Over the past three decades, the number of obese people has risen steadily. The chronic low-grade inflammatory state and the non-specific activation of the immune system have contributed greatly to the development of obesity-related immunology. Food allergy as a kind of inflammatory disease with abnormal immune response may be associated with obesity. This review begins with the pro-inflammatory immunological effects of adipose tissue in obesity, and explains the possible effects of obesity on food allergy. In short, obesity not only directly causes imbalance of allergic-related immune cells in adipose tissue, but also indirectly causes this consequence through affecting expression of adipocytokines and peroxisome proliferator-activated receptor gamma (PPARγ) in adipose tissue. As a result, circulating levels of pro-inflammatory factors which are partly derived from adipose tissue increase, which might cause intestinal barrier injury. Therefore, obesity may increase the risk of food allergy.
Since the 21st century, health problems caused by the increasing number of obese people have become more serious.1 More and more epidemiological evidence suggests that obesity increases the risk of asthma and allergic diseases.2,3 Among them, food allergy is a common type of allergic disease in the world. Its symptoms vary in intensity, ranging from mild (itch) to severe (vomiting, diarrhea, urticaria, dyspnea, etc.) and may even be life-threatening. Depending on the severity of food allergy symptoms, there are also differences in treatment and management.4 It has been reported that about 5% of adults and 8% of infants and young children worldwide are allergic to some foods and the incidence rate continues to rise, seriously affecting human living quality.5
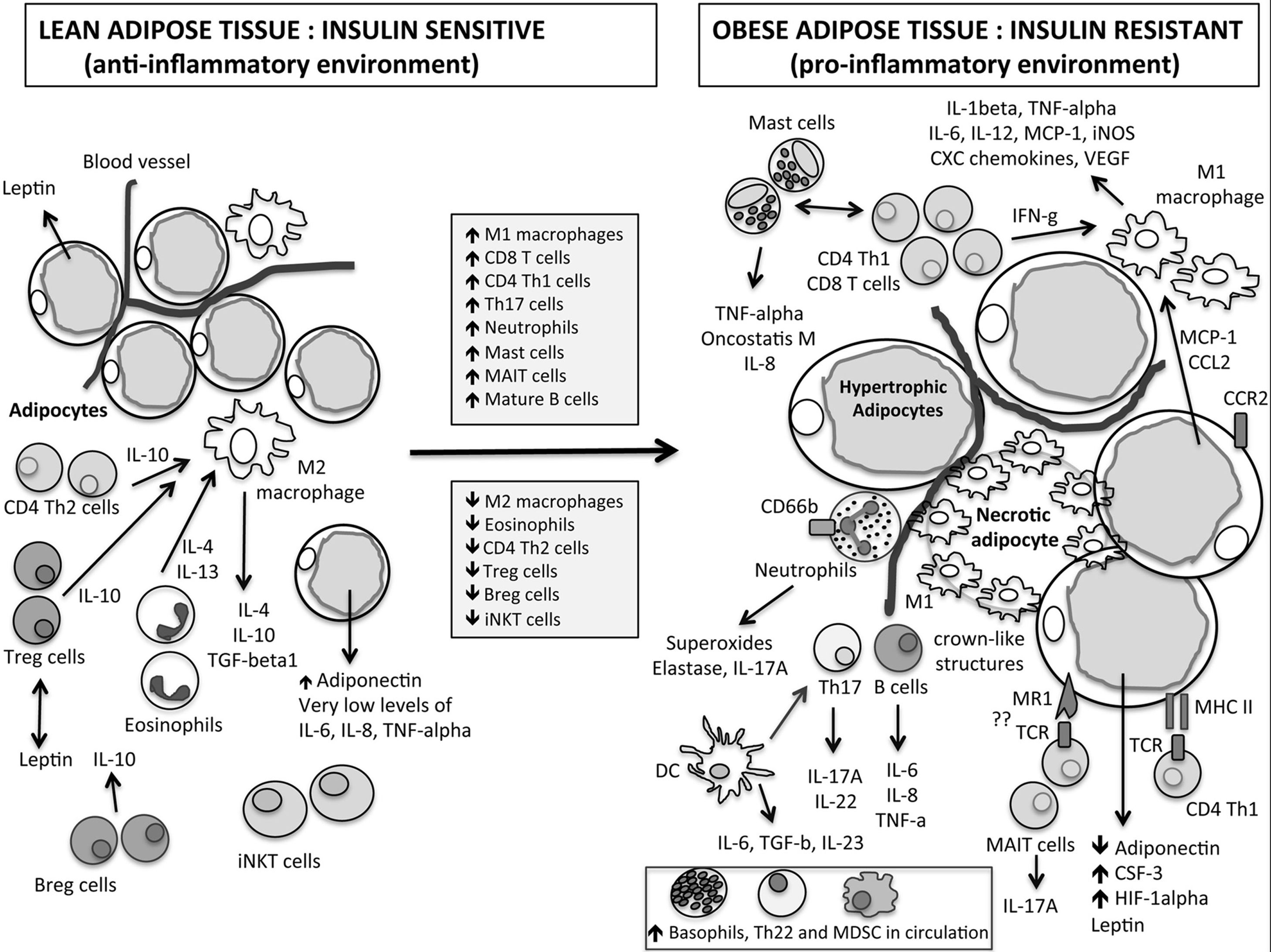
Obesity, as a persistent low-grade inflammatory state, may be related to food allergy which is an inflammatory disease caused by allergen ingestion.6 Obesity is an excess of energy in adipose tissue. Adipose tissue is both an organ that stores energy and an immune organ. Adipocyte hypertrophy and pro-inflammatory activation of dormant immune cells in adipose tissue play an important role in the pathology of obesity. These cells cause an increase in circulating pro-inflammatory cytokines.7 In adipose tissue of lean individuals, pro-inflammatory cells are inhibited by T-regulatory cells (Treg cells), whereas in adipose tissue of obese individuals, there is no immunosuppression. Aggregation of pro-inflammatory cells promotes the production of pro-inflammatory cytokines.8 In addition, adipose tissue has been shown to be an endocrine organ, which secretes a variety of adipocytokines (such as leptin and adiponectin) to regulate adipose tissue homeostasis, including immune homeostasis. Obesity breaks this balance by affecting the levels of adipocytokines. Interestingly, receptors expressed in adipocytes, such as PPARγ, are involved not only in the activation and proliferation of adipocytes, but also in the inflammatory response and play a role in inhibiting inflammation.9 Therefore, downregulation of PPARγ expression in obesity also affects the immune balance of adipose tissue.10 A variety of immune cells present in adipose tissue, cytokines secreted by adipose cells, and receptors expressed in adipose tissue, such as PPARγ, affect the immune status in adipose tissue.10,11 Pro-inflammatory immunological effects of adipose tissue in obesity can enter to circulation and reach intestines, and which may therefore be associated with food allergic reactions.7
Past population studies on obesity and allergy have been controversial. Visness et al. reported that obese and overweight children aged 2–19 years have higher levels of major antibody immunoglobulin E (IgE), which is involved in allergic reactions, than normal-weight children.12 That is, obese and overweight children are more likely to develop food allergic reactions. Beyond that, the probability of allergic reactions in obese children is increased compared with normal-weight children, and this association is mainly caused by food allergy. Similarly, Luo et al. found that in Harbin, China, obesity is positively correlated with adult atopic disease.3 Specifically, obesity is significantly associated with atopic dermatitis and rhinitis, suggesting that obesity increases the risk of allergic disease. In contrast, in a study of 486 students (more than 98 students who were overweight or obese), the results showed that obesity is not associated with allergic disease.13 In a study about Body Mass Index (BMI) and obesity complications, Bruno et al. reported that atopic disease and IgE levels that play an important role in allergic reactions are not associated with adult BMI. This means that obesity does not affect allergic reactions.14
In order to clarify whether obesity affects food allergic reactions, this review provides the latest information on the relationship between obesity and allergic diseases, mainly food allergy, focusing on the immunological effects of the low-grade inflammatory state in obesity.
Association between pro-inflammatory immunological effects of adipose tissue and food allergy in obesityA variety of immune cells present in adipose tissue, cytokines secreted by adipose cells, and receptors expressed in adipose tissue, such as PPARγ, constitute a complex immune network in adipose tissue. In obesity, the immune network tilts toward pro-inflammatory direction and produces pro-inflammatory immunological effects in adipose tissue causing pro-inflammatory immune cells to aggregate and secrete a large number of pro-inflammatory cytokines, adipocytokines, and at the same time the function of PPARγ is affected, thereby breaking the immune homeostasis. Thus, obesity may be associated with diseases such as food allergy that are accompanied by abnormal immune responses.
1. Inflammation induced by imbalanced immune cells in adipose tissue may be associated with reduced allergen tolerance and intestinal barrier damage.
There are many immune cells in adipose tissue, such as mast cells, T helper cells (CD4+T cells, Th cells), cytotoxic T cells (CD8+T cells, Tc cells), regulatory T cells (Treg cells), regulator B cells (Breg cells), invariant natural killer cells (iNKT cells) and M2 macrophages that balance local inflammation, which act to maintain the immune balance of adipose tissue.15 Dynamic immunological response changes to pro-inflammatory state in obesity with an influx of pro-inflammatory immune cells (such as mast cells, basophils, etc.) which secrete a large number of pro-inflammatory cytokines (such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), etc.) and a decrease of anti-inflammatory Treg cells as shown in Fig. 1.11 Meanwhile anti-inflammatory M2 macrophages are converted into pro-inflammatory Ml macrophages. The imbalance of immune cells in adipose tissue may be the core of the development of local and systemic inflammation associated with obesity.16 It is known that a large number of pro-inflammatory cytokines can activate the nuclear factor-kappa B (NF-κB) signaling pathway, induce the production of pro-inflammatory cytokines, chemokines and so on, and aggravate the pro-inflammatory immunological effect.17 Activated NF-κB-induced proteins, such as TNFα, also activate NF-κB, creating a vicious circle that enlarges inflammation again.18 Meanwhile, studies have shown that TNF-α can increase the permeability of the epithelial barrier by disrupting tight junctions in Caco-2 cells.19 Therefore, when a large number of adipose tissue-derived pro-inflammatory cytokines enter the bloodstream and reach the intestines, they may cause intestinal barrier injury and increase the susceptibility of obese individuals to food allergens.
Immunity complexity in obesity leads to low chronic inflammation.11
An important feature of food allergic reactions is the imbalance of T helper cell type-1 (Th1)/T helper cell type-2 (Th2), and the immune response tilts toward Th2-type immune response. Apostolopoulos et al. found that both Thl cells and Th2 cells have different levels of accumulation in adipose tissue in obese mice and humans compared to normal-weight ones.11 Among them, the number of Th1 cells in adipose tissue increased, while the number of Th2 cells decreased in obesity. That is, the immune response may tilt toward the Th1-type immune response, which is different from the feature of food allergic reaction. Therefore, obesity may affect food allergic reaction by interfering with other immune cells in adipose tissue.
As an anti-inflammatory T cell subtype, Treg cells can inhibit Th2 cells proliferation, reduce allergen-specific IgE secretion, inhibit the migration of T cells to tissues, and limit the interaction of eosinophils, mast cells and neutrophils with resident tissue cells, thereby inhibiting an inflammatory response.20 Dysfunction of Treg cell is one of the important links in the development of allergic diseases. This change will lead to reduced immune tolerance to allergens, which will promote CD4+T cells (Th0 cells) to differentiate into Th2 cells, triggering allergic reactions.21 Studies have shown that the number of Treg cells in diet-induced obese mice is significantly reduced.22 In addition, Jeffrey et al. reported that Foxp3 transcripts (important symbols of Treg cells) in visceral adipose tissue were negatively correlated with obesity markers.23 Therefore, the decrease of Treg cells is involved in the formation of pro-inflammatory immunological effects in adipose tissue under the condition of obesity. In addition, the increased circulating levels of adipose tissue-derived pro-inflammatory cytokines mentioned above may cause damage to the survival and/or maturation of circulating Treg cells.24 Based on the above results, it can be inferred that both obese mice and humans may have decreased tolerance to allergens due to the decrease of Treg cells, and they are more likely to develop food allergic reactions than the lean control group.
Mast cells are effector cells that release inflammatory mediators, cause symptoms of skin, respiratory tract, and digestive tract when food allergic reactions occur. Mast cells bind to IgE through high-affinity IgE receptors (FcεRIs) on their surface cross-link allergens and play an important role in food allergy.25 Mast cells are abundant in adipose tissue and can interact with adipocytes to recruit inflammatory cells.26 Studies by Altintas et al. have shown that mast cells in visceral fat in obese mice are higher than in lean controls.27 Similarly, it has been reported that obese mice and humans on a high-fat diet have high levels of tryptase (an indicator of mast cell activation) and mast cells in adipose tissue.28 As tryptase can activate protease-activated receptor 2 (PAR-2), and PAR-2 is coupled with activation of beta-arrestins dependent extracellular regulated protein kinases 1/2 (ERK1/2) pathway which can restructure F-actin around tight junctions to increase intestinal epithelial permeability.29 Increased levels of tryptase in adipose tissue caused by obesity may enter the bloodstream and reach intestines, resulting in intestinal barrier injury and increased susceptibility to food allergens. These studies suggest that there are more activated mast cells in obese animals or humans. This condition is not only involved in the formation of pro-inflammatory immunological effects in adipose tissue, but may also lead to increased sensitivity to food allergens, resulting in more severe allergic reactions.
2. Changes in adipocytokine levels down-regulate the activity of Treg cells
Adipose tissue, as an endocrine organ, secretes a variety of adipocytokines including leptin, adiponectin, IL6 and TNF-α. Adipokines act on different organs such as the brain, kidney, liver, pancreas and skeletal muscle to regulate metabolic homeostasis.30 In individuals with normal metabolic status, there is a balance between pro-inflammatory (such as leptin) and anti-inflammatory adipocytokines (such as adiponectin). When adipose tissue expansion (due to excessive nutrition or lack of physical activity) leads to dysfunction of adipocytes, adipocytokines may be dysregulated, possibly causing local or systemic effects on the inflammatory response, leading to inflammation-related pathological processes.30
Leptin is a kind of hormone involved in regulating satiety and has an effect on many aspects of immune function, mainly to stimulate pro-inflammatory response.31 Leptin activates CD4+T cells, which leads to the secretion of pro-inflammatory cytokines (TNF-α, IL-6, IL-12) and up-regulates activation markers on monocytes (CD11B, CD11C, major histocompatibility complex (MHC) class II, CD25, CD38, CD69, etc.), stimulates the production of reactive oxygen and chemotaxis of multinucleated cells.11 Adipose-derived plasma protein adiponectin increases fatty acid degradation, decreases blood glucose levels and increases insulin sensitivity. It also has anti-inflammatory, anti-oxidant and anti-cancer activities, and antagonizes the expression of TNF-α in adipocytes and macrophages. However, under the condition of obesity, circulating adiponectin levels are decreased, which are associated with various obesity complications and get involved in the formation of pro-inflammatory immunological effects.32
When gaining weight, the levels of IL-6, leptin and TNF-α in the circulation increase, and IL-6 and leptin down-regulate the activity of Treg cells. In addition, adiponectin, which decreases in obesity, down-regulates interleukin 10 (IL-10) secreted by macrophages and adipocytes. These changes in IL-6, leptin and IL-10, all impair immunoregulation function of Treg cells, leading to reduced antigen tolerance.33 Therefore, it can be speculated that the immunological changes caused by obesity may lead to reduced food allergen tolerance and increased risk of food allergy.
3. Deregulation of PPARγ and allergic reaction
PPARγ is a class of ligand-dependent nuclear receptors expressed in adipose tissue, kidney, stomach, heart, liver, spleen and brain.34 Expression and activation of PPARγ has been shown to be sufficient to induce adipogenesis.35 In addition, PPARγ is also expressed in various immune cells, and it not only regulates genes involved in lipid metabolism, but also regulates immune and inflammation-related genes.36 PPARγ exerts anti-inflammatory effects by inhibiting the expression of pro-inflammatory genes.37 It has been reported that expression of PPARγ decreased in high-fat diet group in the late phase of obesity.38 In addition, in the diet-induced canine obesity model established by Gayet et al., both visceral adipose tissue and skeletal muscle PPARγ mRNA expression decreased.39 Therefore, the pro-inflammatory immunological effects of adipose tissue in obesity may attenuate the anti-inflammatory effects of PPARγ by down-regulating its expression.
The possible mechanisms of PPARγ down-regulation in obesity include phosphorylation, epigenetic events and fatty acid composition in diet. Studies have shown that the phosphorylation of PPARγ in obesity can lead to dysfunction of PPARγ.40,41 Epigenetic events are also involved in the down-regulation of PPARγ expression. The up-regulation of microRNA (microRNA-27b, 130b and 138) induced by obesity is negatively correlated with the expression of PPARγ, but obesity does not affect the methylation of PPARγpromoter.42 In addition, conjugated linoleic acid (CLA) and conjugated linolenic acid (CLNA) decrease the expression and activity of PPARγ in obesity.43 However, another study showed that the addition of CLA to a high-fat diet can activate the expression of PPARγ in rats.44 Also, the addition of n-3 and n-6 polyunsaturated fatty acids to high-fat diets containing partially hydrogenated vegetable oils can alleviate inflammation in rats by up-regulating the expression of PPARγ.45 Interestingly, the effect of dietary fatty acids on PPARγ also mediates changes in adipocytokine levels. For example, fish oil can increase the expression of anti-inflammatory adiponectin in a PPARγ-dependent manner.46 Therefore, fatty acid composition in diet may become the research focus of regulating PPARγ expression and improving inflammation in obesity.
At present, several anti-inflammatory mechanisms of PPARγ have been proposed, including inhibiting signal transducers and activators of transcription (STAT) and NF-κB factors, both of which are closely related to food allergic reactions.47 In the Janus-activated kinase-signal transducers and activators of transcription (JAKSTAT) pathway, STAT5 plays a key role in IgE-mediated cytokine production and release of histamine and leukotriene.48 Transcription factor NF-κB has a marked impact on coordinating innate and acquired immunity.49 Studies have shown that NF-κB is over-activated in mouse colonic mucosa in allergic reactions, interfering immune tolerance mechanism and producing a continuously expanding inflammatory response.50 Due to the decrease of PPARγ level in adipose tissue in obesity, its inhibitory effect on STAT and NF-κB is reduced, which may indirectly lead to increased activation of these two transcription factors. Increased activation of NF-κB in adipose tissue will produce more pro-inflammatory factors (such as TNF-α) and inducible nitric oxide synthase (iNOS), which will aggravate inflammation, damage intestinal barrier, and may increase the risk of food allergy.19,51 Increased STAT activation in adipose tissue may promote mast cell activation, leading to more tryptase secretion.48 These tryptase may enter to circulation and reach the intestines, resulting in intestinal barrier injury and increased susceptibility to food allergens.29 In addition to its anti-inflammatory effects by inhibiting STAT and NF-κB transcription factors, PPARγ is also involved in regulation of the number and function of Treg cells in adipose tissue.52 Therefore, the down-regulation of PPARγ in adipose tissue caused by obesity may affect the activity of Treg cells, indirectly promoting the accumulation of pro-inflammatory immune cells and the secretion of pro-inflammatory cytokines, leading to intestinal barrier damage and increased risk of food allergy.19
In addition, studies have shown that PPARγ is expressed in mast cells, and PPARγ agonist reduces the phenotypic markers and viability of mast cells, inhibits degranulation, and induces cell apoptosis.53,54 As PPARγ is one of the main regulators of mast cell maturation, decreased expression of PPARγ in obesity will accelerate the differentiation of mast cells.55 As mentioned above, increased activation of mast cells can lead to intestinal barrier injury and increased risk of food allergy by secreting more tryptase.29
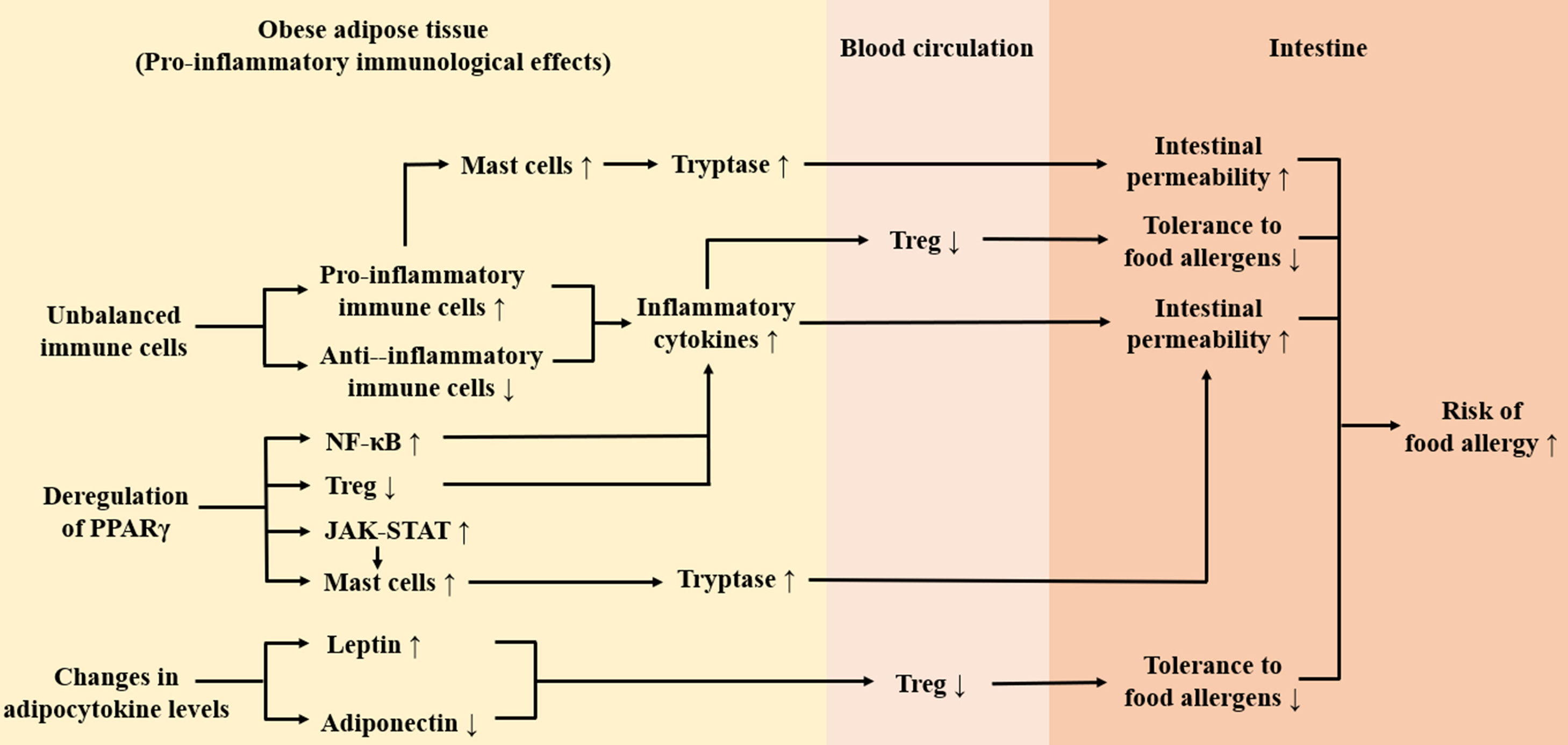
ConclusionThis review takes the pro-inflammatory immunological effects of adipose tissue in obesity as a breakthrough point, expounding that obesity may increase the risk of food allergy. As shown in Fig. 2, firstly, obesity leads to the accumulation of inflammatory immune cells in adipose tissue and these cells secret a large number of inflammatory factors (TNF-a, tryptase, etc.) which may damage the intestinal barrier, thus increasing the risk of food allergy. Secondly, the imbalance of circulating adipocytokines in obesity inhibits functions of Treg cells, which may lead to decreased tolerance to food allergens. Thirdly, obesity-induced deregulation of PPARγ weakens its anti-inflammatory effect and has an impact on activation of Treg cells and mast cells, which may contribute to intestinal barrier injury and increased risk of food allergy. It is noteworthy that except for the pro-inflammatory immunological effects of adipose tissue, gut microbiome imbalance caused by obesity may also increase susceptibility to food allergens.56 In order to explain the link between obesity and food allergy accurately, further research on the effects of obesity on intestinal immunity is needed.
Ethical disclosuresConfidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Protection of human subjects and animals in research. The authors declare that no experiments were performed on humans or animals for this study.
Funding sourcesThis work was supported by National Natural Science Foundation of China (No.81573158).
Conflict of interestsThe authors have no conflict of interest to declare.