There is little understanding of the mechanisms by which food allergy (FA) develops into persistent disease, or by which symptoms it regresses. Food allergy is a major health problem in developed countries, where the prevalence reaches up to 6% in children and 3% in the adult population.
ObjectiveChildren with food allergy remission (FAR) and those without FAR below five years of age, were compared 7–10 years with respect to clinical data and expression of glycoprotein A repetitions predominant (GARP) on peripheral blood mononuclear cells.
MethodsForty children with FAR and 40 children without FAR at age 7–10, in whom FA was previously diagnosed at age below five years were evaluated. In this prospective study, demographic and clinical data were taken, patients were classified as atopic based on history and serum specific IgE (sIgE) for a specific allergen. Blood samples were obtained from all patients to assess expression of GARP.
ResultsWe observed higher expression of GARP in children with FAR compared to children without FA (p=0.005); optimal cut-off for GARP prediction of the remission was 20.1%. Children with FAR and food-specific IgE in serum had higher expression of GARP compared to children with low food specific IgE (<0.35kU/L). Keeping pets at home decreased, and presence of allergic rhinitis increased ORs for high expression of GARP (hGARP) in our patients.
ConclusionhGARP (>20.1%) is related with FAR in school children. Allergic rhinitis, and pets at home modify this effect of GARP. Children with allergic rhinitis have less chance of developing remission despite maintaining immune tolerance (hGARP); quite the opposite case with pets at home.
There is little understanding of the immunological mechanisms by which food allergy develops, or by which its symptoms regress. These mechanisms by which immune responses to food antigens lead to either allergy or tolerance involve complex interactions between genetic background and the exposure. Food allergy is a major health problem in developed countries, where the prevalence reaches up to 6% in children and 3% in the adult population.1,2 Natural history of FA remains unknown.3
Glycoprotein A repetitions predominant (GARP), a receptor on activated regulatory T cells that binds latent transforming growth factor – β, is involved in the regulation of peripheral tolerance.4 GARP encodes a surface receptor on activated Tregs that binds latent TGF-β (transforming growth factor-β) and modulates peripheral tolerance and T effector cell function. TGF-β are a group of cytokines that control many biological processes and contribute to protection against FA. TGF-β-producing Br3, and TGF-β-producing Th3, cells seem to be involved in immune tolerance, including allergy tolerance.5,6
The current study was designed to investigate the expression of protein GARP in children 7–10 years with FA and those with FAR. Children were diagnosed towards FA below five years of age, and then compared at the age of 7–10 years with respect to clinical data.
Patients and methodsPatientsThis study was a prospective study based on 40 children with food allergy (FA) and 40 children with food allergy remission (FAR) at age 7–10, in whom FA was previously diagnosed at age below five years. Children were registered and followed up in our outpatient allergy clinic. Patients with other chronic diseases such as congenital or acquired heart and lung disease, rheumatologic diseases and immunodeficiency were excluded from the study.
Parents were requested to attend the clinic with their children by phone call. They were interviewed personally. At the first visit they were informed about the purpose of the study, and a blood sample was drawn for IgE testing. Demographic data and medical history (clinical data) were taken, patients were classified as atopic based on history and serum specific IgE (sIgE) for a specific allergen. At this visit blood samples were obtained from all patients to assess immunological parameters.
Food allergy in early childhoodThe childhood FA was diagnosed by a specialist based on clinical symptoms (such as eczema, wheezing, abdominal pain, diarrhea, OAS) and presence of serum IgE of ≥0.35 KU/L specific for any food allergens (egg yolk and white, betalactoglobuline, casein).
Remission of food allergyRemission of FA in our patients was defined as the absence of clinical symptoms.
IgE measurementsMeasurement of serum specific IgE was employed if necessary. Allergen sensitization was defined as specific IgE of ≥0.35KU/L for at least one of the tested allergens (chemiluminescence method (CLIA), Immulite 2000, XPI, Siemens, Germany). For the purposes of the study we defined allergy as the presence of serum IgE of ≥0.35 KU/L specific components for food allergens: milk, egg, soya, fruits, vegetables, meat, grain.
Immunological assessmentThe antibodies conjugated with allophycocyanin (APC) were used for Anti-GARP APC assay (antibodies from Becton Dickinson, San Diego, CA, USA). All procedures were carried out according to the manufacturer’s instructions. The isolated peripheral blood mononuclear cells (PBMC) were incubated with surface monoclonal antibodies against GARP in the dark, at room temperature for 30min. The samples were then washed twice in buffered saline (PBS) (5min 140g) (PBS) (PAA Laboratories GmbH, Austria). Cells for evaluating the expression of intracellular antigens were fixed using an intracellular staining kit according to the manufacturer’s protocol (BD, San Diego, CA, USA).
The study was approved by the Medical Ethics Committee of the Medical University of Lodz, the committee’s approval number is RNN/173/14/KE. All parents or legal guardians gave their oral and written consent for the evaluation of data from medical documentation of their children.
Statistical methodsStatistical analysis was conducted in three steps. First, simple comparisons with Mann–Whitney test of GARP expression between groups of participants were done. Second, ROC curve analysis according to Youden method was used to calculated optimal cut-off for GARP expression in the prediction of the remission of FA. Finally, dichotomized GARP was included into the logistic regression analysis in univariate and multivariate model to verify the independence of previous findings. Variables associated with GARP in univariate models (p<0.1) were included according to a stepwise approach into the final, multivariate model. The significance threshold of p-level was set at 0.05. Statistical analysis was performed with the STATISTICA 13.1 (StatSoft Polska, Kraków, Poland).
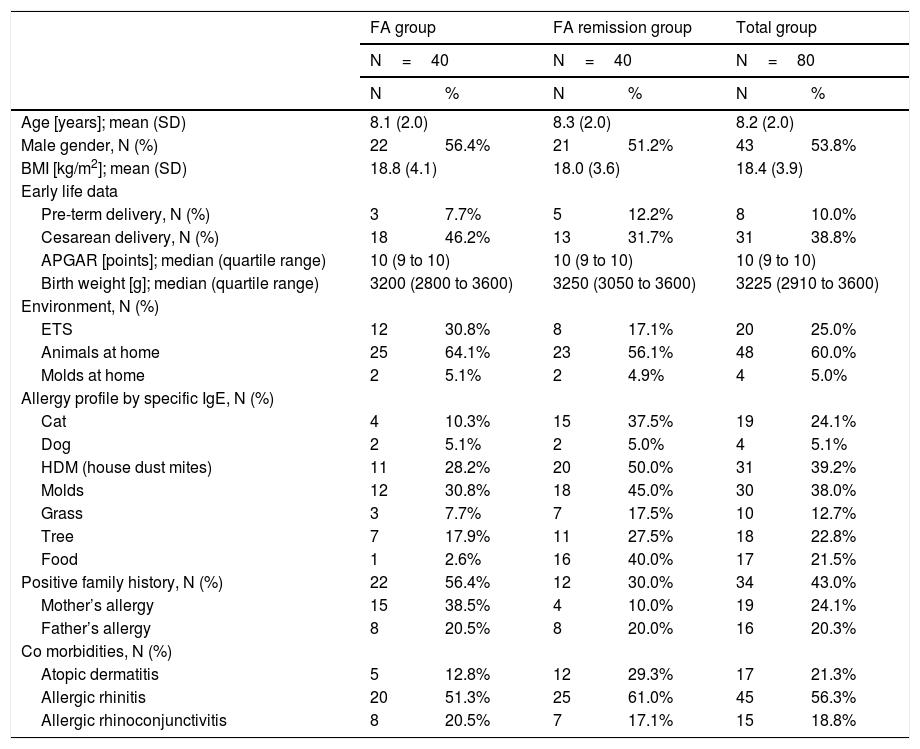
ResultsEighty patients were included into the analysis. Clinical characteristics of the study group are presented in Table 1.
Baseline characteristics of study population at the age of 7–10 years.
| FA group | FA remission group | Total group | ||||
|---|---|---|---|---|---|---|
| N=40 | N=40 | N=80 | ||||
| N | % | N | % | N | % | |
| Age [years]; mean (SD) | 8.1 (2.0) | 8.3 (2.0) | 8.2 (2.0) | |||
| Male gender, N (%) | 22 | 56.4% | 21 | 51.2% | 43 | 53.8% |
| BMI [kg/m2]; mean (SD) | 18.8 (4.1) | 18.0 (3.6) | 18.4 (3.9) | |||
| Early life data | ||||||
| Pre-term delivery, N (%) | 3 | 7.7% | 5 | 12.2% | 8 | 10.0% |
| Cesarean delivery, N (%) | 18 | 46.2% | 13 | 31.7% | 31 | 38.8% |
| APGAR [points]; median (quartile range) | 10 (9 to 10) | 10 (9 to 10) | 10 (9 to 10) | |||
| Birth weight [g]; median (quartile range) | 3200 (2800 to 3600) | 3250 (3050 to 3600) | 3225 (2910 to 3600) | |||
| Environment, N (%) | ||||||
| ETS | 12 | 30.8% | 8 | 17.1% | 20 | 25.0% |
| Animals at home | 25 | 64.1% | 23 | 56.1% | 48 | 60.0% |
| Molds at home | 2 | 5.1% | 2 | 4.9% | 4 | 5.0% |
| Allergy profile by specific IgE, N (%) | ||||||
| Cat | 4 | 10.3% | 15 | 37.5% | 19 | 24.1% |
| Dog | 2 | 5.1% | 2 | 5.0% | 4 | 5.1% |
| HDM (house dust mites) | 11 | 28.2% | 20 | 50.0% | 31 | 39.2% |
| Molds | 12 | 30.8% | 18 | 45.0% | 30 | 38.0% |
| Grass | 3 | 7.7% | 7 | 17.5% | 10 | 12.7% |
| Tree | 7 | 17.9% | 11 | 27.5% | 18 | 22.8% |
| Food | 1 | 2.6% | 16 | 40.0% | 17 | 21.5% |
| Positive family history, N (%) | 22 | 56.4% | 12 | 30.0% | 34 | 43.0% |
| Mother’s allergy | 15 | 38.5% | 4 | 10.0% | 19 | 24.1% |
| Father’s allergy | 8 | 20.5% | 8 | 20.0% | 16 | 20.3% |
| Co morbidities, N (%) | ||||||
| Atopic dermatitis | 5 | 12.8% | 12 | 29.3% | 17 | 21.3% |
| Allergic rhinitis | 20 | 51.3% | 25 | 61.0% | 45 | 56.3% |
| Allergic rhinoconjunctivitis | 8 | 20.5% | 7 | 17.1% | 15 | 18.8% |
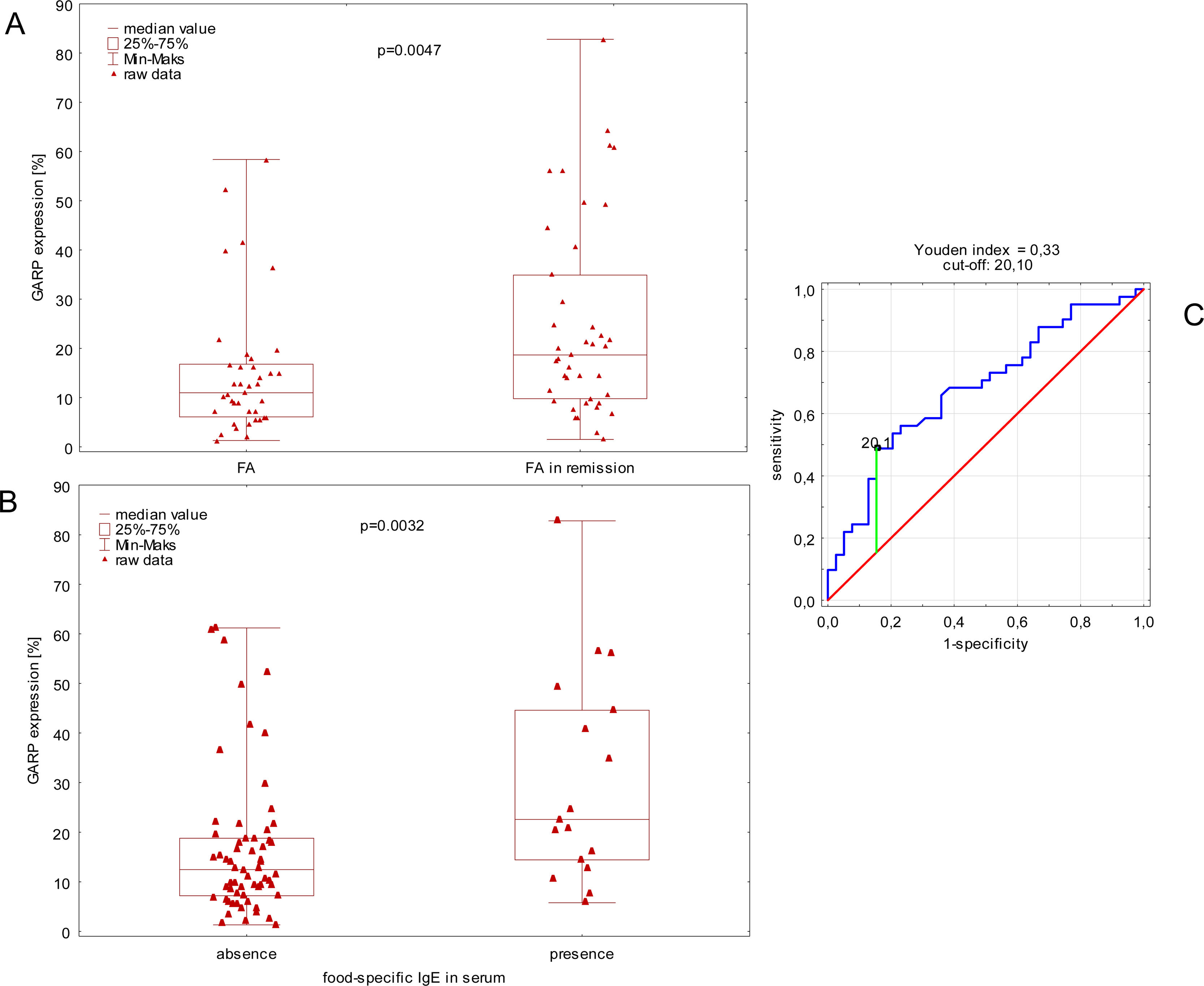
In the first step, we observed higher expression of GARP in children with remission of FA compared to children without remission of FA (Fig. 1A). Moreover, children with FAR and food-specific IgE in serum had a higher expression of GARP compared to children with FAR with low food specific IgE (<0.35kU/L) (Fig. 1B).
Comparison of GARP expression between children with remission of food allergy and children without medical history of food allergy (A). Comparison of GARP expression between children with and without food-specific IgE in serum (B). ROC curve visualization with cut-off estimation (C).
ROC curve analysis and calculation of Youden index showed that optimal cut-off for GARP prediction of the remission of FA is 20.1. Next, GARP was dichotomized for each participant according to the following rule: i) expression below 20.1% is defined as low, lGARP, ii) expression higher or equal 20.1% is defined as high, hGARP. Dichotomized GARP was finally included as a dependent variable into the logistic regression analysis.
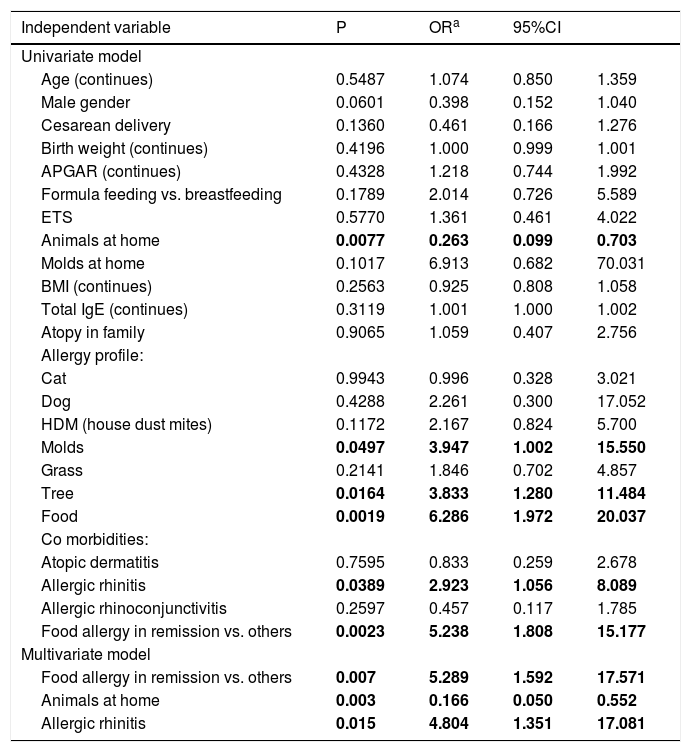
Logistic regression analysisAll available clinical data as independent variables were included into the univariate model of logistic regression analysis. The results are shown in Table 2. Multivariate model of logistic regression showed that remission of FA is independently associated with hGARP. Keeping pets at home decreased and the presence of allergic rhinitis increased ORs for hGARP in our patients.
Associations between GARP expression above 201%, defined as dependent variable and group of independent variables in univariate and multivariate model of logistic regression analysis.
| Independent variable | P | ORa | 95%CI | |
|---|---|---|---|---|
| Univariate model | ||||
| Age (continues) | 0.5487 | 1.074 | 0.850 | 1.359 |
| Male gender | 0.0601 | 0.398 | 0.152 | 1.040 |
| Cesarean delivery | 0.1360 | 0.461 | 0.166 | 1.276 |
| Birth weight (continues) | 0.4196 | 1.000 | 0.999 | 1.001 |
| APGAR (continues) | 0.4328 | 1.218 | 0.744 | 1.992 |
| Formula feeding vs. breastfeeding | 0.1789 | 2.014 | 0.726 | 5.589 |
| ETS | 0.5770 | 1.361 | 0.461 | 4.022 |
| Animals at home | 0.0077 | 0.263 | 0.099 | 0.703 |
| Molds at home | 0.1017 | 6.913 | 0.682 | 70.031 |
| BMI (continues) | 0.2563 | 0.925 | 0.808 | 1.058 |
| Total IgE (continues) | 0.3119 | 1.001 | 1.000 | 1.002 |
| Atopy in family | 0.9065 | 1.059 | 0.407 | 2.756 |
| Allergy profile: | ||||
| Cat | 0.9943 | 0.996 | 0.328 | 3.021 |
| Dog | 0.4288 | 2.261 | 0.300 | 17.052 |
| HDM (house dust mites) | 0.1172 | 2.167 | 0.824 | 5.700 |
| Molds | 0.0497 | 3.947 | 1.002 | 15.550 |
| Grass | 0.2141 | 1.846 | 0.702 | 4.857 |
| Tree | 0.0164 | 3.833 | 1.280 | 11.484 |
| Food | 0.0019 | 6.286 | 1.972 | 20.037 |
| Co morbidities: | ||||
| Atopic dermatitis | 0.7595 | 0.833 | 0.259 | 2.678 |
| Allergic rhinitis | 0.0389 | 2.923 | 1.056 | 8.089 |
| Allergic rhinoconjunctivitis | 0.2597 | 0.457 | 0.117 | 1.785 |
| Food allergy in remission vs. others | 0.0023 | 5.238 | 1.808 | 15.177 |
| Multivariate model | ||||
| Food allergy in remission vs. others | 0.007 | 5.289 | 1.592 | 17.571 |
| Animals at home | 0.003 | 0.166 | 0.050 | 0.552 |
| Allergic rhinitis | 0.015 | 4.804 | 1.351 | 17.081 |
Values given in bold indicate p < 0.05.
Our study provides novel insights into the differences between children with FAR and those with symptomatic FA. We observed higher expression of GARP in children with remission of FA compared to children without FAR. Children with FAR and food-specific IgE in serum had higher expression of GARP compared to children with FAR with low food specific IgE (<0.35kU/L). Further analysis showed that the optimal cut-off for GARP prediction of the remission of FA is 20.1%. Finally, keeping pets at home increased, and allergic rhinitis decreased, the possibility of FAR in school children with high GARP expression in our patients.
Allergic rhinitis was a strong predictor of asthma persistence into adulthood,1,7,8 and was also a predictor of FA persistence in our children. The expression of GARP in both groups (remission vs. control group) was different and much higher in the remission group, which suggests that natural remission of clinical symptoms of FA in children is related to immunoregulatory processes. hGARP however, was less related to FAR in the presence of allergic rhinitis, and much more related in the presence of pets at home, which suggests its possible role in the regulation of peripheral tolerance.
In our study, children with FAR and food-specific IgE in serum had higher expression of GARP compared to children with FAR with low food specific IgE (<0.35kU/L). We hypothesized that GARP binding latent TGF- β (transforming growth factor-β) modulates peripheral tolerance and is therefore related with remission of clinical symptoms of FA in children with the presence of IgE in the time of remission. Many studies addressed predictors of remission or persistence of FA in early childhood through adulthood.1,9 Immunoregulatory parameters related to the outcome of childhood FA are widely discussed, however they are not yet well established.6,10 Clinical observations in the present study were surprising. The presence of allergic rhinitis in school children correlated with hGARP, however it lowered its effect on food remission. Conversely, the presence of pets at home increased this effect of GARP. Studies showed that glycoprotein A repetitions predominant (GARP) has strong anti-inflammatory and regulatory effects on human cells in vitro as well as in vivo and is involved in the regulation of peripheral tolerance,4,11,12 playing an essential role in mediating suppressive effects of Treg.4 Moreover, treatment with sGARP reduced airway hyperresponsiveness (AHR), influx of neutrophils and macrophages into the bronchoalveolar lavage (BAL), and human CD45(+) cells in the lungs.4 Authors showed that sGARP had a synergistic effect together with Treg by preventing inflammation.4 In our study (data not published), we showed a higher expression of GARP positive cells in patients without AR, which stays in agreement with our present study.
Our study has some limitations. We recognize that FA in childhood is a heterogeneous phenotype, however, our study was not powered to differentiate various phenotypes, but only to characterize factors that contribute to FA persistence or remission. The study limitation is that no control group was included. Additionally, we did not perform oral food challenge in our subjects; they were diagnosed based on serum IgE level and clinical symptoms. Another limitation is that GARP expression before reaching food tolerance is unknown.
This study is the first showing that high expression of GARP (>20.1%) is related with FAR in school children. We revealed that allergic rhinitis, and the presence of pets at home modify this effect of GARP. It is clearly visible that children with allergic rhinitis have less chance of developing remission despite maintaining immune tolerance (hGARP); it is quite the opposite in the event of having pets at home. We can hypothesize that the modification of immune regulation in food allergic children takes place under different interactions. Our results suggest that natural remission of clinical symptoms of FA in children is related to immunoregulation processes. The underlying mechanisms for the tolerance and remission/relapse of clinical symptoms remain unclear, and future research should therefore integrate standardized molecular approaches in identical ways in both pediatric and adult populations and in longitudinal studies.1
Financial informationThis study was funded by grant 503/2-056-01/503-01 and 502-03/2-056-01/502-14-311 from the Medical University of Lodz, Poland.