Although hazelnut consumption is very high in Turkey, the prevalence of hazelnut allergy is still unknown. This study's objective was to investigate the prevalence of hazelnut sensitisation and to verify its clinical importance using double-blind, placebo-controlled challenge (DBPCFC) in an adult population.
MethodsPrick-to-prick skin tests were performed with fresh hazelnut in 904 patients admitted to the allergy department. Among the 904 subjects, 20 patients with a history of allergic reactions to hazelnut and/or positive skin tests were recalled for further evaluation. Specific IgE was measured in these subjects. Eleven (11/20) patients accepted to undergo DBPCFC with hazelnut.
ResultsAmong the 904 individuals, the history of reactions to hazelnut was positive in 16 subjects (1.8%); prick-to-prick skin tests were positive in 13 (1.4%); prick tests with the commercial product were positive in nine (0.9%); and history plus skin tests were positive in 16 (1.8%). Specific IgE to hazelnut was positive in only three patients. DBPCFC was conducted in 11 subjects with a positivity rate of 63.6% (7/11). We observed six mild and one moderate systemic reactions during the DBPCFC. Among seven subjects with a positive DBPCFC, six (85.7%) had a history of hazelnut allergy, and five (71.4%) had both history and skin test positivity.
ConclusionSkin test sensitisation to hazelnut was found to be 1.76% (16/904) which is similar to the sensitisation rate in previous reports. However, DBPCFC was positive in 63% of cases with a history of hazelnut allergy and/or positive skin tests in this study. These results indicate that the presence of history with a positive skin test can be suggestive of hazelnut allergy; however an oral food challenge is needed to confirm the diagnosis.
Allergic reactions induced by food are characterised by clinical manifestations such as anaphylaxis, urticarial angio-oedema, rhinitis and asthma, flare-up of atopic eczema, gastrointestinal symptoms, and oral allergy syndrome (OAS).1 Food allergy is an important health problem and there is increasing evidence that the prevalence of food allergies is increasing in parallel to the other forms of atopic disease.2–8 A “2008 Centers for Disease Control and Prevention Report” indicated an 18% increase in childhood food allergy from 1997 to 2007, with an estimated 3.9% of children currently affected.9
The food stuffs which are responsible for most allergic reactions in adults are peanuts, tree nuts, fish, and shellfish.10 There are also many others that are known to cause allergy, depending on the geographical region (e.g. celery, kiwi fruit and rice, etc.).11,12 The actual prevalence of food allergy is not well known. Most of the investigations assessing the prevalence of food allergy have focused on paediatric populations.13–18 However, similar data are scarce for adults and the rate of perceived adult food allergy shows great variability between countries (e.g. Spain 4.6%, Australia 19.1%).19 While Woods et al. have found that 1.3% of adults in Australia were consistently sensitised to food and perceived adverse reactions to the same allergen; perceived hypersensitivity reaction to peanut and tree nut was reported to be observed in 1.1% of the population in USA.20,21Hazelnut is also a common food which is frequently implicated in severe anaphylactic reactions. In Denmark, hazelnut allergy was recently reported at 6.6% in population of young adults.22 Although hazelnut production and consumption is very high in our country, the prevalence of hazelnut allergy is still unknown in the adult population. Most studies of food allergy in adults were case reports which describe anaphylactic reactions after ingestion of a specific food, or retrospective reports based on clinical history supported by positive allergy skin testing, and in vitro studies. Although double-blind, placebo-controlled food challenge (DBPCFC) is the gold standard for the diagnosis of food allergy, few reports exist in which DBPCFC was used.1,23–30
Therefore, the objective of this study was to investigate the prevalence of hazelnut sensitisation based on DBPCFC in adult patients who attended an outpatient allergy clinic.
MethodsPatient selection and study designA total of 904 patients who attended the outpatient allergy clinic with a complaint such as cough, sneezing, itching, nasal obstruction, shortness of breath, and fatigue were randomly selected to be included in the study at Ankara University, Medical School, Department of Allergy, between 2001 and 2003. The mean age of the patients was 35.2±14.9 years (range: 13–72 years), 631 females and 273 males.
In the first phase of the study a detailed history of allergy and physical examination were followed by skin prick tests (SPTs) with commercial extracts of hazelnut and prick-to-prick skin tests with fresh hazelnut. Among this patient population subjects with a history of allergic reactions to hazelnut and/or positive skin tests with hazelnut were called back for further evaluation. In the second phase patients with either skin test positivity to hazelnut or clinical history of hazelnut allergy or both underwent DBPCFC with hazelnut to confirm the diagnosis of food hypersensitivity. Specific IgE was also measured in these selected subjects.
Skin testsSkin prick test were performed using either a commercial extract (Stallergèns, France) or fresh hazelnut. The prick-to-prick technique was used for the fresh fruit according to the Dreborg and Foucard method.31 All patients were also tested with a standardised panel (Stallergèns, France) of airborne allergens including Dermatophagoides pteronysinus and Dermatophagoides farinae; grass, tree pollens (alder, birch, hazel), and weed pollens; moulds, and cat and dog allergens. Histamine dihydrochloride (10mg/ml) and glycerol diluent were used as positive and negative controls, respectively. A wheal size larger than 3mm or greater than that produced by the control solution was considered a positive reaction.
In vitro testsTwenty patients who had a positive history and/or skin test positivity to hazelnut were tested for specific IgE antibodies for hazelnut. Allergen-specific IgE antibodies to hazelnut were measured by the UniCAP system according to the manufacturer's instructions (Pharmacia; Sweden). Results equal to or greater than class II (IgE level of ≥0.7kU/ml) were considered positive according to the instructions of the manufacturer.
Challenge testingDBPCFCHazelnut sensitivity was evaluated by DBPCFC in 11 patients who declined the informed consent. Nine patients refused the challenge test because they did not have time. DBPCFCs were carried out at the hospital between November 2002 and March 2003 in Ankara, as previously described. The challenge meals were prepared in the form of pudding. The test pudding included 20g of hazelnut, 100ml of water, 15g of sugar, 50ml of peppermint syrup, 10g of rice flour and one tablespoon of rice grains according to the Ortolani et al. method.1 The placebo meals consisted of the same ingredients except hazelnut. Apart from the hazelnut, all ingredients were known to be tolerated by each patient.
DBPCFC procedureOn the first test day, patients were given the placebo pudding, the second day they ate pudding containing 20g of hidden hazelnut. The test meal was given in gradually increasing doses, beginning with an initial dose of 2g. The dose was doubled every 15min up to a final dose of 20g, and the test was finalised at the end of 3h. The patients were under constant observation during the test. Before administering each dose, the oral cavity and skin were carefully inspected for allergic reactions. The challenge was stopped at the appearance of cutaneous, respiratory, digestive, or cardiovascular symptoms. The severity rating of the reactions observed to challenge was adapted from Bock et al.; scores of 0 (none), 1 (mild), 2 (moderate) and 3 (severe) were coded as none to severe.32
AnalysisStatistical analysis was performed by Statistical Package for Social Sciences (SPSS, v. 11.0 for Windows, Chicago, IL, USA). Data are expressed as means±SD or percentages of the positivity of history, SPT, specific IgE, and DBPCFC to hazelnut. Comparison of pollen sensitisation between patients with hazelnut allergy and controls (no history, negative SPT) was analysed by Fisher Chi-square test. Difference associated with p<0.05 was considered statistically significant.
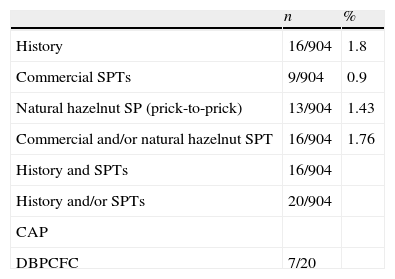
ResultsStep 1Clinical history of reaction to hazelnut was positive in 16 subjects (1.8%, 16/904). Among these patients, four subjects experienced urticaria, angio-oedema, or both; three subjects had urticaria and rhinitis; two subjects had rhinitis; four subjects described OAS; one subject had OAS and urticaria; one subject had dyspnoea and one subject had abdominal discomfort. Skin prick tests with commercial hazelnut extract were positive in nine subjects (0.99%, 9/904), prick-to-prick skin tests with natural hazelnut were positive in 13 patients (1.43%, 13/904). Prick and/or prick- to- prick were positive in 16 patients (1.76%, 16/904).
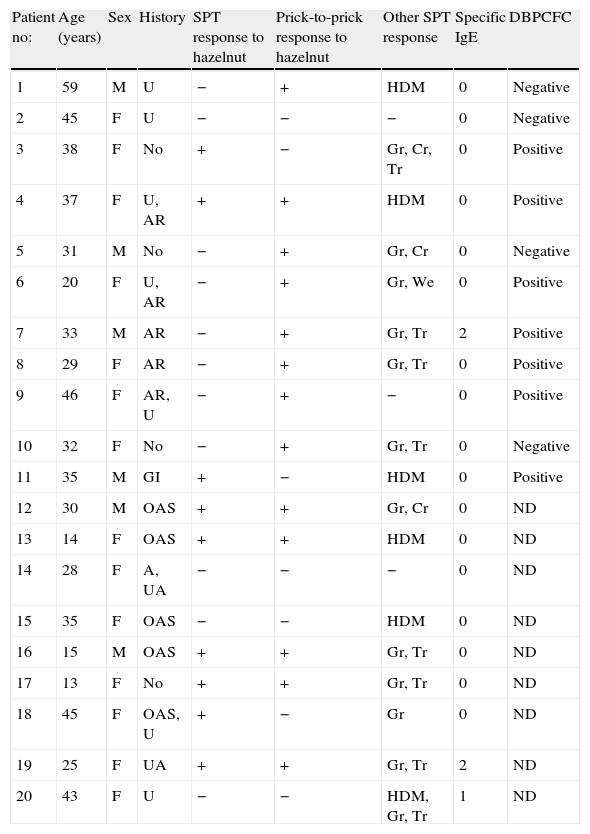
Step 2Selection of phase two patientsAmong 904 patients, 20 subjects had positive clinical history and/or positive skin tests with hazelnut. The mean age was 32.6±11.89 years (range: 13–59 years; 6 males and 14 females). The characteristics of phase 2 patients are shown in Table 1.
Demographic, clinical, and immunological characteristic of the study patients with positive history and/or SPTs.
| Patient no: | Age (years) | Sex | History | SPT response to hazelnut | Prick-to-prick response to hazelnut | Other SPT response | Specific IgE | DBPCFC |
| 1 | 59 | M | U | − | + | HDM | 0 | Negative |
| 2 | 45 | F | U | − | − | − | 0 | Negative |
| 3 | 38 | F | No | + | − | Gr, Cr, Tr | 0 | Positive |
| 4 | 37 | F | U, AR | + | + | HDM | 0 | Positive |
| 5 | 31 | M | No | − | + | Gr, Cr | 0 | Negative |
| 6 | 20 | F | U, AR | − | + | Gr, We | 0 | Positive |
| 7 | 33 | M | AR | − | + | Gr, Tr | 2 | Positive |
| 8 | 29 | F | AR | − | + | Gr, Tr | 0 | Positive |
| 9 | 46 | F | AR, U | − | + | − | 0 | Positive |
| 10 | 32 | F | No | − | + | Gr, Tr | 0 | Negative |
| 11 | 35 | M | GI | + | − | HDM | 0 | Positive |
| 12 | 30 | M | OAS | + | + | Gr, Cr | 0 | ND |
| 13 | 14 | F | OAS | + | + | HDM | 0 | ND |
| 14 | 28 | F | A, UA | − | − | − | 0 | ND |
| 15 | 35 | F | OAS | − | − | HDM | 0 | ND |
| 16 | 15 | M | OAS | + | + | Gr, Tr | 0 | ND |
| 17 | 13 | F | No | + | + | Gr, Tr | 0 | ND |
| 18 | 45 | F | OAS, U | + | − | Gr | 0 | ND |
| 19 | 25 | F | UA | + | + | Gr, Tr | 2 | ND |
| 20 | 43 | F | U | − | − | HDM, Gr, Tr | 1 | ND |
U: urticaria; UA: urticaria, angio-oedema; AR: allergic rhinitis; A: asthma; OAS: oral allergy syndrome; GI: gastrointestinal symptom; Gr: grasses, Cr: cereals, Tr: trees pollens (alder, birch, hazel); We: weed; HDM: house dust mite. ND: not done.
Seventeen patients (85%, 17/20) were also allergic to common inhaled allergens; 12 (60%) had pollen allergy (12 to grasses, 8 to Birch, Alder and Hazel, 3 to Cereales, 1 to Pariateria) and 6 (30%) to house dust mites (HDM). Three patients were mono-sensitised to hazelnut.
There was no significant difference in the rate of pollen sensitisation between hazelnut allergic subjects and controls (history and SPT to hazelnut were negative) (p=0.285).
Specific IgE to hazelnut was only positive in 3 of 20 patients (15%).
DBPCFC with hazelnutAmong the 20 subjects with positive history and/or skin prick tests 11 patients accepted to undergo DBPCFC with hazelnut. DBPCFC was positive in 63.6% of patients (7/11). Six patients were observed to have mild (occasional scratching, rare burst, some blockage or occasional sniffles) systemic reactions and one patient experienced a moderate reaction (>2min each time of continued scratching, intermittent nose rubs and blocked nostrils). None of the patients had a severe reaction during the challenge test.
Among the seven patients with positive DBPCFC, five were allergic to pollens. Three subjects in this group were only sensitive to birch. Among seven subjects with a positive challenge test six (85.7%) had a history of hazelnut allergy and five (71.4%) had both history and skin test positivity.
The results of patient history, commercial and natural hazelnut SPTs, CAP for hazelnut and DBPCFC are summarised in Table 2.
Positivity rate of history, SPT with commercial hazelnut extract and natural hazelnut, CAP and DBPCFC tests.
| n | % | |
| History | 16/904 | 1.8 |
| Commercial SPTs | 9/904 | 0.9 |
| Natural hazelnut SP (prick-to-prick) | 13/904 | 1.43 |
| Commercial and/or natural hazelnut SPT | 16/904 | 1.76 |
| History and SPTs | 16/904 | |
| History and/or SPTs | 20/904 | |
| CAP | ||
| DBPCFC | 7/20 |
Although hazelnut is one of the most frequent allergic-reaction-creating foods, prevalence of hazelnut allergy is not exactly known. It has been reported that 0.5% of adults in the American population have allergy to hazelnut.25 Sicherer et al. have reported tree nut allergy prevalence for adults to be 0.7%,3 while for Schafer et al. the ratio was even higher (11.3%).26 During the last two decades, although it has been reported that there have been several cases of serious systemic reaction to hazelnut – defined by history and skin tests33–36 – there are very few studies in which hazelnut allergy is evaluated by DBPCFC, which is accepted as the gold standard for diagnosing food allergies. Following an earlier study which demonstrated that hazelnut could cause allergic reactions such as urticaria and asthma by the use of DBPCFC,37 Ortolani et al. found a positive response to hazelnut in 77.9% of 86 patients who were tested with DBPCFC.1 Orhan et al. have found positive SPTs to hazelnut in 2.2% of Turkish children while DBPCFC tests were negative in all of the children with positive skin tests.38 The prevalence of hazelnut sensitisation by the use of SPTs was found to be 1.76% among the patients of our outpatient allergy clinic. Hazelnut sensitisation was verified in 63% of this special group with a history of hazelnut allergy and/or skin test reactivity when we further tested them with DBPCFC. This seems to be quite low for our country as hazelnut consumption is known to be high. Furthermore DBPCFC could be performed in 55% of the patients with either a clinical history or a positive skin test or both, this rate should also be taken into consideration while coming to a decision on the prevalence of clinically relevant food allergy in our patient population. It is hard to speculate on the low incidence of hazelnut allergy in our patient population; however, it is well known that many factors may influence sensitisation including the magnitude and duration of allergen exposure. Although we reported hazelnut consumption to be high in our country we did not assess the individual hazelnut consumption of the patients included in the study. On the other hand, factors related to the mucosal immune system of the host could have effects on sensitisation to foods.39–42 Taken together in a selected population we found a quite low rate of sensitivity to hazelnut which needs further evaluation with the documentation of above discussed individual factors. Recently, studies have suggested the development of oral tolerance by the mucosal immune system. The mucosal immune system regularly encounters enormous quantities of antigen and must suppress immune reactivity to food and harmless foreign commensal organism (i.e. develop oral tolerance). Antigen-presenting cells, including intestinal epithelial cells and dendritic cells, and regulatory T cells play a central role in the development of oral tolerance. Defects in that gastrointestinal barrier, however, can lead to the development of aberrant immunological responses, including hypersensitivity reactions.39–42 In the light of such information, we could explain that the rate of hazelnut sensitivity is low in Turkey.
It has been reported that hazelnut allergy could be related to sensitisation to pollens.43 There seems to be geographic variation regarding allergen recognition and severity of symptoms in patients with plant food allergy. Some studies suggest different routes of sensitisation between pollen-associated plant food allergy and plant food allergy without pollinosis. In patients with pollen-related hazelnut allergy, the sensitisation is more likely to occur through inhalation of pollen allergens and subsequent development of food-induced allergic symptoms caused by cross-reactivity between IgE raised against pollen allergens with homologous proteins in plant foods.43,44 In patients with hazelnut allergy without pollen sensitisation, the sensitisation to the plant food is estimated to occur through ingestion of the food (i.e. oral route).43 It is reported that hazelnut allergy is more frequently seen in individuals who are allergic to birch pollen.1,45–47 While its frequency was 7% for those without sensitivity to other allergens, it was found to be over 55% in patients with allergy to birch pollen.47 Ortolani et al. reported that OAS due to hazelnut in their study group was strongly related to the birch pollen sensitivity in study patients.1 Regarding geographic and climate properties, plantation in Turkey is similar to the south-eastern part of Europe. This similarity also reflects in clinical expression.48 In our study the rate of sensitisation to pollens in patients with hazelnut allergy was not different from that of patients without hazelnut allergy. When birch pollinosis was assessed it was seen that 42% of the patients with a positive DBPCFC test had concomitant birch pollen sensitivity while none of the four patients with negative test results were sensitive to birch pollen. The low ratio for concomitant birch pollen allergy in our study group may be explained by regional differences in pollen load. Recently, in addition to birch pollen-hazelnut sensitivity relation, an association between Platanus, Artemisia, hazel pollens and hazelnut, peanut and celery has been reported.49,50 Regional pollen exposure of our study group could be estimated with the data coming from two regional studies which demonstrated that tree pollens were the most common pollen source consisting of 95% of the total amount, followed by grasses (3%) and weeds (2%) in Ankara.48,51 Grass pollen was mostly the cause of seasonal allergic rhinitis, followed by tree and weed pollen in Ankara. The most prominent tree pollens were Cupresseceae, Pineceae, Quercus, Populus, Betula, Acer and Salicacea; however, the most common sensitivities among tree pollens were due to the Platanus, Tilia, Oleaceae, Aesculus and Acer. They explained the discrepancy that higher concentrations of airborne pollens may not always result in a higher prevalence of clinical allergy to those pollens.48,51 Generally it is believed that tree pollens are not as allergic as Gramineae pollens.52 In this study, birch sensitivity was evaluated in relation to hazelnut allergy; it seems that further evaluation is needed on plant food-pollen relations in the light of regional data and recent literature.
Clear differences in IgE reactivity were shown between symptomatic patients with hazelnut allergy and hazelnut-tolerant control subjects with pollen allergy but also between different European regions. Thus, they indicated that the diversity of IgE patterns between the involved regions could suggest the need for a geographically differentiated use of allergens both for diagnostic procedures and in the future also for therapeutic purposes in patients with plant food allergy.43 Although the prevalence of pollen allergy is reported as an estimated 8.9–27.75% in adult Turkish population,53–59 the association between pollens and plant food has not yet received investigation in Turkey.
Patient history, skin tests and/or in vitro tests support the diagnosis of IgE-mediated food allergies. Patients with food allergies are frequently evaluated by skin tests. But, since the positive predictive value of skin tests is lower than 50%, its negativity might be much more useful in excluding the diagnosis.60 If qualified extracts are used, the negative predictive value might be over 95%.60–62 Prick-to-prick skin tests with fresh foods give more reliable results compared to skin tests performed with commercial extracts, as allergens may degrade in their preparation.25,63 In this study, the rate of positive history of hazelnut allergy was 85.7% and both history and skin test positivity was 71.4% among patients with a positive DBPCFC. Thus, in line with several previous studies64,65 positive clinical history and skin tests seems to be important steps preceding challenge tests in our study group.
Radioallergosorbent tests support IgE-mediated food allergy data. Correlation has been found between specific IgE antibody levels to food and reaction to food.25 However, Hill et al. reported that skin tests are more sensitive than food-specific antibody level in diagnosis of food allergy.66 In another study, clinic sensitivity could be demonstrated with peanut sIgE in only 30% of patients.61 Similarly, in our study, hazelnut specific IgE was found to be positive in only 3 of 20 patients with positive history and skin prick tests which corresponds to a quite low ratio (33%).
Challenge tests are known to be important tools in diagnosing food allergy. Despite the fact that DBPCFC is a good indicator of food hypersensitivity clinically, it is time consuming, hazardous and more expensive.60,66 It is stated that when a careful history, skin tests and IgE antibody levels are combined for diagnosis, challenge test is required in fewer patients.25 We could verify hazelnut allergy with DBPCFC in about two thirds of patients with positive history and positive skin tests in our study. According to our data it seems that history and skin tests may be sufficient for the diagnosis of hazelnut allergy in the majority of subjects. However, there is still need for challenge tests in some patients in whom history and/or skin tests are positive.
The major limitation of our study is the low proportion of DBPCFC tests performed in a group highly suspected of hazelnut allergy. Challenge tests could be performed in 55% of patients with positive history and/or skin tests. Therefore, it is hard to speculate on the exact rate of prevalence of hazelnut allergy in our study population, but our results indicate that there is need for further challenge tests in a small proportion of patients with positive history and skin tests. Another limitation of our study is that the study population is selected among the patients who attended to the outpatient allergy clinic, thus this is not a population-based study. However patients from many parts of the country are referred to the study centre which is a tertiary clinic located in the capital city. Although the study group was a selected population with allergic complaints we think our results may reflect country data rather than Ankara due to the origin of the patients.
In conclusion, among subjects who admitted to an allergy department, the rate of hazelnut sensitisation was found to be 1.76% which seems to be comparable to previous reports. On the other hand, DBPCFC was shown to be positive in 63% of cases with a history of hazelnut allergy and positive skin tests. These results indicate that the presence of history with a positive skin test can be suggestive of hazelnut allergy; however an oral food challenge is needed to confirm the diagnosis.