95th percentile range); differences between cord and capillary were compared using the Mann–Whitney U test. p significant (bold font), p=<0.05; very significant, p=<0.01; and highly significant, p=<0.001.
In this work, to see if there was any difference in leukocyte subtypes between genders, we stratified the data into capillary blood and capillary blood female (n=21) and cord blood and cord blood male (n=28).
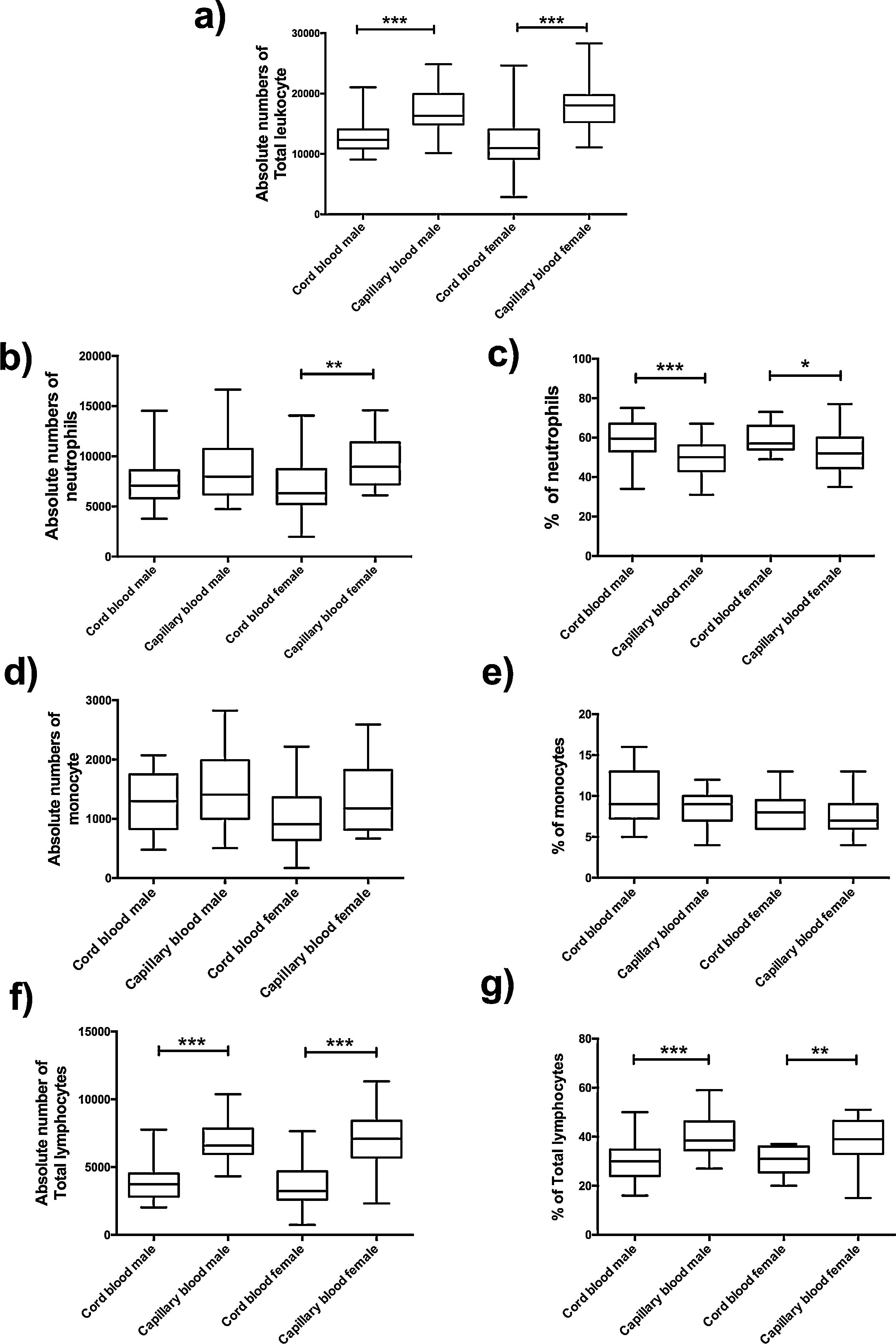
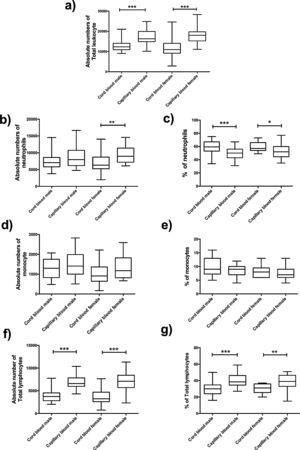
The absolute number of total leukocyte was higher in capillary blood in groups of males and females (p=<0.0001, p=<0.0001, respectively) (Fig. 3a); for this reason, a significant difference was found between cord blood and capillary blood in some leukocyte subpopulations of the groups of male or female, but there were no differences between genders.
Flow cytometric analysis of neutrophils, monocytes and total lymphocytes, in absolute numbers (cell/mm3) and percentages (%) from the cord and capillary blood female neonates (n=21) and male neonates (n=28). a) Total leukocytes (cell/mm3); b) neutrophils (cell/mm3), c) neutrophils (%); d) monocytes (cell/mm3); e) monocytes (%); f) total lymphocytes (cell/mm3); g) total lymphocytes (%). Differences between cord and capillary were compared using the Mann–Whitney U test. (*) significant, p=<0.05; (**) very significant, p=<0.001; and (***) highly significant, p=<0.0001.
In the leukocyte populations (neutrophils, total lymphocytes, and monocytes) a significant difference was found, in both male and female groups. Significant differences were found in the absolute number of neutrophils between cord blood and capillary blood in females (p= 0.0022) (Fig. 3b); the percentage of this population in capillary blood was lower, in the group of male as well as female (p=0.005; p=0.0117, respectively) (Fig. 3c). Lymphocytes of capillary blood were higher in absolute numbers (p=<0.0001; p=<0.0001, respectively) as in percentages (p=<0.0001; p=0.0016, respectively) (Fig. 3f and g). In the monocyte population, no significant difference existed (Fig. 3d and e).
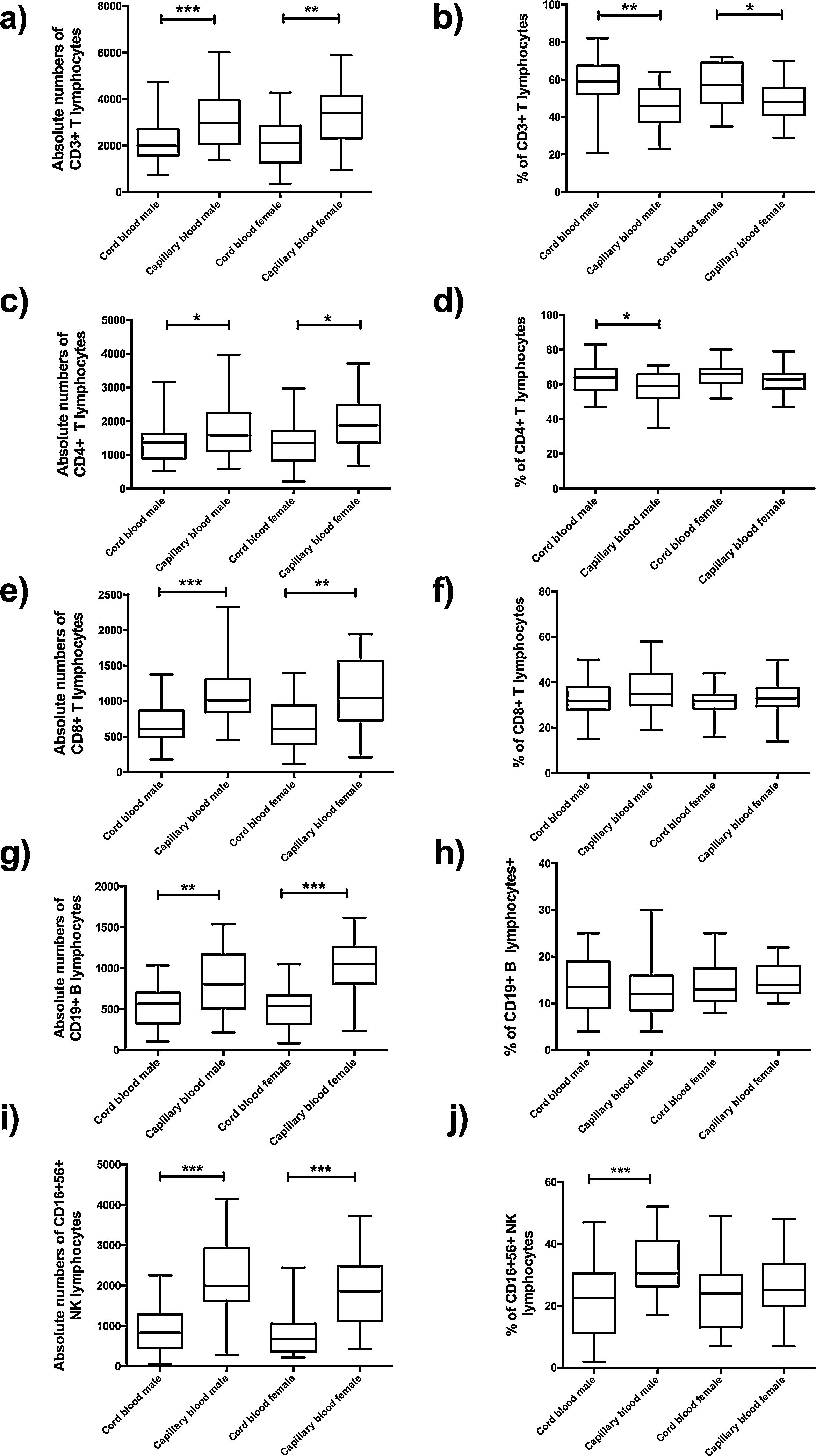
In absolute numbers, a significant difference was found between cord blood and capillary blood in all lymphocyte subpopulations of the groups of male or female (Fig. 4a, c, e, g, and i); in the percentage of lymphocyte subpopulations, only T lymphocyte CD3+ (p=001; p=0.0171, respectively) (Fig. 4b), T lymphocyte CD4+ (p=0.0227, male-only) (Fig. 4d) and NK lymphocyte CD16+56+ (p=0.009, male-only) (Fig. 4j).
Flow cytometric analysis of T lymphocytes, B lymphocytes and NK lymphocytes in absolute numbers (cell/mm3) and percentages (%) from the cord and capillary blood female neonates (n=21) and male neonates (n=28). a) CD3+T lymphocytes (cell/mm3); b) CD3+T lymphocytes (%); c) CD4+T lymphocytes (cell/mm3); d) CD4+T lymphocytes (%); e) CD8+T lymphocytes (cell/mm3); f) CD8+T lymphocytes (%); g) CD19+ B lymphocytes (cell/mm3); h) CD19+ B lymphocytes (%); i) CD16+ 56+ NK lymphocytes and CD16+ 56+ NK lymphocytes (%). Differences between cord and capillary were compared using the Mann–Whitney U test. (*) significant, p=<0.05; (**) very significant, p=<0.001; and (***) highly significant, p=<0.0001.
A newborn represents the culmination of developmental events from conception, implantation, and organogenesis. Dramatic changes occur in the blood and bone marrow of the newborn infant during the first hours and days after birth, and there are rapid fluctuations in the quantities of all hematologic elements. Neonates process a developing immune system, which is different from adults, additionally, neonates are highly susceptible to infections since they change from a semi-allogeneic sterile to being exposed to a microbial environment.1,8
The umbilical cord provides the fetus with oxygen, glucose, amino acids, water, vitamins, and hormones. The flow of fetal blood from the umbilical cord ultimately derives in the chorionic hair space. The space between one hairy column and the other contains maternal blood, separated from the fetal blood within the villus by a membrane created by the trophoblast, composed of specialized epithelium called syncytiotrophoblast and – more internally – cytotrophoblast. This epithelial barrier allows the passage of nutrients into the fetus and the passage of wastes such as CO2, urea and creatinine into the maternal circulation but the passage of blood cells is not usual.9 The umbilical cord blood cells differ from peripheral blood in composition, number, and properties, in the cord blood there is the hematopoietic precursor of stroma.10,11
Fluctuations in the numbers of leukocytes are frequent at all ages but are greatest in infancy. Leukocytosis is typical at birth for full-term and preterm infants, with a wide range between the limits of normal values. However, at birth, neonates have an increased susceptibility to infectious agents due to the functional immaturity of their immune system. Immunophenotyping of blood cell populations to identify these cells is an essential tool in the diagnosis and follow-up of children with immunodeficiency and other immune disorders. A correct interpretation of the results obtained from patients requires knowledge of the normal development in the first hours of life.12 Cord blood is an ideal source for laboratory examination in just-born neonates; it reveals not only normal or abnormal development of human fetal hematopoiesis but also the clinical conditions of these babies.13 On the other hand, capillary blood is easily collected by a minimally invasive procedure and has excellent potential use in point-of-care (POC) health monitoring.3 Point-of-care testing enables rapid diagnosis or monitoring of chronic conditions that can facilitate clinical decisions. Such technologies have the potential to significantly change the healthcare and diagnostic pathways. Patients can obtain immediate results in the hospital, physician’s office, or even at home. This approach saves patients’ a tremendous amount of time, resulting in a faster application of efficient treatment or referral for further investigation.7 It is important to implement the analysis of leukocyte populations to diagnose primary and secondary immunodeficiencies or infections in the newborn in time. In these conditions, early detection and rapid initiation of treatment are key to reducing morbidity and mortality.
We determined reference values for the absolute and relative values of leukocyte subsets in newborns, by analyzing cord and capillary blood samples. Our data will contribute to the development of reference standards for the sampled ethnic-geographic population in this study. These data may also permit the development of clinical strategies to treat infections or diagnose diseases such as immunodeficiencies among others.
Neutropenia is defined as the reduction in the number of circulating neutrophils to less than 1.5×109/L. Neutropenia represents a decrease in neutrophil production or an increase in consumption. Neutropenia can result from infection, impaired bone marrow production or abnormal distribution. Sepsis in neonates is a common cause of morbidity, particularly in premature and low-birth-weight infants.14 Neutropenia is more predictive of neonatal sepsis than in neutrophilia, but it can also be associated with birth asphyxia, intrauterine growth restriction, Rh hemolytic disease or peri-ventricular hemorrhage. Neutrophilia can result from inflammation, certain malignancies.15
Leukopenia refers to decreased counts; it can be seen with viral or bacterial infections, as well as in infants born from women with pregnancy-induced hypertension (PHI). Neonatal patients with lymphopenia may be due to chronic infection with viruses such as cytomegalo virus or Epstein-Barr virus.16 Initiation of prophylactic treatment of opportunistic infections and monitoring responses to antiviral therapy are well established, so immunophenotyping of blood lymphocyte subpopulations is an important tool to evaluate the neonate’s immune system.
Leukocytosis refers to an elevated cell count and it might be observed in patients with infections and leukemias. Lymphocytes constitute about 30% of the leukocytes at birth and may be increased to 60% at four to six months. Immunophenotyping of the blood cell population to identify these cells is an essential tool in the diagnosis and follow-up of children with immunodeficiencies and other immune disorders.17,18
Primary Immunodeficiency (PID) is a heterogeneous group of inherited disorders of the immune system. Immunophenotypic evaluation of PIDs using flow cytometry provides important clues in the identification of diagnosis of these diseases. There are significant phenotypic variations in patients with certain PIDs.7,19
Quantitative assessment of leukocytes populations and subpopulations in neonates is useful for the diagnosis of X-linked agammaglobulinemia (XLA) characterized by the absence of B cells in the peripheral blood (<1%). Patients with severe combined immunodeficiency (SCID) lack T cells while the impact on B and NK cells is variable depending on the genetic defect. Most of the children with SCID die of infections very early in life unless they are treated with hematopoietic stem cell transplantation (HSCT); studies have clearly shown that affected infants diagnosed with SCID as neonates had better survival than patients with delayed diagnosis and this also results in a significant reduction in the cost of therapy.20,21
Patients with Combined Immunodeficiency (CID) have a defect in T lymphocytes in protein that does not affect their development, but affects their function. The patients with ZAP-70 deficiency and MHC I deficiency help guide the defects, when CD8+T lymphocytes are at least 7%. The patients with MHCII deficiency have CD4+T lymphocytes very low.22
NK cells play a major role in the resolution of severe acute respiratory viral infections caused by influenza or respiratory syncytial virus. Although the information on NK cells in bacterial infections in children is limited, it has been shown that young children with recurrent otitis media and sinusitis have NK cell genetic defects.23 The patients with profound monocytopenia, B lymphocytes and NK lymphocytes low, could guide a diagnosis to a Gata 2 deficiency.24 Therefore, establishing baseline values of leukocyte populations in neonates helps guide early diagnosis for some PIDs.
In the present work, we consistently observed differences in the absolute number of different leukocyte populations among cord and capillary blood neonates. We observed higher frequencies and absolute counts of total lymphocytes in capillary blood compared with cord blood. In absolute numbers, it was the capillary blood which had significant differences in all the leukocyte populations. In neonates, the cellular immune system matures rapidly in the first three months of life. The development of neonatal immunity is influenced by multiple factors including maternal cytokines, antigen exposure and precursor frequency of lymphocytes. Fluctuations in the number of leukocytes are common at all ages but are greatest in neonates.8 Our hypothesis as to why there are more lymphocytes, monocytes, and neutrophils in capillary blood is that the newborn needs to mobilize a higher number of cells to the periphery in order to face possible infectious agents and also start the activation and proliferate process to become a mature leukocyte. These data allow us to identify new interesting differences in certain specific leukocyte subsets between the cord and capillary blood of neonates.
Previous studies provided important information about reference values of cord blood for flow cytometry25–27; while reference values for the major immune cells categories that are necessary for clinical purposes have been generated for both adults and neonates.26 In this work, we compared frequencies and the absolute number of cord and capillary blood leukocytes; also establishing reference values in a Mexican population of neonates. Our data provide interesting differences between the blood and identify specific leukocyte subpopulations. Diversity in the phenotypes of leukocyte proportions and concentrations in blood is thought to have variable contributions from genetic, environmental and epigenetic factors.
Conflicts of interestThe authors have no conflict of interest to declare.
The authors wish to thank Dr. Rafael Figueroa Moreno for technical assistance. This study was partially supported by grants from Consejo Nacional de Ciencia y Tecnología CONACyT (project CB-2016-256471), Ciudad de México, México.