Atopic dermatitis (AD), the most common chronic relapsing skin condition of infancy and childhood, is a complex multifactorial disease, which arises from the interaction between strong genetic and environmental factors.
ObjectiveTo investigate the roles of several factors on the severity of AD including FLG R501X gene mutation, serum immunoglobulin (Ig) levels, atopy and accompanying allergic disorders.
MethodChildren were genotyped for the mutation in FLG R501X gene. Serum levels of major Ig isotypes, atopy and accompanying allergic disorders were assessed.
ResultsStudy group consisted of 49 patients (M: 26, F: 23) with a mean age of 4.9±3.6 years and control group consisted of 50 children (M: 30, F: 20) with a mean age of 3.8±2.8 years. Genotyping of R501X mutation revealed risk alleles in none of the children in study group or control group. IgG z-scores were significantly higher in patients with AD compared to controls (−0.97±1.13 vs 1.48±1.02, p=0.026). There was a positive trend in IgG z-scores and a negative trend in IgA z-scores across the severity of AD. History of recurrent infections was significantly associated with asthma and/or AR (47.8% in patients with asthma/AR vs 3.8% in those without). Children with low IgG or IgA levels presented at an earlier age with lower rates of atopy and mild type AD.
ConclusionIn a sample of Turkish children, FLG R501X genotyping revealed no risk alleles in variable severities of AD or healthy controls. Our data suggest that IgG and IgA levels might have a role in phenotypic features of AD in terms of severity and atopic sensitisation.
AD is complex, chronic, recurrent and the most common skin disease in infancy and childhood that is caused by the combined influence of genetic and environmental factors. It occurs in approximately 10–20% of children around the world.1 Chronic skin inflammation develops as a result of impaired skin barrier, decrease in the skin's natural immune response and increased T cell response to environmental allergens and germs.1,2 Recently, studies have focused particularly on epithelial barrier dysfunction in AD.3–5 The relationship between the subtypes of AD and ichthyosis vulgaris due to loss-of-function mutations of the epidermal barrier protein filaggrin (FLG) has been demonstrated in America, Europe, Asia and Japan.6–8 FLG mutation is the most common mutation in AD, accounting for 18–48% of cases.6,9,10 The risk of asthma is also higher in these patients.9,11–15 Even though mutations and frequency of FLG are specific to a population, R501X is the most common mutation in Europe and America.6,9,11,16 FLG mutations have not been investigated in Turkey yet. There are few studies investigating the relationship between AD and immune status. While some of these studies have reported that the severity of AD increases with a decrease in Ig levels,17,18 others have reported that the severity of AD decreases with a decrease in Ig levels.19 Some studies have suggested that patients with FLG gene mutations are predisposed to some infections, for which however, no screening studies have been conducted.20,21
ObjectiveTo investigate the roles of several factors on the severity of AD including FLG R501X gene mutation, serum Ig levels, atopy and accompanying allergic disorders.
Materials and methodsA total of 49 patients aged between 2 months and 16 years who presented to Yeditepe University, Pediatric Allergy Department and who were diagnosed with AD according to the criteria of Hanifin and Rajka22 between April 2010 and November 2010 were enrolled in this study. The control group consisted of 50 healthy age-matched children who presented to the Department of Pediatrics with no active diseases and who had no history of chronic disease such as AD, asthma, allergic rhinitis, renal or kidney disease, and diabetes mellitus on admission. The study was approved by the Ethics Committee of Yeditepe University. All families were informed about the study and an informed consent form was obtained. The severity of eczema was assessed using the SCORAD index: as a mild form <15 points, moderate 15–40 points, and severe >40 points.23 The data recorded included family history of allergy, school absences in the last six months and length of absences from school, requirement for oral corticosteroids and duration of oral corticosteroid use, and use of intravenous immunoglobulin (IVIG). Of the children with recurrent wheezing, those who were diagnosed with asthma according to the GINA (The Global Initiative for Asthma) criteria were classified according to the severity of asthma as intermittent, mild persistent, moderate persistent, and severe persistent asthma, based on current clinical characteristics and daily treatment they received. The number of asthma attacks that they experienced in the last six months was recorded.24
Patients diagnosed with allergic rhinitis according to “The Allergic Rhinitis and its Impact on Asthma (ARIA)” criteria were classified as mild intermittent, moderate-severe intermittent, mild persistent and moderate-severe persistent rhinitis.25
In all children, 2.5ml blood was drawn from an antecubital vein into EDTA tubes for DNA analysis and blood samples were stored at 4°C. In all children, 5ml venous blood was collected in a dry tube and the separated serum samples were stored at −20°C.
Measurement of immunoglobulins and specific immunoglobulin ESerum levels of total IgE were determined using the Electrochemiluminescence/sandwich immunoassays on a Cobas C 411 device whereas eosinophil count was measured using a Coulter counter (Pharmacia, Kalamazoo, MI, USA). The children in whom skin prick test (SPT) could be performed underwent testing for 38 allergens including eight food allergens. Histamine was used as positive control and normal saline as negative control. A wheal of more than 3mm was considered a positive test according to negative control. Children with at least one positive SPT were considered to be atopic. Specific IgE to aeroallergens was analysed using a phadiotop test (CAP; Phadia) whereas food panel (FX5 CAP; Phadia) consisting of cow's milk (f2), egg white (f1), peanut, hazelnut, house dust mite (D1, D2), mould mix was measured by specific IgE (CAP, Phadia) and values greater than 0.35IU/ml were considered positive. Children with at least one positive blood test were considered to be atopic. In each child, a history of frequent disease relapses was investigated to evaluate the immune system. Frequent disease relapse was defined as upper respiratory tract infections more than six times and/or otitis media more than three times and/or acute sinusitis more than once and/or bronchopneumonia more than once per year.26
In all children, IgA, IgM, and IgG were measured in serum by a immunonephelometric method on the Dade Behring BN ProSpec. These measurements were compared with normal distributions for age in Turkish children. Values of −2SD or below were considered low, whereas those of greater than −2SD were considered normal.27 In the comparison of Ig values, z scores were calculated to eliminate the effect of age. z score was calculated using the formula: z-score=(x−x−μ/δ) (x=observed Ig level of the patient, μ=mean Ig value of the population for that age, δ=(−2SD−μ)/2). Lymphocyte count and percentage were obtained from the complete blood count by Coulter counter. These values were compared with normal distribution for age in Turkish children. Values lower than 5% were considered low and those between 5 and 95% normal.28
DNA isolationInvitrogen iPrep™ Purelink™ gDNA blood kit was used for DNA isolation from blood. The procedure in the kit was performed in accordance with the Invitrogen iPrep™ Purification instrument. DNA isolation in a closed system took about 30min and 200μl of DNA was obtained at the end of this procedure.
Spectrophotometric measurement of genomic DNAGenomic DNAs were measured using UV spectrophotometry at 260–280nm wavelengths and cases with a ratio of A260/A280nm=1.6–1.8 were included in the study. As a result of measurements, it was found that DNA values of the patients and controls included in this study ranged between 25 and 250ng/mL.
Analysis of filaggrin gene mutation R501XAnalysis of FLG gene mutation R501X was performed using the “5′-Exonuclease Allelic Discrimination Assay (Taqman)” on an Applied Biosystems 7500 Fast Real Time PCR system. For the analysis of FLG gene mutation R501X, primer and probe sequences that were previously defined by Palmer et al.6 were used. In addition, allelic discrimination analysis with real time PCR was performed using FAM for wild-type allele and VIC fluorescent probes for mutant allele.
Statistical analysis was performed using SPSS 13.0. The Hardy–Weinberg equilibrium was tested using chi-square analysis. It was found that age, eosinophil count and percentage, lymphocyte count and percentage and total IgE, IgA, IgM, IgG levels were not normally distributed. For this reason, values between the median and quartiles were used in the results and all statistics were performed using non-parametric Mann–Whitney U test and Kruskal–Wallis test. A p value of less than 0.05 was considered statistically significant. The factors affecting the severity of AD were investigated using logistic regression analysis. The factors included in the logistic regression analysis were as follows: age, gender, age of onset of AD, skin test positivity, eosinophil count, lymphocyte count, IgE, IgM, IgG and IgA levels, family history of allergic disease, mutation R501X, history of frequent disease relapses, asthma, allergic rhinitis and school absences. In univariate logistic regression analysis, multivariate logistic regression analysis was performed for p values less than 0.05. As a result of this analysis, p values less than 0.05 were considered statistically significant.
ResultsThe study group consisted of 49 children with AD (M: 26, F: 23) with a mean age of 4.9±3.6 years and 50 healthy children (M: 30, F: 20) with a mean age of 3.8±2.8 years. Table 1 shows demographic, biochemical and immunological characteristics of the study group. None of the children in the patient and control groups had risk of FLG R501X alleles and all children were (AA) homozygous.
Demographic and laboratory characteristics of patients with atopic dermatitis and the control group.
| Atopic dermatitisN=49 (%) | ControlN=50 (%) | p | |
| Age (mean±SD) | 4.85±3.64 | 3.77±2.79 | 0.24 |
| Gender | |||
| Male | 26 (53.1) | 30 (60.0) | 0.48 |
| Female | 23 (46.9) | 20 (40.0) | |
| Kinship | 3 (6.1) | 0 (0.0) | 0.12 |
| Frequent disease relapses | 12 (24.5) | 0.0 (0.0) | <0.001 |
| Family history of allergy | 24 (49.0) | 0.0 (0.0) | <0.001 |
| Asthma and/or rhinitis | 23.0 (47.9) | ||
| Asthma – yes | 19.0 (38.8) | 0.0 (0.0) | <0.001 |
| Asthma – no | 30.0 (61.2) | 0.0 (0.0) | |
| Severity of asthma | |||
| Intermittent | 8.0 (42.1) | ||
| Persistent | 11.0 (57.9) | ||
| Asthma attacks | 8.0 (42.1) | ||
| Severity of rhinitis | |||
| Intermittent | 2.0 (20.0) | ||
| persistent | 8.0 (80.0) | ||
| Atopy | 22.0 (44.9) | 0.0 (0.0) | <0.001 |
| House dust mite+ | 18.0 (36.7) | ||
| Food+ | 5.0 (10.4) | ||
| Total IgEMedian (25–75 percentile) | 114.9 (35.8–1122.4) | 29.4 (10.5–65.1) | <0.001 |
| Absolute eosinophil countMedian (25–75 percentile) | 400.0 (200.0–827.5) | 150.0 (107.5–227.5) | <0.001 |
| Eosinophil percentageMedian (25–75 percentile) | 3.9 (2.4–8.6) | 1.7 (1.0–2.6) | <0.001 |
| Absolute neutrophil countMedian (25–75 percentile) | 3.9 (2.7–6.1) | 3.1 (2.4–4.5) | 0.055 |
| Neutrophil percentageMean±SD | 41.4±13.6 | 38.1±17.0 | 0.31 |
| Absolute lymphocyte countMean±SD | 4.5±1.9 | 5.0±2.5 | 0.21 |
| Lymphocyte percentageMean±SD | 43.9±15.1 | 50.2±16.6 | 0.055 |
| IgA z scoreMean±SD | 0.37±1.61 | 0.84±1.27 | 0.12 |
| IgM z scoreMedian (25–75 percentile) | 1.40 (0.64–1.99) | 1.04 (0.04–1.95) | 0.34 |
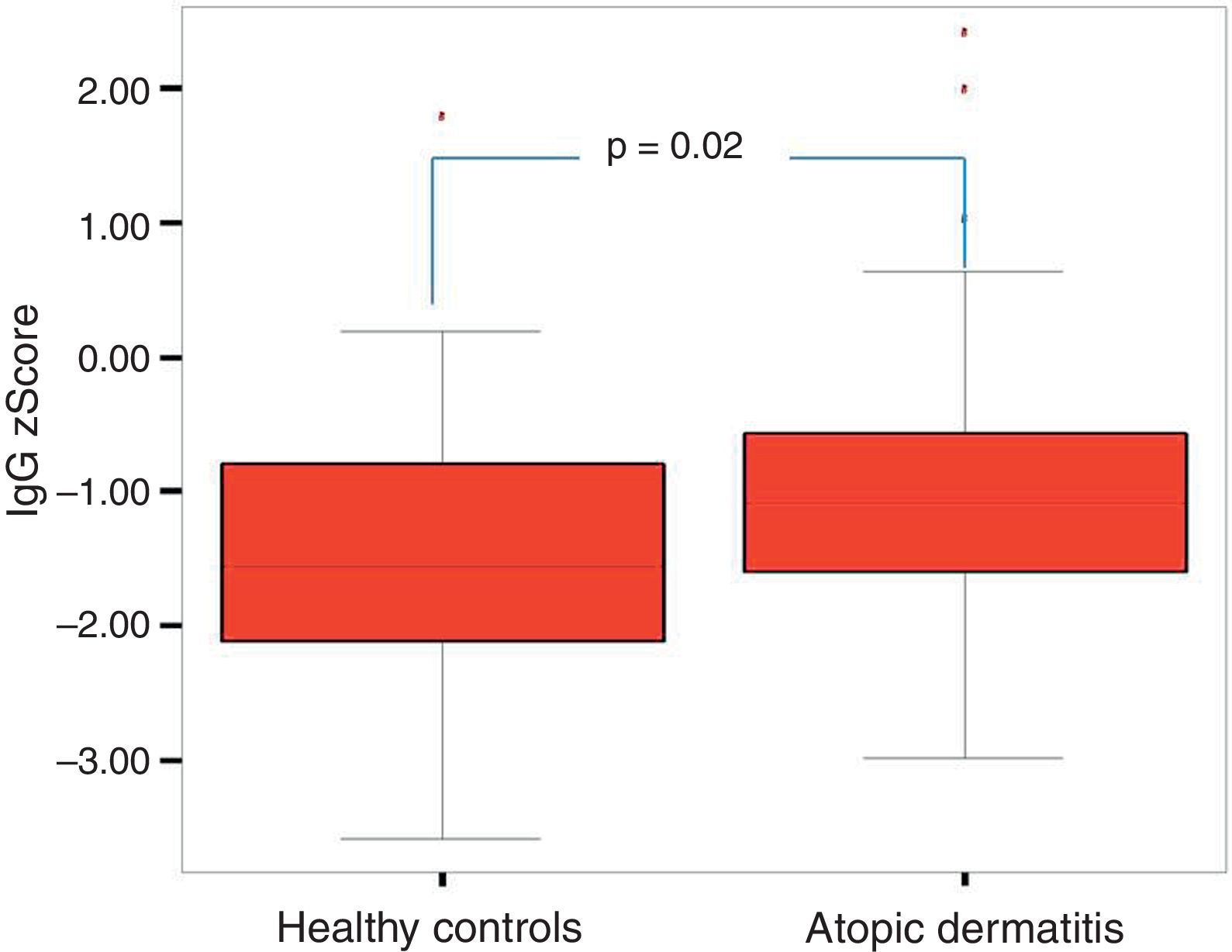
| IgG z scoreMean±SD | −0.97±1.13 | −1.48±1.02 | 0.026 |
| IgA decrease | 9.0 (19.1) | 7.0 (15.6) | 0.78 |
| IgM decrease | 9.0 (21.4) | 10.0 (22.7) | 1.00 |
| IgG decrease | 7.0 (14.9) | 14.0 (31.8) | 0.081 |
| CRPMedian (25–75 percentile) | 0.6 (0.3–1.7) | 0.6 (0.3–2.6) | 0.92 |
| Filaggrin mutation | |||
| AA | 49 (100.0) | 50 (100.0) | >0.05 |
Bold indicates statistical significance p<0.05.
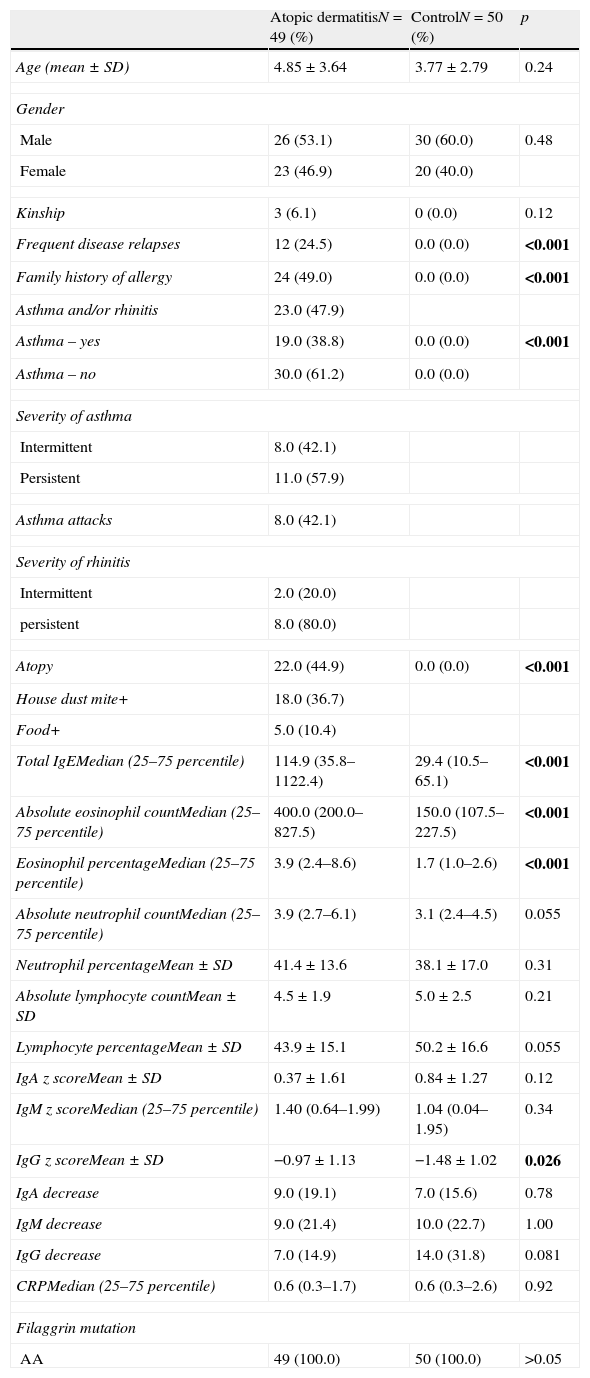
The comparison of the patients with AD and the control group revealed that total IgE, eosinophil count and percentage, family history of allergy and history of frequent disease relapses were statistically significantly higher in patients with AD compared to the control group. There were no statistically significant differences between the absolute and percentage lymphocyte and neutrophil counts and the results of the measurement of IgA, IgM, IgG in patients with AD and healthy controls. IgG z score was statistically significantly higher in patients with AD compared to healthy controls (p=0.03, Fig. 1).
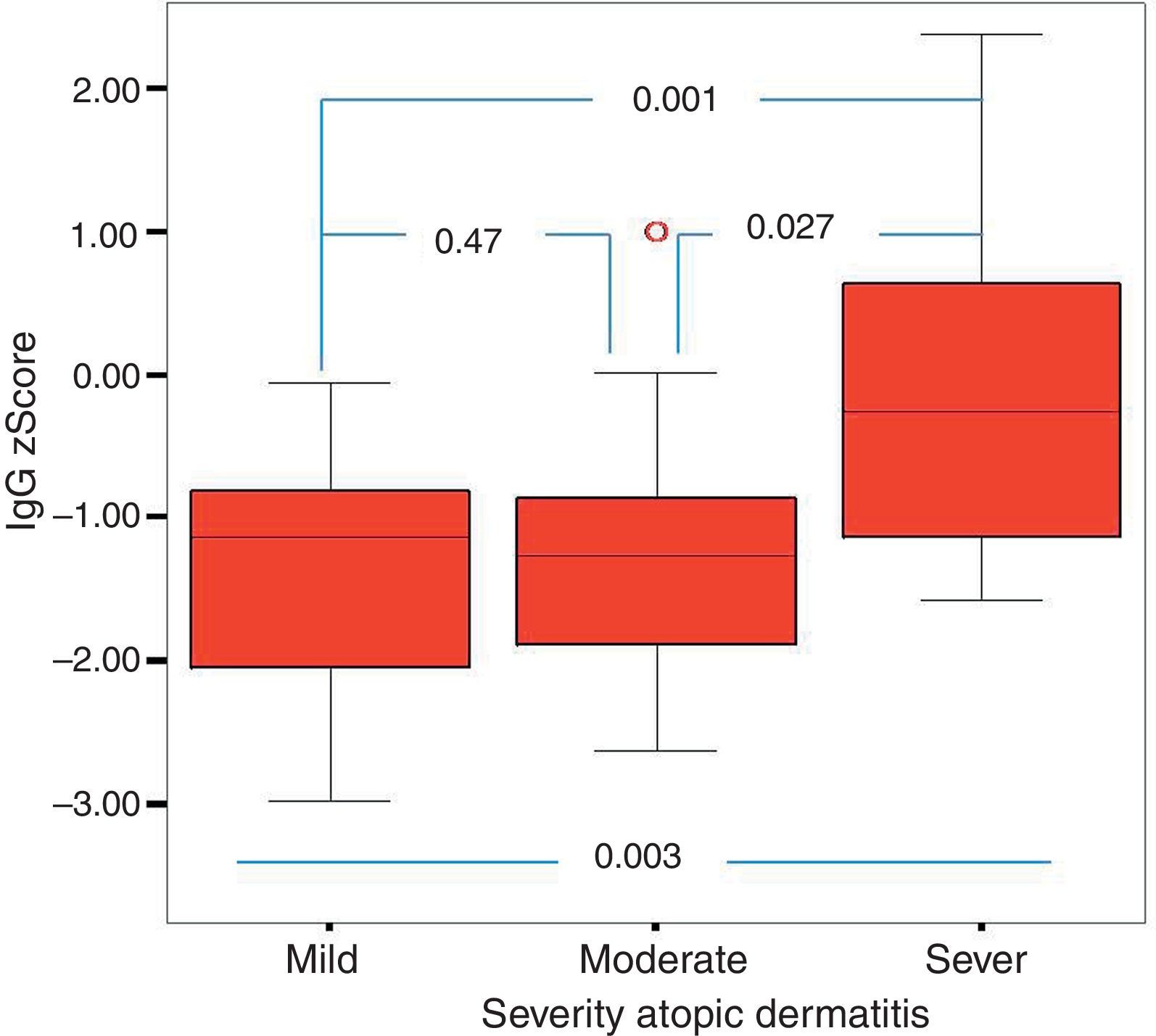
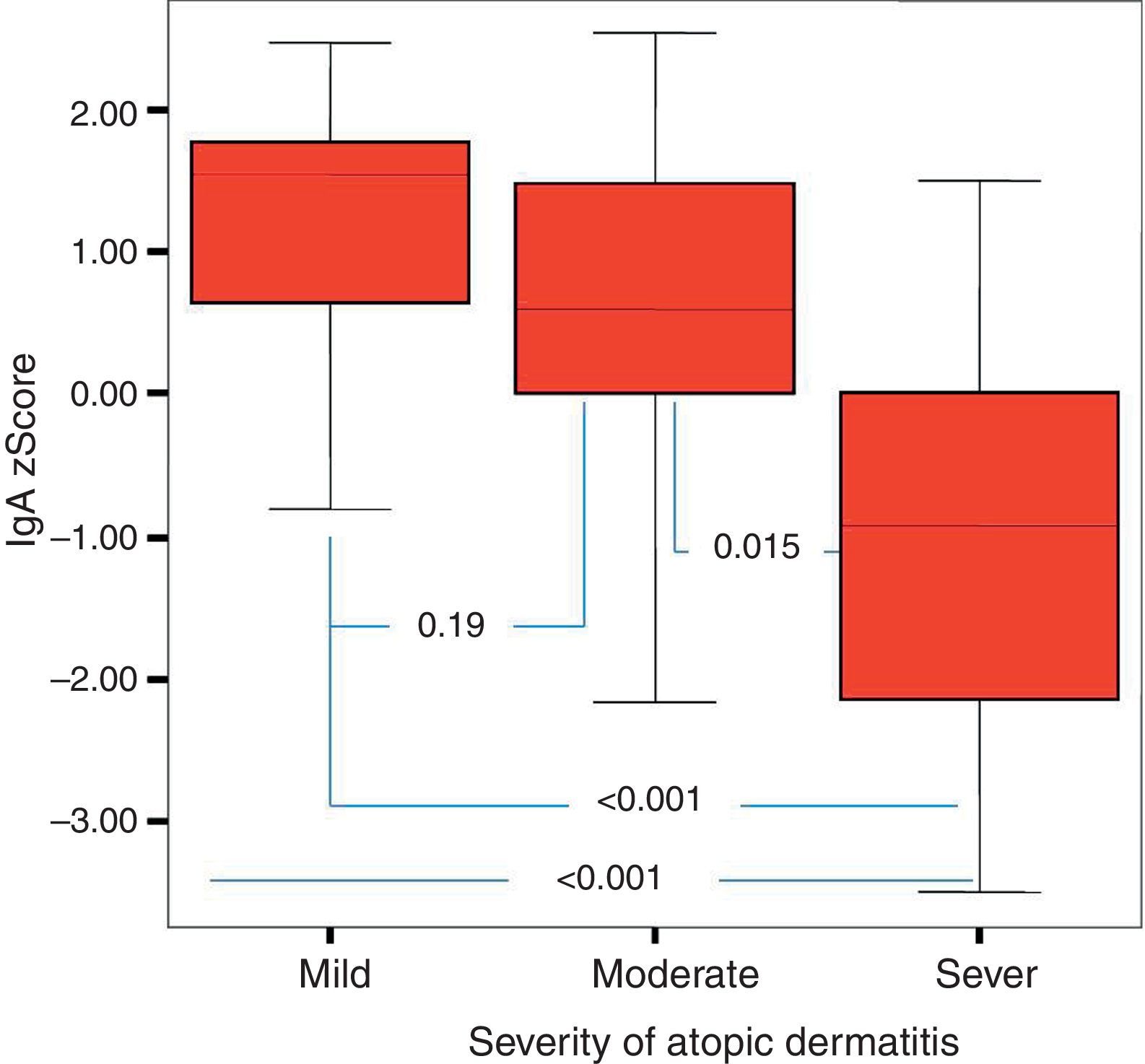
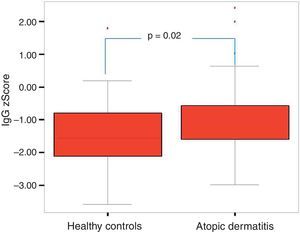
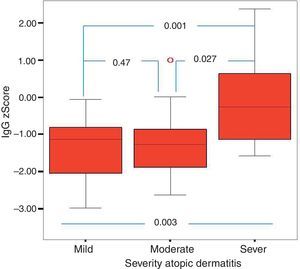
Of the 49 patients, 21 had mild AD, 14 moderate AD, and 14 severe AD. Analysis was performed according to the severity of the disease. Patients’ age, total IgE, eosinophil count and percentage and need for systemic treatment such as oral corticosteroids and IVIG were significantly increased in parallel to the increase in the severity of AD. It was found that as the severity of AD increased, absolute lymphocyte count and percentage in peripheral blood statistically significantly decreased (p=0.003, p=0.017). The lymphocyte percentage was lower in patients with AD compared to the controls, which however did not reach statistical significance (p=0.055). The IgG z scores were statistically significantly higher, whereas IgA z scores were statistically significantly lower in patients with severe AD compared to those with mild and moderate AD (Figs. 2 and 3).
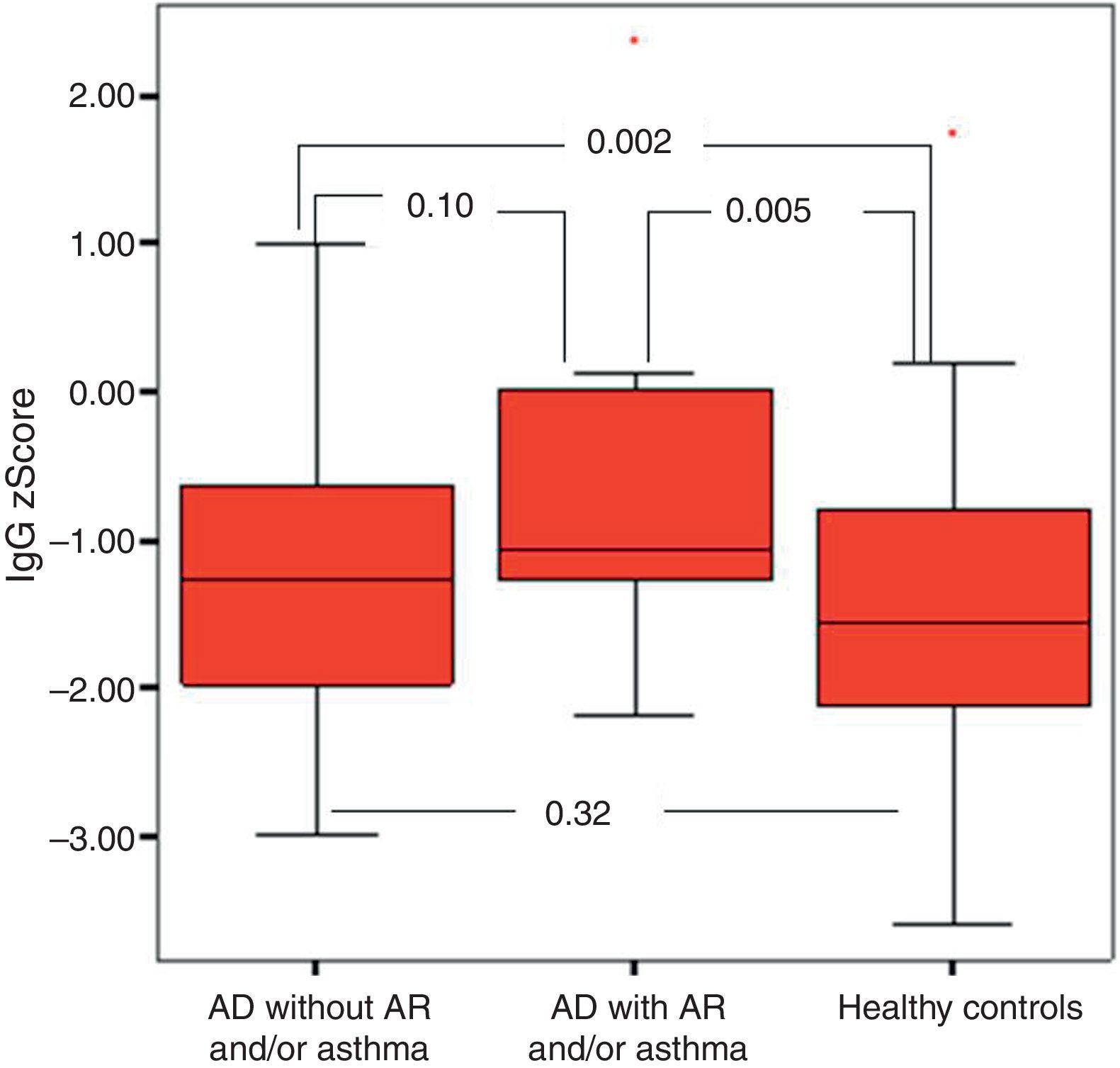
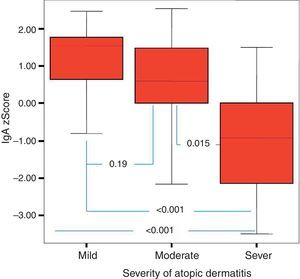
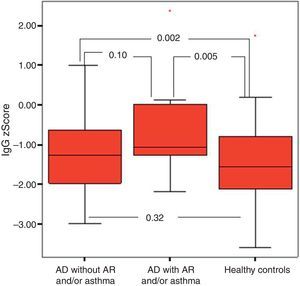
It was found that the most effective factor in frequent disease relapses was the comorbidity of AD and asthma or the comorbidity of asthma and allergic rhinitis in patients with AD (p=0.001). School absences were higher in this group (p=0.018). Patients who had AD with asthma and/or AR had higher sensitivity to mite allergen and a higher rate of atopy compared to patients with AD alone (p=0.01, p=0.043). IgG z score was lower in the control group compared to children who had AD with asthma and/or AR (Fig. 4).
It was found that the rate of atopy was statistically significantly higher in patients with no decrease in IgA and IgG levels compared to those with decreased IgA and IgG levels (p=0.030, p=0.012). Similarly, AD patients with decreased IgA and IgG levels had statistically significantly lower IgG levels (p=0.003, p=0.008).
DiscussionThis study demonstrated that 21 patients with mild AD, 14 with moderate AD and 14 with severe AD and 50 healthy children had no FLG mutation R501X which was reported to have an effect on the development of AD, worsening of the severity of the disease and progression of the disease to asthma in a number of studies and that all children enrolled in this study were homozygous wild type (AA). A total of 22 known mutations in the FLG gene have been documented so far. The most common FLG mutation R501X in Europe and America has not been reported in Japan and China and different mutations have been described in these populations.6,8–10 The prevalence of each mutation in the FLG gene varies among different races and new mutations specific to a particular population in each race can be described. In Italy, FLG mutations R501X and 2282del4 were investigated in 178 children with AD, 195 with psoriasis and 210 healthy children and it was reported that these mutations did not confer a risk for AD.29 This study did not attempt to reach the conclusion that the Turkish and Italian populations have the same genetic background. The FLG R501X mutation seen in the community constitutes an example and to show that we were not alone, the studies from Italy were included. As this study researched the risk factors from one gene of patients with severe AD, all FLG gene mutations were not investigated, but the FLG R501X mutation was observed as the most frequent cause of mutation. We believe that another study should evaluate the immunohistochemical analysis with FLG signals in the skin in the Turkish population. There might be several reasons for the inability to detect this mutation in Turkish population. This result may indicate that the FLG mutation undergoes negative selection on chromosomes in the Turkish population, as in the Italian population. Thus, for FLG and other 21q genes, mutations specific to the Turkish population can be investigated. Therefore, sequence analysis of FLG and 21q region should be performed and mutations specific to the Turkish population should be investigated. On the other hand, this result also suggests that FLG is not the only or the most effective gene group in the epidermal differentiation complex (EDC) region harbouring 21q.
IgG z scores were statistically significantly higher in patients with AD compared to the healthy controls. The analysis performed according to the severity of AD revealed that the severity of the disease significantly increased in parallel with increasing age. This finding suggests that patients with AD improve over time and that as the severity of the disease increased it persists and does not improve at advanced ages. There was no difference in the distribution of patients who had AD with asthma and/or allergic rhinitis according to the severity of AD. However, it was found that the persistency of asthma and rhinitis increased consistently with increasing severity of AD. It was found that AD children with asthma and/or AR had disease relapses more frequently and were more atopic.
In this study, it was found that as the severity of AD increased the absolute lymphocyte count and percentage in peripheral blood decreased significantly. None of the children enrolled in this study had lymphopenia. To our knowledge, an association between AD and the control group or between the severity of AD and lymphocyte count or percentage has not been reported in the literature. However, it is known that T-cell count is increased or unchanged, total T cell count is decreased or unchanged, B and NK cells remain unchanged and suppressor T cell count is decreased in patients with AD.30–32 These results may suggest that defects in lymphocyte count and function are encountered in AD and these defects may be associated with the severity of the disease, although not at the level of immunodeficiency. Further studies on this issue are warranted. Further studies should also investigate whether lymphocyte count, like eosinophil count, can be a marker of the severity of the disease. In this study, as the severity of AD increased IgA z scores decreased, whereas IgG z scores increased. To be able to evaluate this result as a part of the pathogenesis, it would have to be supported by many studies in literature. However, there are very few studies in the literature. It was observed, however, in this study that when the severity of AD increased, the IgA z score decreased and the IgG z score increased. Whether this is a coincidental result or an as yet unclarified cause or result of immunopathological mechanisms of AD requires further study. As this study is one of the first on this subject, it is important in respect of drawing attention to this situation.
In this study it was shown that in infants with low IgA, the dermatitis seen was non-atopic and mild type AD. This situation was not observed in older children. The reason for this is not absolutely clear as there are no similar studies with adequate results in the literature. This should be supported by other studies. However, even in a study by Baris et al.19 low IgG in young children was seen together with non-atopic mild type asthma. In the same study, it was indicated that hypogammaglobulinaemia was seen together with AD. The mentioned study concluded that there was a different phenotype of asthma named as early-onset non-atopic asthma in which hypogammaglobulinaemia might be accompanying. There was a statistical significance between AD patients with hypogammaglobulinaemia and AD patients with normogammaglobulinaemia (p=0.040). The same study also found that the rates of eosinophilia and IgE levels were lower in patients with hypogammaglobulinaemia compared to those with normogammaglobulinaemia (p=0.030). Immunological assessment of hypogammaglobulinaemia patients revealed that TGF-b production of lymphocytes was higher compared to that of the normogammaglobulinaemia group. A plausible explanation might be that an infection-mediated induction of T cell responses may be contributing to the generation of TGF-b. It is known that this cytokine reduces pro-inflammatory cytokine release from various inflammatory cells and enhanced T regulatory cells differentiation which may be contributed to by non-atopic conditions.19
A case study by Yasuno et al.33 included five AD infants with transient hypogammaglobulinaemia. Protein loss was not considered in these patients. Transient hypogammaglobulinaemia was attributed to a disorder in Ig production.
Transient hypogammaglobulinaemia and AD is a condition that occurs during infancy and can improve with age and be simultaneous. Cases of AD accompanied by decreased IgG levels can be encountered. The use of IVIG in the treatment of patients with severe dermatitis is associated with the improvement of AD. Two hypotheses have been proposed regarding this issue. The first hypothesis suggests that along with protein loss from skin lesions or the gastrointestinal tract a loss of Igs can also occur and hypogammaglobulinaemia develops in patients with AD. The second hypothesis states that skin infections and inflammatory cytokines are increased in skin with AD and the CD4/CD8 ratio leads to transient hypogammaglobulinaemia. Walker et al.17 (88) reported that 12 of 15 patients with transient hypogammaglobulinaemia had symptoms of either atopic disease or food intolerance/allergy. In the mentioned study, it was suggested that protein loss from the bowel due to allergic inflammation might have contributed to the development of transient hypogammaglobulinaemia. In conclusion, the question of whether transient hypogammaglobulinaemia leads to AD or whether both conditions occur at about the same ages incidentally and improve at similar time intervals remains to be elucidated.
A study by Lúdvíksson et al.18 demonstrated that the relationship between the prevalence and severity of allergic diseases with low-normal IgA levels in two-year-old children was statistically more significant than that with high IgE levels. A cohort study conducted with the same children at four years of age showed that serum IgA, IgG1, IgG2, IgG4 and IgE levels were significantly increased.34 Children with AD had lower salivary IgA and children who developed AD at the age of four had lower salivary IgA than that at the age of two, whereas children who recovered at the age of four had higher IgA levels. All children had normal IgA levels. These findings suggested that children aged four years and younger who are genetically predisposed to allergy are more prone to allergy when IgA levels are close to the lower limit of normal range.
In conclusion, FLG mutation R501X does not confer a risk for the development of AD in Turkish children. It is considered that IgA and IgG levels play a role in the severity of AD and the development of atopy, thus having an effect on AD phenotype.
ContributorsDr. Hulya Ercan contributed with the conception and design of the study, data generation, analysis and interpretation of the data and preparation or critical revision of the manuscript. Turgay Ispir carried out the genetic analysis. Deniz Kirac carried out the genetic analysis. Dr. Safa Baris contributed with the data generation. Dr. Ahmet Oguzhan Ozen contributed with the analysis and interpretation of the data and preparation or critical revision of the manuscript conception. Dr. Serdar Oztezcan evaluated biochemical analysis. Dr. Mehmet Reha Cengizlier contributed with the conception and design of the study and preparation or critical revision of the manuscript.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresPatients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.