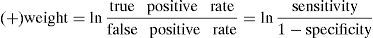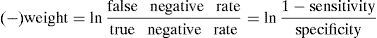To use probability theory to establish threshold values for total serum IgE and eosinophil counts that support a diagnosis of allergic rhinitis and to compare our results with previously published data.
MethodsProspective study of rhinitis patients using a modified version of Bayes’ theorem. Study included 125 patients at the West Los Angeles VA Medical Center diagnosed with rhinitis who completed allergy consultation and immediate hypersensitivity skin testing.
ResultsEighty-nine of 125 patients were atopic by prick and/or intradermal skin testing. Using a modified version of Bayes’ theorem and positive and negative probability weights, calculations for different thresholds of serum IgE and eosinophil counts were summated and a posttest probability for atopy was calculated. Calculated posttest probabilities varied according to the threshold used to determine a positive or negative test; however, IgE thresholds greater than 140IU/ml and eosinophil counts greater that 80cells/ml were found to have a high probability of predicting atopy in patients with rhinitis. Moreover, IgE had a greater influence than eosinophil count in determining posttest probability of allergy in this population. Considerable differences were noted in the IgE levels of atopic and non-atopic patients, including those with asthma or a history of smoking. However, these differences were not observed with eosinophil levels.
ConclusionsUsing a modified version of Bayes’ theorem to determine posttest probability, IgE threshold levels greater than 140IU/ml and eosinophil counts greater than 80cells/ml in an individual with clinical signs and symptoms of rhinitis are likely to correlate with an atopic aetiology. This model of probability may be helpful in evaluating individuals for diagnostic skin testing and certain types of allergy-modifying treatment.
Many investigators have used total serum immunoglobulin E (IgE) and eosinophil count for evaluating allergic disease.1–4 While attempts have been made to define normal/abnormal thresholds for each,5,6 there is no consensus in the literature for a given threshold value of IgE or eosinophil count that confirms atopic disease. Currently, there is great interest in the measurement of total IgE levels given the advent of omalizumab, a recombinant DNA-derived humanised IgG1κ monoclonal antibody against human IgE. Clinical studies with omalizumab required patients to have a baseline IgE between 30 and 700IU/ml.7–10 Total IgE levels can be affected by race,11 smoking history,12–15 age,16–18 season,19 and non-atopic disease.20–22 To confirm the presence of atopy, physicians have relied on serum antigen specific IgE testing, prick or intradermal skin testing.23,24 In this study we have used probability theory to evaluate total serum IgE and eosinophil count as factors for predicting the likelihood of atopy in rhinitis.
Bayes’ theorem calculates the probability of disease after performing a test, given the test's sensitivity, specificity, and estimated probability of disease before the test.25 A modified version of Bayes’ theorem introduced by Rembold and Watson combines a test's sensitivity and specificity into a single value known as a “weight”.26 Positive tests (i.e. tests whose values exceed a given threshold) will have positive weights and negative tests (i.e. tests whose values are less than a given threshold) will have negative weights. Since positive weights indicate an increase in the likelihood of disease and negative weights suggest a decreased likelihood of disease, the greater the weight (positive or negative), the greater the influence in predicting the likelihood of disease.
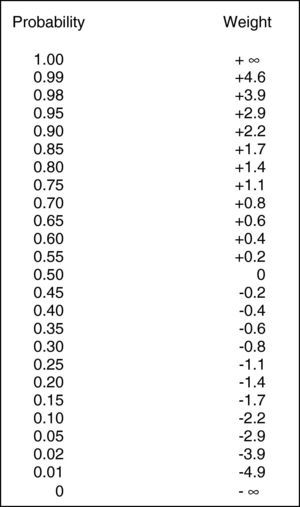
The present study uses this modification of Bayes’ theorem to establish practical thresholds (upper limits of normal) for serum IgE and eosinophil counts for atopy in allergic rhinitis. By altering threshold values using computer-assisted analysis, positive and negative weights were calculated from an array of thresholds and graphed as threshold versus weight. The main advantage of this process is that the sum of the weight factors can be converted to a posttest probability using a simple conversion table (Fig. 1). Thus, for allergic rhinitis, two factors (IgE and eosinophil count) can be taken into consideration to screen or determine whether atopy is likely to be present.
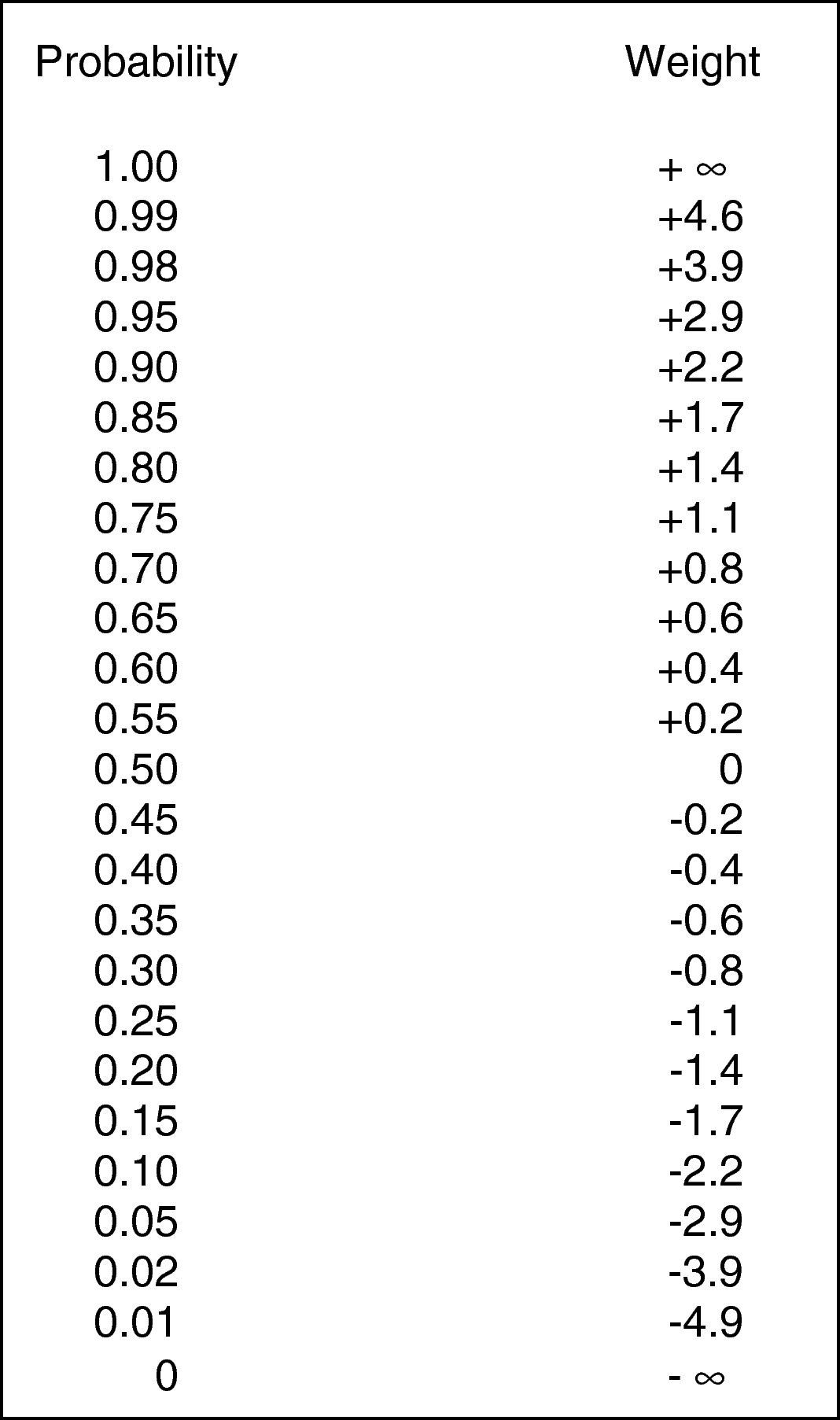
A statistical calculation of probability and weight. This figure is used to convert positive and negative weights into posttest probabilities (expressed in percentage probability). Note that the scale is non-linear (taken from Rembold and Watson26).
Three hundred and fifty-eight consecutive patients were referred to the Allergy/Immunology clinic at the West Los Angeles VA Medical Center over a 24-month period. One hundred and eighty-four of 358 patients (51%) were diagnosed with rhinitis based on a history of seasonal or perennial symptoms with exposure to common allergens. One hundred and twenty-five of 184 (67%) completed immediate hypersensitivity skin testing using a prick and/or graded intradermal technique to determine the presence of atopy. The criteria for atopy were as follows: (1) a positive prick test with any routine or screening antigen; (2) positive intradermal test at 1:10,000 dilution (or greater) with two or more routine antigens; and (3) a positive intradermal test at 1:10,000 dilution (or greater) with any screening antigen. A positive reaction was defined according to previously described criteria.27 Atopy was defined as a positive skin test reaction and a correlating clinical history. Rhinitis patients with skin test results (n=125, 89 atopic, 36 non-atopic) and either serum IgE (n=117), eosinophil count (n=110), or both were included in the data analysis. Geometric mean values were calculated for serum IgE and eosinophil counts.
Data analysisA test's sensitivity and specificity are defined as follows28:
Moreover, according to the modified version of Bayes’ theorem,26 the positive and negative weight of a test is determined from the sensitivity and specificity as follows:
Using these definitions, the more positive the weight, the greater the likelihood that the disease is present; the greater the negative weight, the greater the likelihood that the disease is not present.However, because sensitivity and specificity vary according to the threshold (upper limit of normal) of a test, the weight of a test will also vary with the threshold. A computer-assisted analysis was used to calculate positive and negative weights from an array of thresholds and the results were graphed as threshold vs. weight.
ResultsOne hundred and eighty-four of 358 (51%) patients diagnosed with rhinitis after initial evaluation were scheduled for skin testing and 125 of 184 (67%) patients completed skin testing to determine the presence of atopy. Eighty-nine of 125 (71%) patients were atopic, of whom 83 were male and 6 female (Table 1). Sixty-eight of 89 (76%) atopic patients had a history of smoking (past or current) and 34 of 89 (38%) had asthma. For the non-atopic patient group, 27 of 36 (75%) had a history of smoking and 10 of 36 (27%) had asthma. The mean age of patients who had skin testing was 57 years with a range of 21 to 87. There were no significant differences noted between atopic and non-atopic patients, nor between the subgroups of patients with a history of smoking or asthma, with regard to distribution of age.
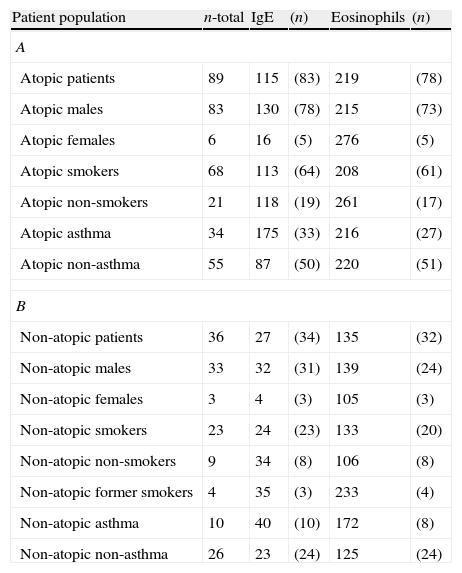
Geometric means of IgE and eosinophils for atopic and non-atopic patients with rhinitis.
| Patient population | n-total | IgE | (n) | Eosinophils | (n) |
| A | |||||
| Atopic patients | 89 | 115 | (83) | 219 | (78) |
| Atopic males | 83 | 130 | (78) | 215 | (73) |
| Atopic females | 6 | 16 | (5) | 276 | (5) |
| Atopic smokers | 68 | 113 | (64) | 208 | (61) |
| Atopic non-smokers | 21 | 118 | (19) | 261 | (17) |
| Atopic asthma | 34 | 175 | (33) | 216 | (27) |
| Atopic non-asthma | 55 | 87 | (50) | 220 | (51) |
| B | |||||
| Non-atopic patients | 36 | 27 | (34) | 135 | (32) |
| Non-atopic males | 33 | 32 | (31) | 139 | (24) |
| Non-atopic females | 3 | 4 | (3) | 105 | (3) |
| Non-atopic smokers | 23 | 24 | (23) | 133 | (20) |
| Non-atopic non-smokers | 9 | 34 | (8) | 106 | (8) |
| Non-atopic former smokers | 4 | 35 | (3) | 233 | (4) |
| Non-atopic asthma | 10 | 40 | (10) | 172 | (8) |
| Non-atopic non-asthma | 26 | 23 | (24) | 125 | (24) |
The units for IgE are in IU/ml and for eosinophils are in cells/ml. The (n) refers to the number of patients for whom data were available.
Total serum IgE was measured in 83 atopic patients and 36 non-atopic patients. The mean IgE level in atopic patients was 115IU/ml and the mean IgE level in the non-atopic patients was 27IU/ml. Atopic patients with asthma had the highest mean IgE (175IU/ml) and female non-atopic patients had the lowest mean IgE (4IU/ml). Atopic male patients had a mean IgE of 130IU/ml compared to atopic female patients with a mean IgE of 16IU/ml; non-atopic males had a mean of 32IU/ml whereas non-atopic females had a mean of 4IU/ml. Differences were also noted in the mean IgE of atopic and non-atopic current smokers, 113IU/ml and 24IU/ml, respectively.
Eosinophils were measured in 78 of 89 atopic patients and 32 of 36 non-atopic patients. The mean eosinophil count in the atopic patients was 219cells/ml and in the non-atopic patients 135cells/ml. The highest and lowest mean values of 276 and 105cells/ml were seen in the atopic and non-atopic female patients respectively. Unlike the case with the IgE level, no significant differences in the eosinophil count were noted in the atopic/non-atopic subgroups with regard to gender or smoking. However, non-atopic patients with asthma did tend to have higher counts than non-atopic patients without asthma (172cells/ml vs. 125cells/ml).
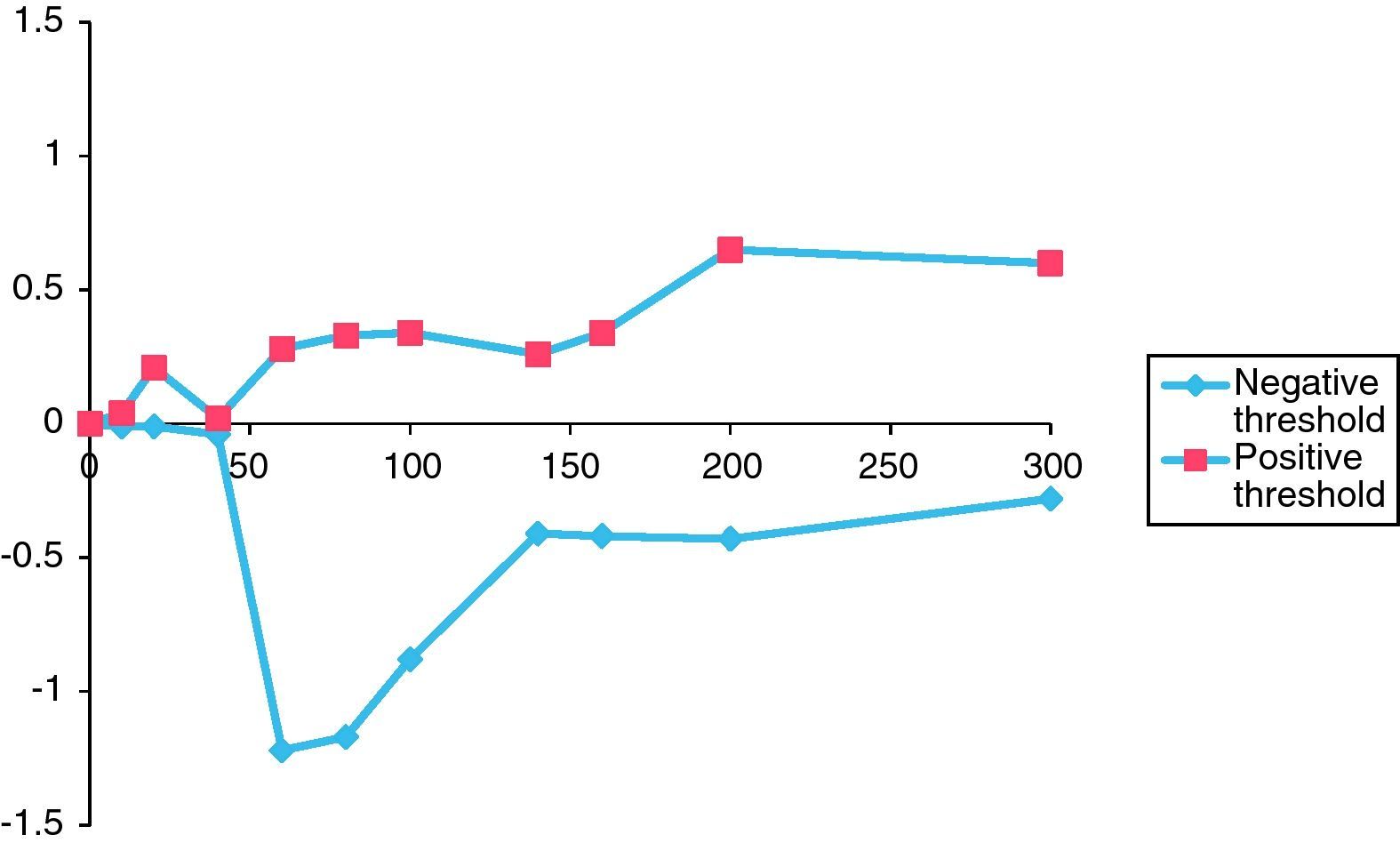
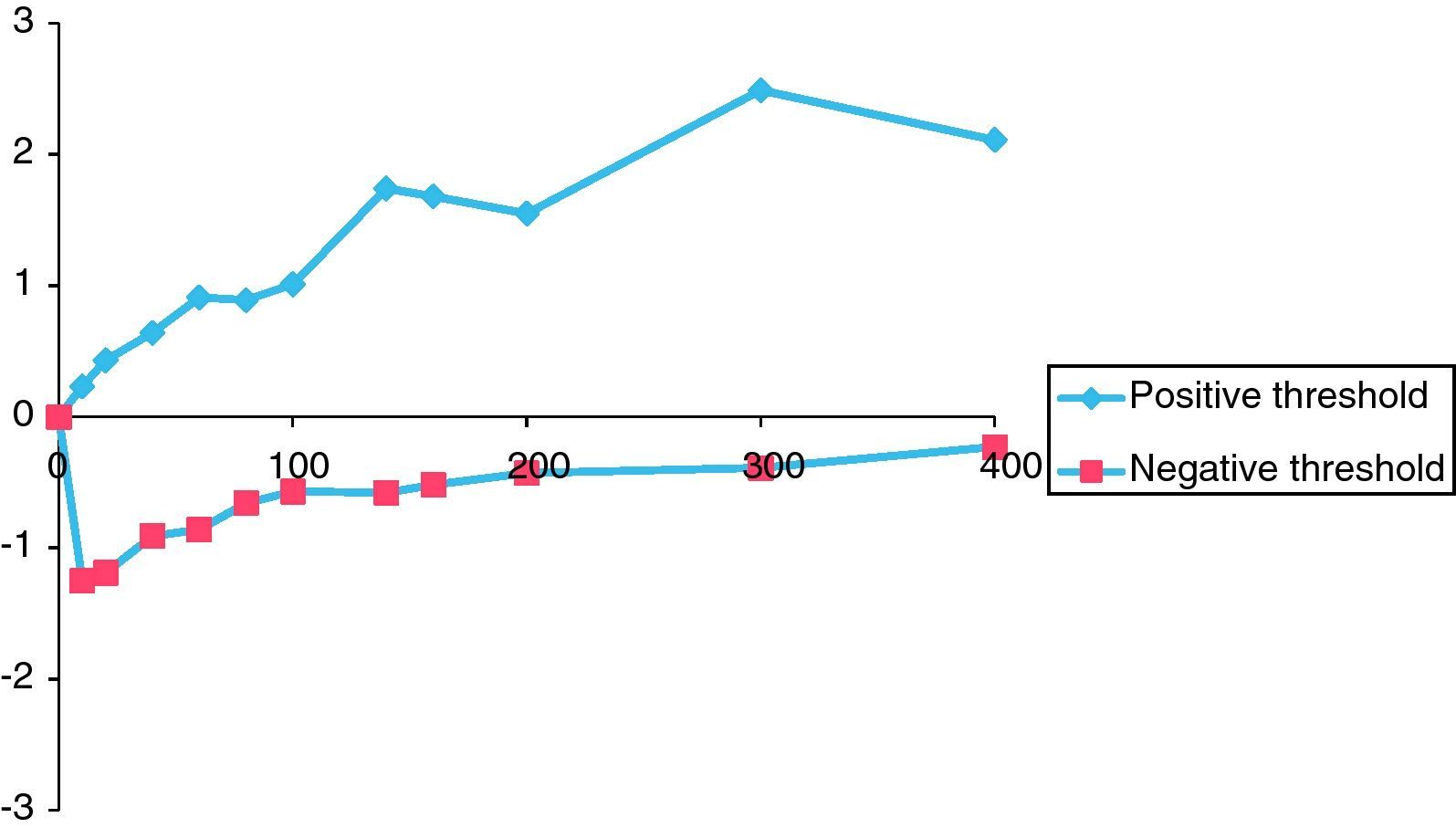
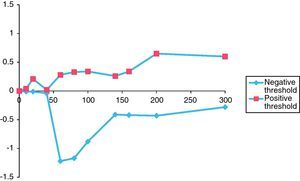
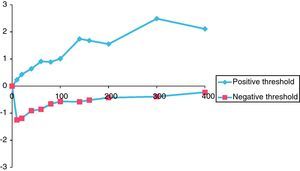
Figs. 2 and 3 graphically depict the calculated positive and negative weights for a range of selected thresholds of eosinophils and IgE levels. For both curves, as the thresholds increase the positive weight becomes more positive and the negative weight becomes less negative. Thus, as the eosinophil and IgE thresholds increase, so does the likelihood of atopic disease. Moreover, beyond an eosinophil count of 300cells/ml and an IgE of 400IU/ml, the positive weight becomes infinitely large, indicating that the probability of atopic disease beyond these thresholds is virtually 100%.
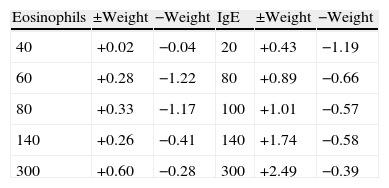
For eosinophils (Fig. 2), there is not much difference in the positive or negative weights until the threshold reaches 60cells/ml. The largest difference between positive and negative weights was found at thresholds of 60cells/ml (positive weight=0.28, negative weight=−1.22, difference=1.50) and 80cells/ml (positive weight=+0.33, negative weight=−1.17, difference=1.50) (Table 2).
Positive and negative weights for randomly selected values of IgE (IU/ml) and eosinophils (cells/ml).
| Eosinophils | ±Weight | −Weight | IgE | ±Weight | −Weight |
| 40 | +0.02 | −0.04 | 20 | +0.43 | −1.19 |
| 60 | +0.28 | −1.22 | 80 | +0.89 | −0.66 |
| 80 | +0.33 | −1.17 | 100 | +1.01 | −0.57 |
| 140 | +0.26 | −0.41 | 140 | +1.74 | −0.58 |
| 300 | +0.60 | −0.28 | 300 | +2.49 | −0.39 |
Fig. 3 shows a similar but more pronounced trend for increasing thresholds of IgE when compared to the curve in Fig. 2. The largest differences between the positive and negative weights were found at IgE thresholds of 140IU/ml (positive weight=+1.74, negative weight=−0.58, difference=2.32) and 300IU/ml (positive weight=+2.49, negative weight=−0.39, difference=2.88) (Table 2).
DiscussionThe distribution of male and female patients was consistent with that expected for a VA population. As such, the number of female patients was significantly reduced (six atopic and three non-atopic). The average age of our patients (57 years) must also be taken into account as serum IgE has been reported to be higher in younger aged groups.29,30
Nonetheless, IgE levels obtained from our general and subset patient populations were not different from what has been previously published. For example, non-atopic patients’ serum IgE level of 27IU/ml was quite similar to that obtained by Burrows et al. for patients without asthma (26IU/ml) and Droste et al.’s value of 25IU/ml in patients without allergic rhinitis.31,32 Similarly, Klink et al. reported a value of 20IU/ml in normal males, age 15–74 and Simoni et al. reported a value of 19IU/ml in females, and 29IU/ml in males ages 8–78.33,34 Moreover, our average value of 115IU/ml obtained for atopic patients was in agreement with that published by Haahtela et al. for skin test positive patients (116IU/ml),35 but somewhat lower than the values of 142IU/ml reported by Hetman et al.,36 204IU/ml by Campos et al.,37 225IU/ml reported by Mullarkey et al.,38 and 326IU/ml reported by Bousquet et al.39 Interestingly, however, when Hetman's data was separated by age, mean values of 103IU/ml and 74IU/ml were obtained for the 41–50-year age group and 51–60-year age group, respectively,36 and atopic rhinitis patients over the age of 40 had a mean IgE of 94IU/ml in Wittig's collection of data.31 It is therefore most likely that the differences in our reported mean values are due to age. With regard to patients with asthma, our atopic asthma patients had a mean IgE of 175IU/ml and our non-atopic asthmatics had an IgE of 40IU/ml. Burrows et al. reported that asthma subjects in the age range of 35–54 had an average IgE of 117IU/ml with 72% having positive skin tests and in the 55 and older group, the mean IgE was 56IU/ml with 39% having positive skin tests.31 Finally, in Wittig's study of IgE levels in asthmatic patients, the mean IgE for patients over the age of 40 was noted to be 172IU/ml, the majority of whom were known to have previous evidence of atopy.40
The influence of cigarette smoking on IgE levels was also evaluated in our study. In the non-atopic non-smoking, smoking and ex-smoking populations, the mean IgE levels in our patients were 34IU/ml, 24IU/ml and 35IU/ml, respectively. In the atopic population, patients who smoked had a mean IgE of 113IU/ml and non-smokers 118IU/ml. This is in agreement with other studies in which smokers tended to have higher IgE levels than non-smokers.41 It has been theorised that smokers may have a higher than average antigenic load due to tobacco smoke or have an increased colonisation of the airways with microorganisms that could stimulate an IgE response.42
Our data also show that eosinophil counts are consistently higher in the atopic population and this trend was not influenced by gender, the presence of asthma, or whether or not the individual smoked. Although the significance of blood eosinophilia is disputed, this trend has been reported by others.43–45 In normal patients with no skin reactivity and in asymptomatic patients with positive skin reactivity, Felarca and Lowell found mean eosinophil counts of 100cells/ml and 202cells/ml respectively.46 These figures are in agreement with our non-atopic (eosinophils=135cells/ml) and atopic (eosinophils=219cells/ml) populations.
The thresholds of 140IU/ml for serum IgE level and 80cells/ml for eosinophil count were chosen because it was at these points on the graphs that the curves made their largest initial divergence and where the largest difference between the positive and negative weights occurred (Figs. 2 and 3). In the ideal situation, as the threshold value is approached, the positive weight becomes more positive and the negative weight less negative. Furthermore, beyond the threshold point, the positive weight should become infinitely positive (i.e., the probability of the disease being present approaches 100%). This trend is certainly seen for the IgE data presented in Fig. 3. Beyond the threshold of 140IU/ml, the positive weights tend to become infinitely positive and the negative weights approach zero. The threshold value of 140IU/ml for atopy is not radically different from what has been previously published. For example, Grater et al. performed 5500 IgE measurements in 7000 referred patients and determined that IgE levels greater than 80IU/ml should be the minimum level required to confirm atopy.47 In addition, in two independent studies, Mullarkey and Zetterstrom et al. reported strong evidence for the presence of atopic disease when IgE values were greater than 100IU/ml.48,49 Hetman et al. found a preponderance of atopic individuals with IgE levels greater than 120IU/ml,36 and Haahetela et al. concluded that IgE values greater than 160IU/ml suggest atopy.35 This trend is not seen as clearly with the data obtained for eosinophils. There is not the large divergence as seen with the IgE data in Fig. 3. The largest difference between the positive and negative weights is 1.50 and is seen at both the 60 and 80cells/ml threshold. This finding is somewhat surprising since the mean eosinophil count in our atopic and non-atopic populations was considerably higher than either of these values (Table 1). Nonetheless, the 80cells/ml threshold was chosen since the positive weight was greater (0.33 vs. 0.28) and the negative weight less (−1.17 vs. −1.22) (Table 2). Unlike mean IgE, there are few reports in the literature that estimate a threshold for eosinophils to suggest atopy. Using the data from Table 2, we randomly summated eosinophil counts and IgE levels and calculated the posttest probability for atopy. The posttest probability for atopy was greatest when both IgE and eosinophil counts exceeded the threshold and lowest when neither exceeded the threshold value. We also determined that IgE levels had a greater influence than eosinophil counts in determining posttest probability for atopy in this veteran population.
Recent novel therapies using monoclonal anti-IgE antibody such as omalizumab in effect utilise threshold values for allergic disease based on total serum IgE level. Using probability theory in our VA population, a total IgE level of 140IU/ml may be a useful threshold as a preliminary screen for allergic disease. We are not aware of any other studies that have used a modified Bayes’ theorem to determine the presence of atopy and suggest that additional trials be conducted in allergic populations with a different prevalence of disease.
This study demonstrates that IgE levels greater than 140IU/ml and eosinophil counts greater than 80cells/ml are suggestive of an atopic aetiology for patients with signs and symptoms of rhinitis. Our data were collected from a referred population where the pretest probability of the disease (i.e., prevalence of atopy) was quite high (70%). Since this has a great influence in determining posttest probability, it is suggested that IgE and eosinophil counts may have an even greater influence in a population with a lower pretest probability of disease. Furthermore, although this study was conducted in a veteran population where the number of younger patients and female patients was quite small, our data were in general agreement with previous reports. However, caution should be used when applying them to the population as a whole since others have reported that both age and sex may influence IgE levels and eosinophil counts. Nevertheless, these data do suggest that IgE levels and eosinophil counts are important factors in the evaluation of the atopic patient, with IgE having the greater impact in determining posttest probability of atopy.
Conflict of interestDr. Rumbyrt is currently on the speaker's bureau for GlaxoSmithKline, Merck, Astra Zeneca and has received research support from GlaxoSmithKline and Schering AG. Dr. Gowda is on the speaker's bureau for GlaxoSmithKline and Meda Pharmaceuticals and has received clinical research support from Novartis.
A posttest probability is determined from a posttest weight using Fig. 1. A posttest weight is calculated by sequentially adding the weights of any/all tests to the pretest weight. The pretest weight is determined from the pretest probability of the disease in question (i.e., the prevalence of disease in the population being studied). In this series, the pretest probability (prevalence of disease in the population being studied) was 70% (89 atopic patients/125 patients skin tested for atopy). The test weight for given thresholds of IgE and eosinophils were added to the pretest weight, and the sum (posttest weight) converted to a posttest probability for atopy using Fig. 1. The weight of a test (positive or negative) was chosen based on whether or not the observed test value exceeded a selected threshold. In other words, if a threshold value “x” is chosen, and the observed test value exceeds “x” the positive weight is used in the determination of posttest probability. If the observed value is less than “x”, the negative weight is used. This five-step process if summarised as follows.
- 1.
Estimation of pretest probability (=prevalence of disease in population).
- 2.
Conversion of pretest probability to a pretest weight (Fig. 1).
- 3.
Selection of positive and negative test weight depending on test threshold.
- 4.
Calculation of posttest weight:
Posttest weight=pretest weight+test 1 weight+test 2 weight + ⋯.
- 5.
Conversion of posttest weight to a posttest probability (Fig. 1).