Autoimmune diseases such as rheumatoid arthritis (RA) are the consequence of a persistent imbalance between pro- and anti-inflammatory immune mechanisms, leading to chronic inflammation. The objective of this study was to determine whether the high sensitive C-reactive protein (hs-CRP) and cytokines are elevated in RA patients and to investigate the relationship between these markers and disease activity in RA, measured by disease activity score 28 (DAS28).
MethodsWe studied 110 RA patients according to American College of Rheumatology revised criteria for RA, and 55 controls matched by age and sex. Serum levels of hs-CRP and cytokines interleukin (IL)-6, IL-10 and tumour necrosis factor-¿ (TNF-¿) were estimated and correlated with the DAS28. Serum hs-CRP was assayed immunoturbidimetrically and cytokines were analysed by commercially available ELISA kit.
ResultsWe found that RA patients had significantly higher levels of serum hs-CRP (p<0.001), IL-6 (p<0.001), TNF-¿ (p<0.001), and IL-10 (p<0.01) as compared to healthy controls. hs-CRP, IL-6 and TNF-¿ correlated positively (p<0.001) and IL-10 correlated negatively (p<0.01) with DAS28.
ConclusionsThese results demonstrate that RA patients have high levels of inflammatory markers, and these levels are correlated with the DAS28. These findings suggest a possible role of these markers in the pathogenesis of RA. Moreover, these biomarkers can be used as markers of disease activity in the diagnosis and treatment of RA.
Rheumatoid arthritis (RA) is a chronic inflammatory disorder that is characterised by polyarthritis with often progressive joint damage and disability, immunological abnormalities, systemic inflammation, increased co-morbidity, and premature mortality. It affects 1% of the adult population worldwide and also occurs among one in a thousand children as juvenile RA. RA is much more common in women and affects women 2–3 times more frequently than men, and during pregnancy 70% of women suffering from RA experience remission, with flare-ups after birth.1 The aetiology of RA is not known, but it is classified as one of the autoimmune diseases.2 It is associated with reduced life expectancy and a major cause of chronic disability and handicap, and conditions become more dangerous with time. Many studies have shown that advance therapy including the use of early, aggressive therapy, and the introduction of anti-cytokines agent have improved patient's quality of life, eased clinical symptoms, retarded the progression of joint destruction, and delayed disability.3
Inflammatory processes play a pivotal role in the pathogenesis of RA. Markers of inflammation such as C-reactive protein (CRP), interleukin (IL)-6, tumour necrosis factor (TNF)-¿ and anti-inflammatory marker IL-10 are highly expressed in synovium fluid and serum of arthritic patients and play an important role in the pathophysiology of RA. CRP is an acute-phase protein produced by hepatocytes, upon stimulation by the cytokines IL-1, IL-6 and TNF-¿, during an acute-phase response.4,5 CRP is a general marker of systemic inflammation and is elevated in patients with RA. Some studies reported a higher frequency of increased CRP concentrations in serum samples of RA patients before the onset of RA.6
In RA, several cytokines are involved in almost all aspects of articular inflammation and destruction.7 Increased levels of pro-inflammatory cytokines lead to the proliferation of synovial tissue, and thereby cause damage in the articular cartilage and bone destruction in the adjacent area. Anti-inflammatory cytokines can also be found in the affected joints, and it has been postulated that chronic synovitis may reflect an imbalance in pro- and anti-inflammatory cytokines production in RA. IL-6 is the most abundantly expressed cytokine in RA patients with biological activities that include regulation of immune response, inflammation, and haematopoiesis. IL-6 stimulates the secretion of immunoglobulin by plasmacytes, activates and promotes the proliferation of T and B cells (thus it is involved in the production of the rheumatoid factor), induces synthesis of acute-phase proteins such as CRP, fibrinogen, haptoglobin and serum amyloid-A, regulates the proliferation and differentiation of osteoclasts, and induces bone resorption.8
TNF-¿ is one of the pivotal pro-inflammatory cytokines responsible for inflammation and joint destruction in RA. TNF-¿ and its two receptors (p55 and p75 TNFR) are readily detected in both synovial fluid and serum of patients with RA. The severity of this disease is correlated with the concentration of TNF-¿ in RA patients.9 TNF-¿ is a potent stimulator of mesenchymal cells, such as synovial fibroblasts, osteoclasts, and chondrocytes that release tissue-destroying matrix metalloproteinases. TNF-¿ also inhibits the production of tissue inhibitors of metalloproteinases by synovial fibroblasts. These dual actions are thought to lead to joint damage. Although, TNF-¿ and IL-6 have overlapping and synergic actions, some of the effects of these two cytokines are regulated by distinct mechanisms.10 IL-10 is a potent immunosuppressive and anti-inflammatory cytokine, produced as a part of the homeostatic response to infection and inflammation, and plays a critical role in limiting the duration and intensity of immune and inflammatory reactions. As an anti-inflammatory cytokine, IL-10 has been shown to inhibit the synthesis of pro-inflammatory cytokines.
In the present study, we have screened 110 RA cases attending the Rheumatology OPD of a tertiary care hospital in Delhi, India. Acute phase protein hs-CRP, pro-inflammatory markers IL-6 and TNF-¿ and anti-inflammatory marker IL-10 were estimated in the serum of RA patients to rule out the levels of these biomarkers during active RA and compared them with healthy controls, and then investigated the correlation between serum levels of these inflammatory markers with the disease activity score 28 (DAS28) in the patient group.
MethodsThe present study was carried out on 110 RA patients, fulfilling the 1987 revised criteria of the American College of Rheumatology (formerly, the American Rheumatism Association).11 All cases were selected from the Rheumatology Department of a tertiary care hospital in Delhi, India, under the guidance of a specialist rheumatologist. All patients had active RA (>3 swollen and >3 tender joints). Some of them had evidence of erosive disease on X-rays of hands or feet. Disease activity in RA patients was measured using the DAS28, which includes the 28 tender and swollen joint counts, the erythrocyte sedimentation rate (ESR) and the patients’ assessment of disease activity measured with a visual analogue scale. The body mass index (BMI) was calculated by dividing the weight (kg) by the square of the height (m2). Fifty-five controls, matched by age and sex were selected from blood donors and hospital staff. The controls were healthy persons who had no personal or family history of a rheumatic disease. These healthy controls were screened for diabetes, hypertension and dyslipidaemia. The study had local Research Ethics Committee and Research and Development approval, and all participants gave their written informed consent.
Overnight fasting blood samples were collected for measurement of all parameters. Blood was allowed to clot at room temperature, and serum was obtained immediately by centrifugation at 3500rpm for 10min. Serum was aliquoted into plastic tubes and stored at −27°C until assayed. Serum levels of hs-CRP were determined by immunoturbidimetric assay with the use of reagents and calibrators from Roche diagnostics. The levels of IL-6, IL-10 and TNF-¿ were estimated by means of commercially available quantitative “sandwich” enzyme-linked immunosorbent assay (ELISA) kits obtained from R&D Systems, according to the instructions of the manufacturer. Rheumatoid factor (RF) and ESR were measured by routine hospital procedure. Statistical Package for the Social Sciences 16 (SPSS 16.0) was used for all statistical analyses. All the descriptive variables were expressed as the mean±standard deviation (SD). Independent sample t-tests were used to compare the mean values of variables between the RA patients and the control groups. The correlations among the concentrations of each parameter and DAS28 were tested using Pearson's correlation. Differences were considered to be significant at p<0.05.
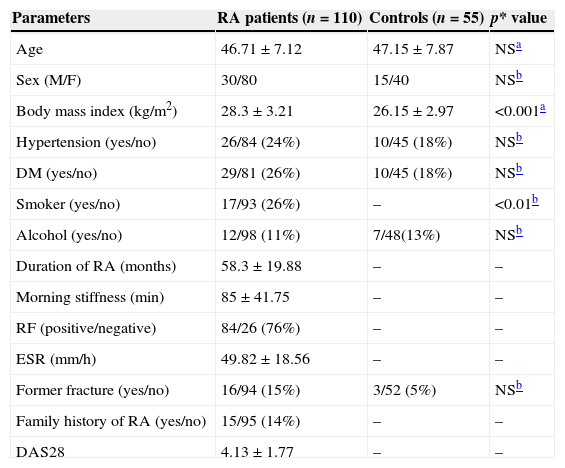
ResultsThe demographic and clinical data of the study groups are shown in Table 1. The mean age of the 110 patients with RA was 46.71±7.12 years and the patient group was comprised of 30 males and 80 females. The mean value of morning stiffness was 85±41.75min. The mean disease duration of the patients was 58.3±19.88 months. The mean DAS28 of RA patients was 4.13±1.77. Fifteen of the 110 patients had a family history of RA or other types of arthritis, and 16 had a former fracture. RA is closely associated with a family history of arthritis and former fracture or injury. Eighty-four RA patients were positive for RF; 29 RA patients were diabetic; and 26 were suffering from hypertension. The mean age of the healthy controls was 47.15±7.87 years and the control group was comprised of 15 males and 40 females.
Demographic characteristics of the study.
| Parameters | RA patients (n=110) | Controls (n=55) | p* value |
|---|---|---|---|
| Age | 46.71±7.12 | 47.15±7.87 | NSa |
| Sex (M/F) | 30/80 | 15/40 | NSb |
| Body mass index (kg/m2) | 28.3±3.21 | 26.15±2.97 | <0.001a |
| Hypertension (yes/no) | 26/84 (24%) | 10/45 (18%) | NSb |
| DM (yes/no) | 29/81 (26%) | 10/45 (18%) | NSb |
| Smoker (yes/no) | 17/93 (26%) | – | <0.01b |
| Alcohol (yes/no) | 12/98 (11%) | 7/48(13%) | NSb |
| Duration of RA (months) | 58.3±19.88 | – | – |
| Morning stiffness (min) | 85±41.75 | – | – |
| RF (positive/negative) | 84/26 (76%) | – | – |
| ESR (mm/h) | 49.82±18.56 | – | – |
| Former fracture (yes/no) | 16/94 (15%) | 3/52 (5%) | NSb |
| Family history of RA (yes/no) | 15/95 (14%) | – | – |
| DAS28 | 4.13±1.77 | – | – |
RA, rheumatoid arthritis; DM, diabetes mellitus; RF, rheumatoid factor; ESR, erythrocyte sedimentation rate; DAS, disease activity score; NS, not significant.
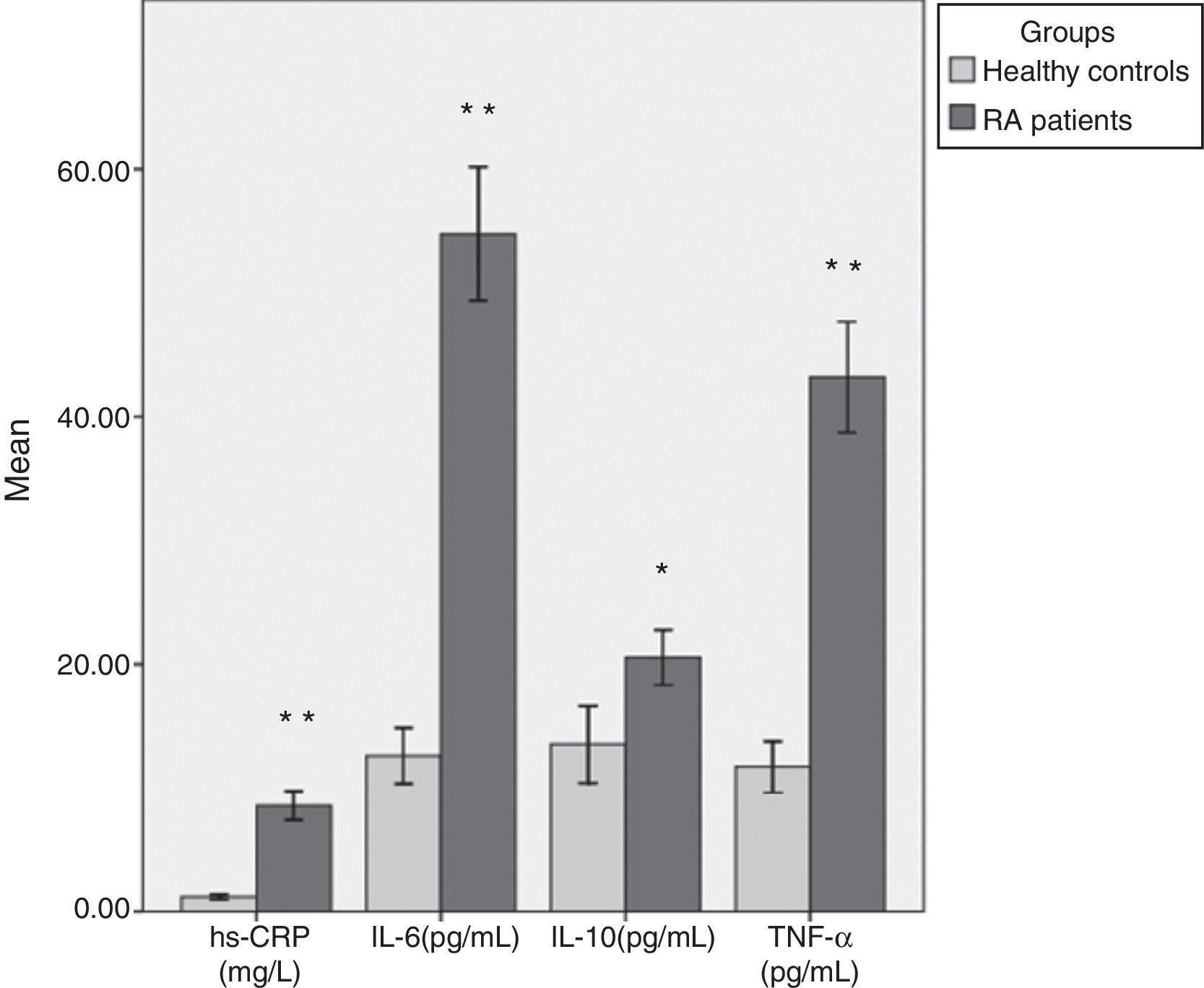
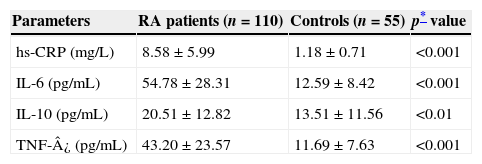
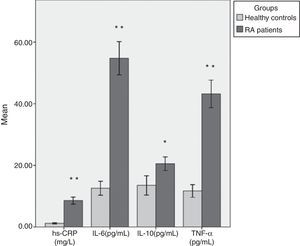
Table 2 shows mean values and SD of all parameters studied in both groups with test of significance using SPSS 16 statistical software (Fig. 1). We found that the mean level of hs-CRP was highly, significantly increased (p<0.001) in RA cases (8.58±5.99mg/L) as compared to the healthy controls (1.18±0.71mg/L). Moreover, there were highly significant increases in serum IL-6 (54.78±28.31 vs. 12.59±8.42pg/mL), and TNF-¿ (43.20±23.57 vs. 11.69±7.63pg/mL) levels in patients with RA as compared to the control group (p<0.001). Levels of anti-inflammatory cytokine IL-10 were also elevated in RA patients (20.51±12.82pg/mL) when compared to the controls (13.51±11.56pg/mL) (p<0.01). There was no significant difference in inflammatory markers level between men and women RA patients.
Mean values of laboratory variables in RA patients and healthy controls.
| Parameters | RA patients (n=110) | Controls (n=55) | p* value |
|---|---|---|---|
| hs-CRP (mg/L) | 8.58±5.99 | 1.18±0.71 | <0.001 |
| IL-6 (pg/mL) | 54.78±28.31 | 12.59±8.42 | <0.001 |
| IL-10 (pg/mL) | 20.51±12.82 | 13.51±11.56 | <0.01 |
| TNF-¿ (pg/mL) | 43.20±23.57 | 11.69±7.63 | <0.001 |
RA, rheumatoid arthritis; hs-CRP, high sensitive C reactive protein; IL, interleukin; TNF, tumour necrosis factor.
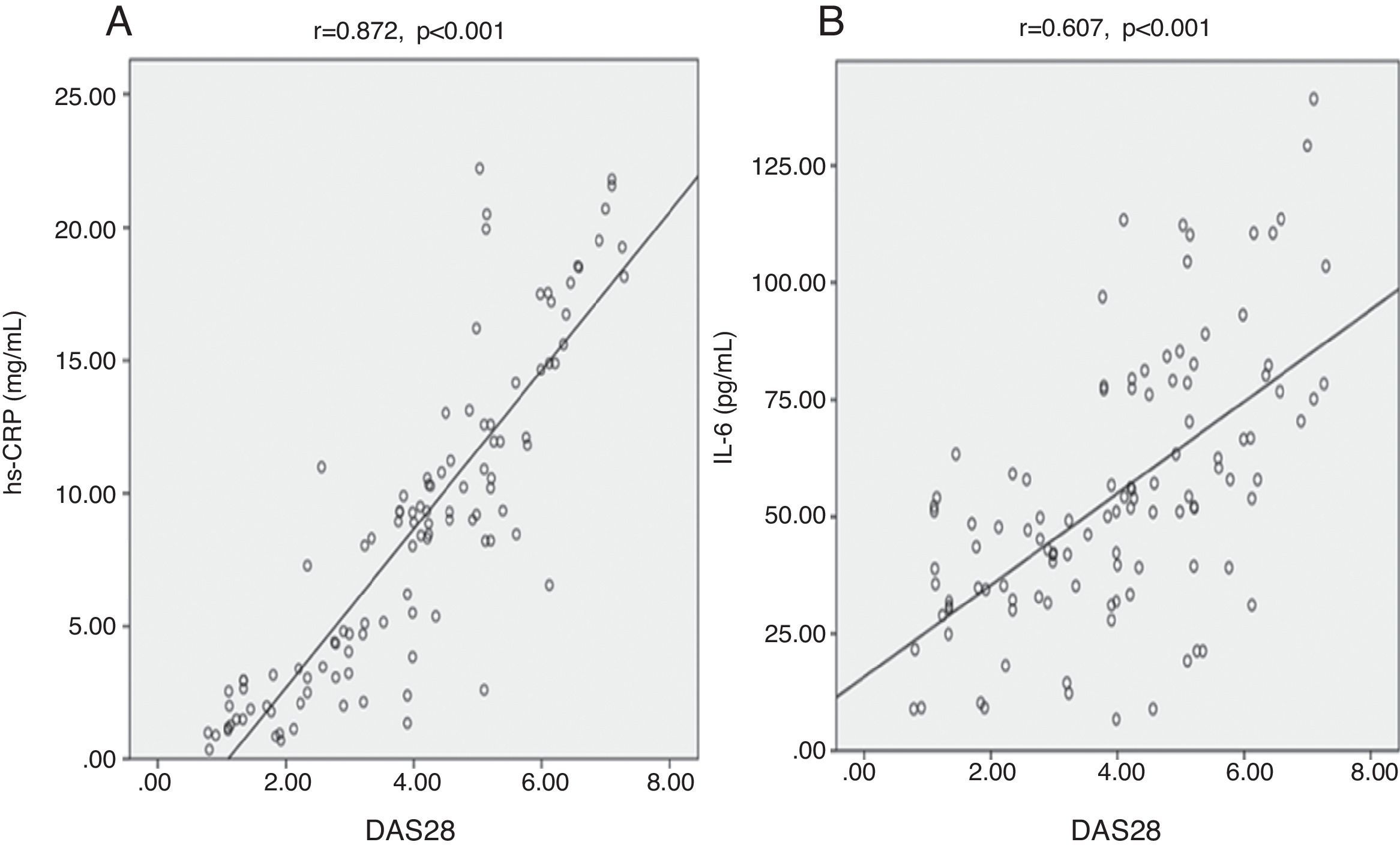
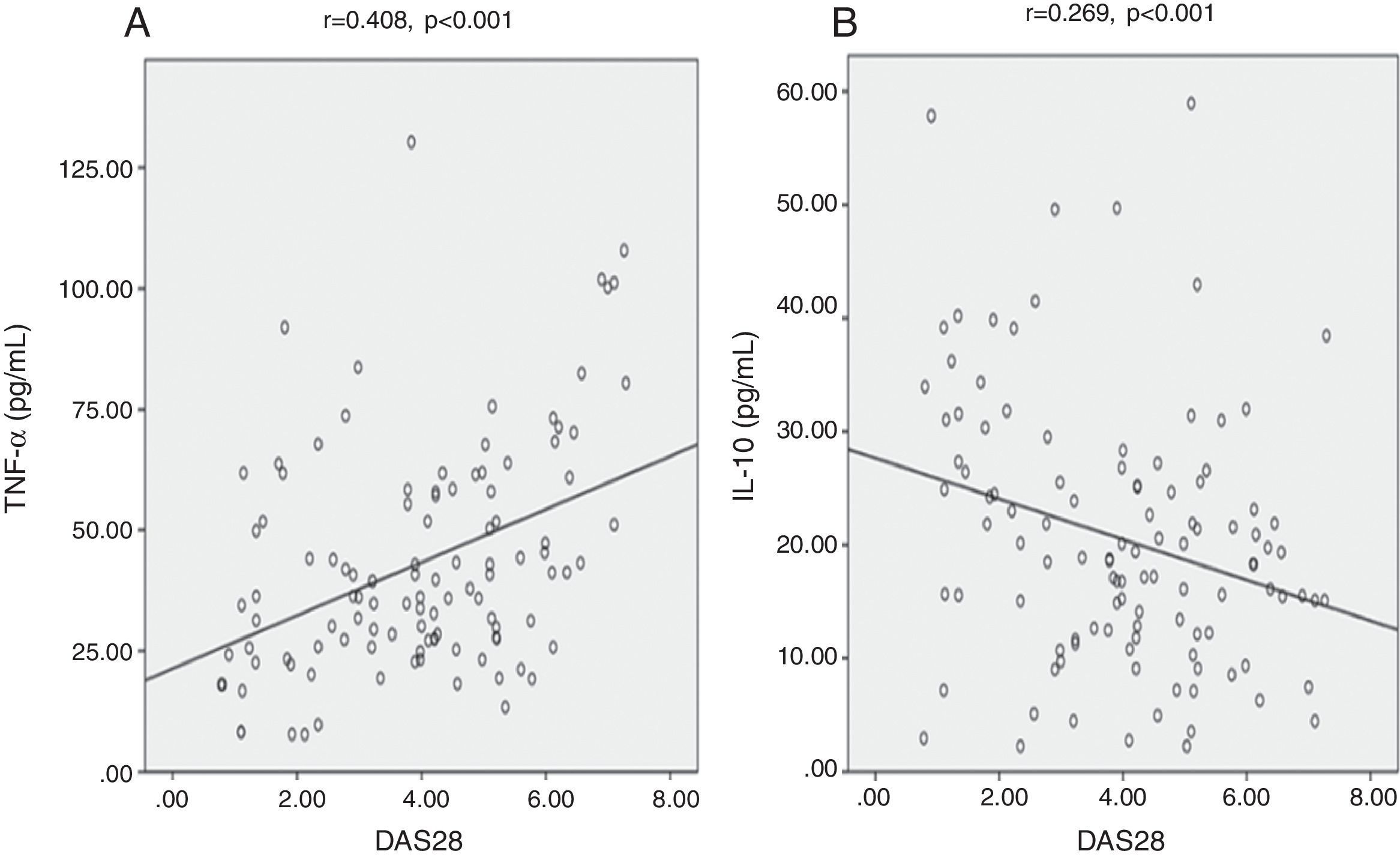
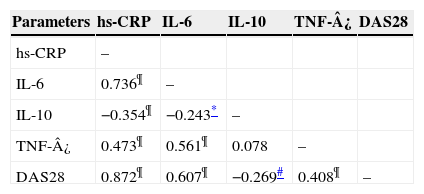
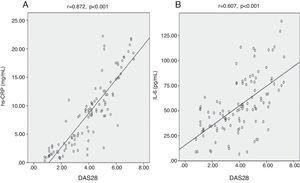
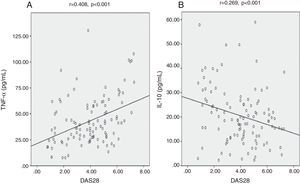
We found highly significant positive correlations between DAS28 and each of hs-CRP (Fig. 2A), IL-6 (Fig. 2B), and TNF-¿ (Fig. 3A) (p<0.001) (r=0.872, 0.607, 0.408) respectively. There was also a negative significant correlation between DAS28 and IL-10 (p<0.01) (r=−0.269) (Fig. 3B). In RA patients, there were highly significant positive correlations between hs-CRP and IL-6 (r=0.736, p<0.001) and hs-CRP and TNF-¿ (r=0.473, p<0.001), and negative correlation between hs-CRP and IL-10 (r=−0.354, p<0.001). IL-6 correlated positively with TNF-¿ (r=0.561, p<0.001), and negatively with IL-10 (r=−0.243, p<0.05). No statistically significant correlations were found between TNF-¿ and IL-10 (Table 3).
Correlation between different parameters among 110 RA patients.
| Parameters | hs-CRP | IL-6 | IL-10 | TNF-¿ | DAS28 |
|---|---|---|---|---|---|
| hs-CRP | – | ||||
| IL-6 | 0.736¶ | – | |||
| IL-10 | −0.354¶ | −0.243* | – | ||
| TNF-¿ | 0.473¶ | 0.561¶ | 0.078 | – | |
| DAS28 | 0.872¶ | 0.607¶ | −0.269# | 0.408¶ | – |
RA, rheumatoid arthritis; hs-CRP, high sensitive C reactive protein; IL, interleukin; TNF, tumour necrosis factor; DAS28, disease activity score 28.
Pearson's correlation test was used.
The results of our study confirm the observation of previous studies that the serum concentrations of inflammatory markers are elevated in the majority of patients with RA. RA is characterised by chronic inflammation and hypertrophy of the synovial membranes. Inflammation of the joints occurs in response to production of growth factors, cytokines, and chemokines by many different types of cells present in synovium and cartilage, in addition to infiltrating cells from the peripheral blood.12 In the present study, we have measured disease activity of RA patients with the help of DAS28. The DAS28 is an index similar to the original DAS, consisting of a 28 tender joint count (range 0–28), a 28 swollen joint count (range 0–28), ESR (or CRP), and an optional general health assessment on a visual analogue scale (range 0–100). It is a useful parameter in daily clinical practice as a treatment goal and to evaluate the actual disease activity. Low DAS28 is an important prognostic factor of persistent remission and DAS28 correlates with radiological progression.
As expected, the level of hs-CRP was significantly increased in RA patients as compared to the controls. The hepatic acute phase protein response is a prominent feature of many inflammatory diseases, including RA. The ESR and CRP concentrations are generally accepted as the most reliable markers of this response and associated with more rapid radiological progression.13 CRP is synthesised by hepatocytes in response to pro-inflammatory cytokines, particularly IL-6.14 It has been shown to be of great value as an inflammatory marker in RA and has been suggested to mediate part of the complement activation in RA. Patients who had serological abnormalities before the onset of symptoms had slightly higher CRP concentrations compared with patients without serological abnormalities, which indicates an intensified inflammatory process in patients with immunoglobulin-M rheumatoid factor and/or anti-citrullinated peptide antibodies positivity before the onset of symptoms.6 Some studies have concluded that new joint involvement and damaged joint progression are increased with increasing CRP and have also shown a significant relationship between the degree of synovial inflammatory infiltration and CRP.15 The use of hs-CRP assays was recently recommended as a measure to identify low disease activity in RA. Systemic low grade inflammation that is generally not detectable by routine CRP testing may be common in RA. Since even mild disease activity is associated with the poor long-term outcome in RA, hs-CRP testing should be helpful in deciding whether disease modifying agent therapy needs intensification.
We found significant increased levels of IL-6 and TNF-¿ in RA patients as compared to the healthy controls and both the inflammatory cytokines correlated positively with DAS28. These findings suggest that IL-6 and TNF-¿ are important mediators of inflammation in RA and play a pivotal role in the development and progression of RA. Many of the biological actions of IL-6 appear relevant to inflammatory diseases such as RA, where chronic immune activation and recurrent acute phase responses are characteristic. TNF-¿ can induce resorption of cartilage and bone by activation of osteoclasts, stimulate expression of adhesion molecules that facilitate the transport of large numbers of leucocytes to areas of inflammation, class I major histocompatibility complex expression, and the stimulation of fibroblast growth via platelet derived growth factor.16,17 TNF-¿, IL-1, and IL-6 have the capacity to induce synovial cells and chondrocytes to release prostaglandins, reactive oxygen, and neutral proteinases such as collagenases and stromelysin. These enzymes degrade proteoglycans and collegen, resulting in cartilage destruction.
Our results show a highly significant increase in serum IL-10 in patients with RA compared to the control group. The mechanisms leading to increased release of anti-inflammatory cytokine IL-10 in RA patients remain unclear; however, it may be assumed that in severe inflammatory processes occurring in RA, more IL-10 is produced as a compensatory phenomenon to inhibit continued pro-inflammatory cytokine production and inflammatory propagation, resulting in elevated levels of IL-10. IL-10 is a potent immune-suppressive and anti-inflammatory cytokine that deactivates macrophages and dendritic cells.18 IL-10 functions as an effective immune-regulatory molecule based on two important functions, inhibition of cytokine synthesis and down-regulation of antigen-presenting cell function. In RA patients, IL-10 rises in response to inflammation as an attempt to keep it in check but falls short of mitigating joint inflammation. In RA the pro-inflammatory cytokines themselves appear to trigger the synthesis of IL-10 as evidenced by many experimental studies.19 In addition to losing anti-inflammatory functions in RA patients, IL-10 can acquire pro-inflammatory functions.18,20
In addition to some previous observations, the significant increase in the hs-CRP and cytokines levels observed in the current study indicates that these markers might play a role in inducing inflammatory responses or mediating anti-inflammatory responses in the pathogenesis of RA, and they correlate with the disease severity. Cytokines and acute phase proteins reciprocally regulate each other's expression and activities, constituting a communication network between fibroblasts, macrophages, lymphocytes and hepatocytes. Activation of the network results in inflammation and the progressive destruction of joints and systemic symptoms characteristic of RA.21 In the case of RA, the presence of high concentrations of acute phase proteins in the circulation is associated with a more severe progressive course of the disease characterised by intense bone resorption,22 which reflects the higher circulating levels of cytokines observed during an acute phase response.
It is well established that continued disease activity leads to joint damage, resulting in reduction of physical functioning and if damage is progressive, to irreversible disability.23 In recent guidelines for RA, it was recommended to evaluate disease activity by composite measures at regular intervals and adapt treatment decisions based on the results. For example, the DAS, along with variants DAS28-ESR and DAS-CRP, has been shown to provide better clinical control, improved long-term physical ability, reduced radiographic progression and lower costs.24 An absolute level of disease activity can be selected as a clinically meaningful goal for therapeutic intervention; with a value of ≤3.2 defined as the threshold for a low disease activity state and ≤2.6 as the threshold for remission.25 A combination of such tools (DAS28) with a novel approach to biomarker evaluation may therefore allow for optimised understanding and prediction of the fate of the individual RA patient.
The hypothesis that inflammation and disease activity are closely linked is supported by some studies. In our study, inflammation measured by hs-CRP and disease activity by DAS28 show a significant positive correlation which also confirms the hypothesis. Previous investigators have also reported a positive correlation between CRP levels and disease activity in RA patients.26,27 Since 1973, assessment of serum concentrations of CRP has been advocated as an objective measure of disease activity in RA.5 CRP level correlates more closely with subjective (morning stiffness, pain and fatigue after walking) and semi-objective (grip strength, articular index) and clinical parameters of RA disease activity. Furthermore, serum level of CRP not only reflects the extent of disease activity but also has prognostic value in terms of progressive joint damage and functional status and outcome.28,29
Because of the critical role of cytokine networks in perpetuating inflammatory responses in the rheumatoid joint, circulating, and/or synovial cytokines are considered to be ideal biomarkers to monitor disease onset, development and progression. Elevated circulating cytokines levels are a common manifestation of the persistent state of inflammation and are predictive of disease severity.30 We found highly significant positive correlations between DAS28 and each of IL-6 and TNF-¿, and negative correlation with IL-10. Milman et al.31 have reported significant correlations between plasma levels of IL-6 and DAS28. The clinical value of IL-6 measurements in the monitoring of disease activity and radiological progression in RA is based on the supposed role of IL-6 as mediator between the inflammatory process in the joints and the systemic acute phase response. In fact, treatment of RA patients with the humanised anti-IL-6 receptor antibody, tocilizumab, is highly effective. TNF-¿ has a wide range of pro-inflammatory activities; it occupies a pivotal role in the initiation and amplification of the inflammatory cascade in RA and high level of TNF correlates positively with DAS28. TNF-¿ blocking therapy has remarkable effects on disease activity, and improves the clinical course and outcome in RA.32 Based on the inhibitory effect of IL-10 on macrophage-monocytes we can conclude that IL-10 downregulates IL-6 synthesis and in this way influences CRP production in RA patients. Accordingly, in the present study, we found a good negative correlation between IL-10 and each of IL-6, CRP and DAS28. Perhaps one of the most interesting observations in this study was the negative correlation between IL-10 and CRP serum levels, suggesting an indirect anti-inflammatory effect of IL-10 on acute phase protein production.
The major limitation of this study is that there was no consideration and analysis of the type of drugs taken by the patients. Some drugs can reduce the concentrations of inflammatory markers; therefore, further study is warranted to accurately compare the correlation between disease activity and the concentrations of these markers, with consideration for the effects of the drugs in a larger patient population. However, the study was designed to measure inflammatory markers in RA patients when subjects initially visited our hospital. We have selected only active RA patients for the study and compared the serum levels of inflammatory markers with age-matched and sex-matched controls. It is widely accepted that early detection of RA and therapeutic intervention are key elements in the prevention of joint damage.33 Biological agents that specifically inhibit the effects of TNF-¿ and IL-6 are effective in the treatment of RA patients. This in turn improves the patient's quality of life by improving their mobility and function.
In conclusion, the results of the present study indicate that the levels of inflammatory markers hs-CRP, IL-6, IL-10 and TNF-¿ are significantly more elevated in RA patients than in healthy controls, and correlated with the disease activity. DAS28 correlates positively with hs-CRP, IL-6 and TNF-¿ and negatively with anti-inflammatory cytokine IL-10. These findings suggest that inflammatory markers may be involved in the pathogenesis of RA, and that levels of these markers reflect the activity of the disease.
Conflict of interestNone of the authors have a competing interest to disclose.
Funding statementNo funding was received for this study.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.