Alpha-Tocopherol (α-TCP), one major form of vitamin E, has been known as a treatment for airway allergic inflammation. However, the role and mechanism of α-TCP in treating allergic rhinitis remains unclear.
ObjectiveIn this study we examined the inhibitory function of α-TCP in a mouse model of allergic rhinitis.
MethodsAllergic phenotype was examined by hematoxylin and eosin staining. Total IgE, OVA-specific IgE, OVA-specific IgG1 and OVA-specific IgG2a levels were examined by ELISA. mRNA expression was measured by qPCR, protein levels were examined by Western Blot.
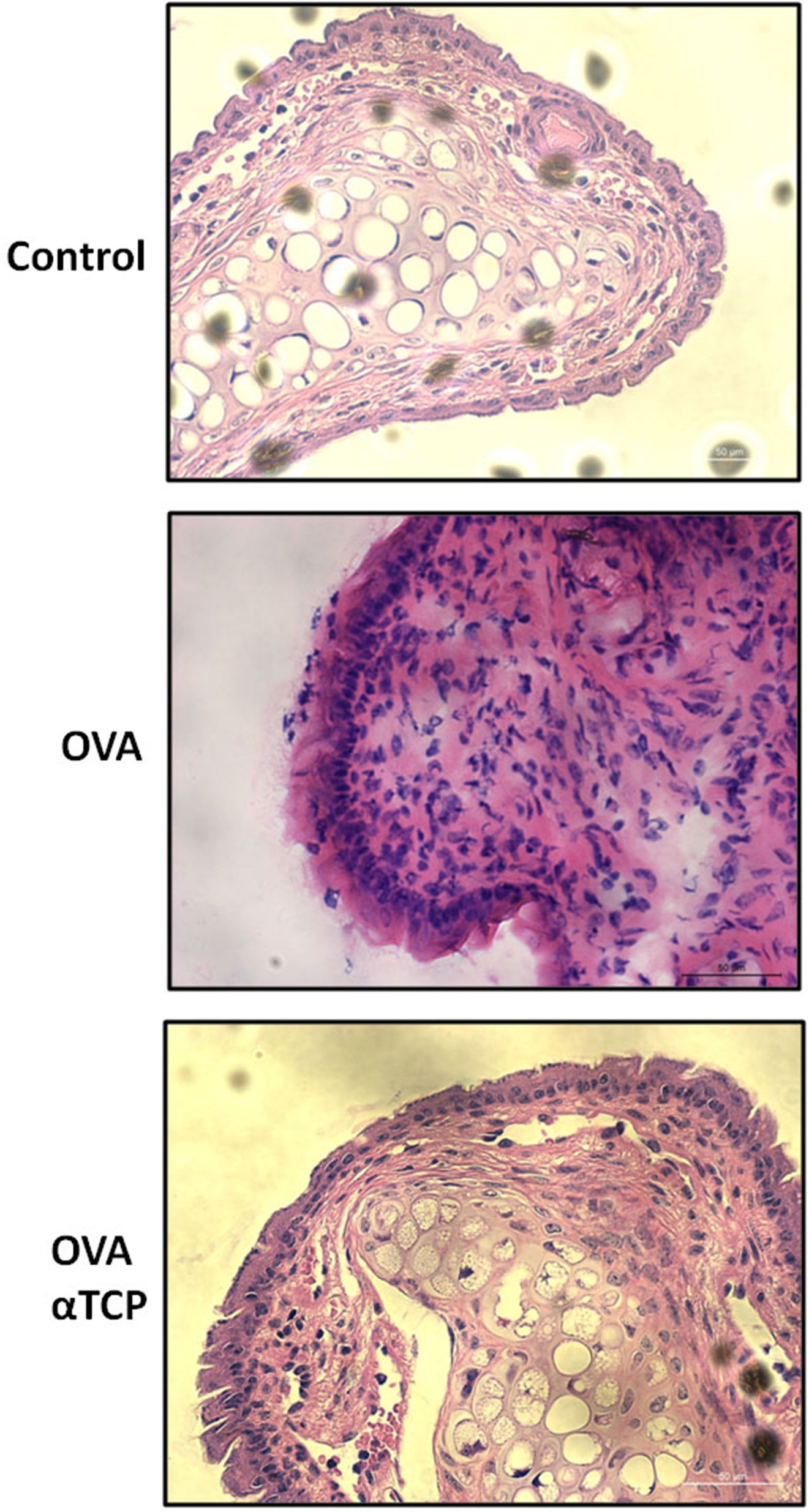
ResultsHistological analysis of the nasal membranes revealed that there was a significant reduction in inflammatory cells appearance in cross-sections in alpha-TCP treatment of Ovalbumin (OVA)-sensitized mice compared to OVA sensitized animals. In addition, eosinophils were significantly reduced in nasal mucosa of alpha-TCP treatment of OVA-sensitized mice compared to the OVA group. Lower total IgE, OVA-specific IgE, OVA-specific IgG1 and OVA-specific IgG2a levels were found in alpha-TCP treatment of OVA-sensitized mice compared to the OVA group. Furthermore, we found that the subepithelial distribution of tryptase positive mast cells was reduced in the alpha-TCP treatment of OVA-sensitized mice. More importantly, the PI3K-PKB pathway was suppressed by α-TCP in mast cells.
ConclusionsOur results demonstrated that α-TCP-mediated suppression of PI3K-PKB activity in mast cells is a potential mechanism of anti-allergic function of α-TCP.
Allergic rhinitis (AR) is the most common debilitating disease of childhood allergy.1,2 Epidemiologic studies demonstrated that AR affects up to half of the population over the world.3 Multiple environmental changes, especially exposure to environmental toxicants, are related to the rapid increase in AR prevalence.4,5 Several cellular processes have important roles in orchestrating AR reactions.6 Mast cells can release a spectrum of inflammatory mediators and be involved in cross-linking of many allergens to the IgE-FcεRI complex.6 Recent studies further demonstrated that eosinophils, basophils, and T lymphocytes can regulate the late allergic reaction.7 There are still adverse side effects with long-term use in AR treatment. Therefore, a safe alternative therapy is needed.
Alpha-Tocopherol (α-TCP) has been considered as a therapy for airway allergic inflammation.8 However, the mechanisms by which α-TCP inhibits inflammation in allergic rhinitis are not yet clear. In this study we further examined the influence of alpha-TCP on the development of allergic rhinitis, using an experimental animal model. Our findings showed that α-TCP reduces eosinophil and mast cell numbers in nasal mucosa in the model. We further speculated that the PI3K-PKB pathway is involved in the inhibitory effect of αTCP in mast cells.
Materials and methodsMurine allergic rhinitis model and treatmentFour-week-old female BALB/c mice were purchased from Shanghai Laboratory Animal Co. Ltd. D-alpha-tocopherol (α-TCP) and ovalbumin (OVA) were obtained from Sigma Aldrich, St. Louis, MO, USA. Mice were sensitized on days 0, 7, 14, and 21 by i.p. injection of OVA (75μg) in 200μL of PBS. Mice were further challenged with nasal instillation of OVA 500μg in 25μL of PBS on days 22−30. α-TCP was dissolved in soya oil and nasally administered (20μL at 1000U/g), beginning 1h before each challenge on days 22−30. The experimental group design included OVA-sensitized animals treated with α-TCP (n=10), positive group: OVA-sensitized animals treated with vehicle (n=10), and negative group: PBS treated animals and challenged with saline aerosol (n=10). We recorded the number of sneezing and nose rubbing motions for 15min after the final allergen challenge.
Eosinophils numberWe sacrificed the mice 24h after the last OVA challenge. The heads were removed en bloc and fixed in 4 % paraformaldehyde (Sigma Aldrich, St. Louis, MO, USA). To evaluate nasal histology, nasal tissues were decalcified, embedded in paraffin, and sectioned coronally (4μm thick), and further stained with HE. We counted eosinophils number in the submucosal area of the whole nasal septum.
Real-time RT-PCRRNA was extracted from nasal mucosa tissues using TriZol reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) was synthesized using Reverse transcriptase and oligo (dT) primers (Applied Biosystems, Foster City, CA, USA). For real-time PCR, 1μl of diluted cDNA (1:20) was amplified for 40 cycles with a master mix (SYBR Green Supermix; Applied Biosystems) using real-time PCR Systems (Bio-Rad, Hercules, CA, USA). Melting curve analysis was done at the end of the reaction to assess the quality of the final PCR products. The threshold cycle C(t) values were calculated by fixing the basal fluorescence at 0.05 unit. Three replicates were used for each sample and the average C(t) value was calculated. The ΔC(t) values were calculated as C(t) sample – C(t) GAPDH. The N-fold increase or decrease in expression was calculated by the ΔΔCt method using the C(t) value as the reference point.
ELISASolid-phase ELISA was used to measure serum levels of total and OVA-specific IgE. To measure the levels of OVA-specific IgG1 and IgG2a, 96-well immuno plates were coated overnight at 4°C. Serum samples were tested at the diluted concentration of 1:250 and 1:500. Detection was performed with biotinylated rat anti mouse IgG1 and IgG2a (BD Pharmingen) and then streptavidin-HRP, and developed using TMB substrate and read at 450nm. Reagents and ELISA kits were purchased from BD Pharmingen.
Immunohistochemistry, IHCSections were deparafinized in xylene and rehydrated. The primary antibodies were anti-mast cell (MC) tryptase (Abcam). Mast cell density was assessed in areas showing the highest concentration of tryptase positive mast cells.
ImmunoblottingProtein samples were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), then transferred onto polyvinylidene fluoride or polyvinylidene difluoride (PVDF) membranes. The membranes were incubated at 4°C overnight with primary antibodies as follows: p-PKB, PKB, GSK3β, and mTOR (Cell signaling technology).
Statistical analysisAll data were expressed as mean±SEM. Results were compared by Kruskal-Wallis and Mann Whitney U test using GraphPad Prism program (La Jolla, CA, USA). A P value <0.05 was regarded as statistically significant.
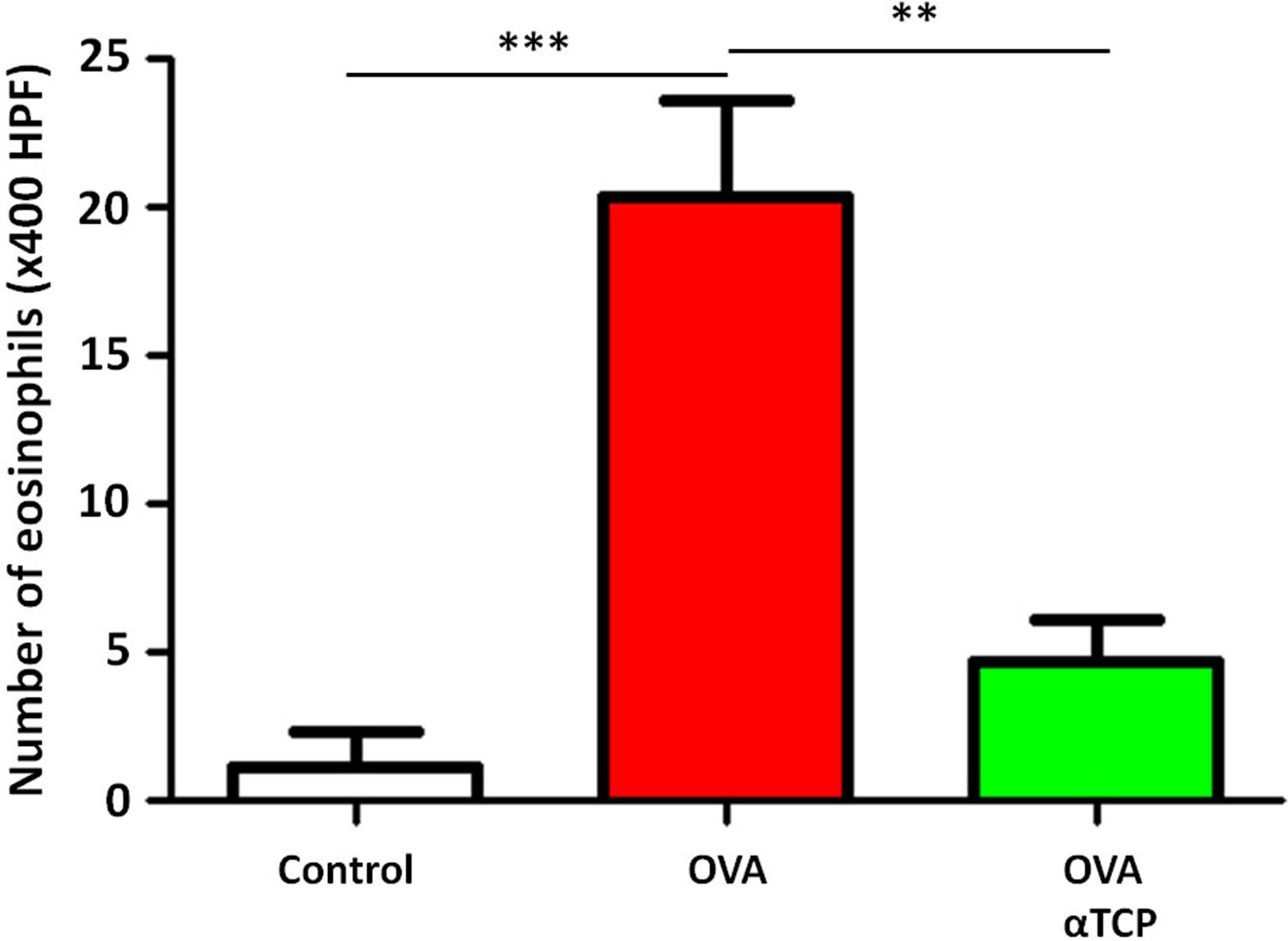
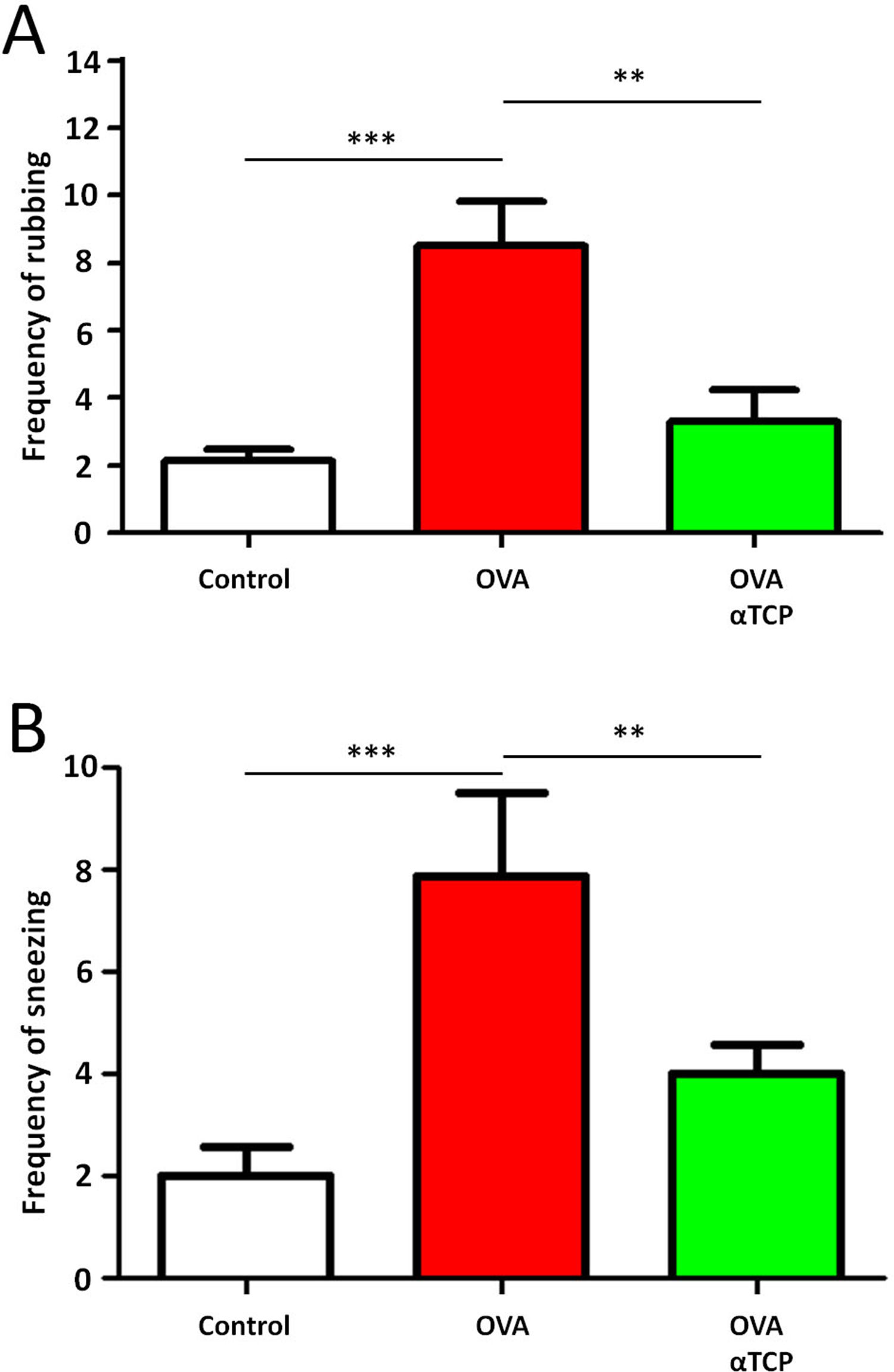
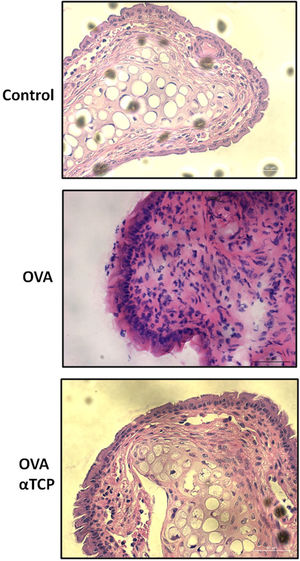
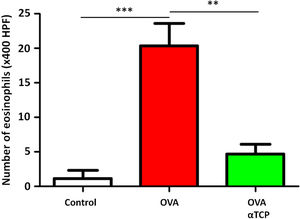
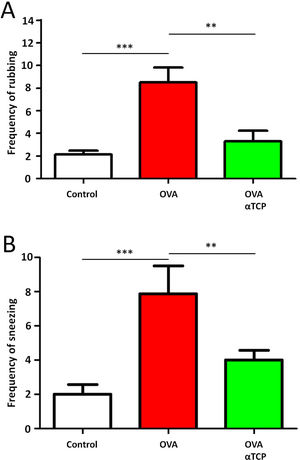
Resultsα-TCP inhibits allergic rhinitis development in mouse modelWe first examined the effects of α-TCP in a mouse model. Histological analysis demonstrated AR lesions in the submucosa. There was a clear reduction in inflammatory cells appearance in cross-sections in alpha-TCP treatment of OVA-sensitized mice compared to the OVA group (Fig. 1). Eosinophil counts in the nasal mucosa are shown in Fig. 2. There was a significant reduction in eosinophils in alpha-TCP treatment of OVA-sensitized mice (n=10) compared to the OVA group (n=10) (OVA: 20±7.4 vs. α-TCP: 4.7±1.1, p<0.01). We further tested the effect of α-TCP on nasal symptoms. We found that nasal rubbing times were significantly increased in the OVA group than in the control group (n=10) (8.2±0.8 vs 2.1±0.3). Sneezing times were also increased in the OVA group than in the control group (7.8±1.6 vs 2.0±0.3). Importantly, nasal rubbing/sneezing scores were decreased in α-TCP treated groups than in OVA group (both p<0.01) (Fig. 3).
The number of eosinophils in the nasal septal mucosa. The number of eosinophils was significantly decreased in alpha-TCP treatment of OVA-sensitized mice. n=10 each group. Data are representative of at least two independent experiments. n.s.: not significant; *: p<0.05; **: p<0.01; ***: p<0.001. Error bar values represent SEM. For comparison between two groups, Student's two-tailed t test was used.
Frequency of rubbing (A) and sneezing (B) for 10min after the last OVA sensitization and α-TCP treatment. n=10 each group. Data are representative of at least two independent experiments. n.s. not significant; *: p<0.05; **: p<0.01; ***: p<0.001. Error bar values represent SEM. For comparison between two groups, Student's two-tailed t test was used.
We found a significantly higher total IgE and OVA-specific IgE levels in OVA groups compared to control group (Supplemental Fig. S1A and 1B). Significant reductions of total IgE and OVA-specific IgE were observed in the alpha-TCP treatment of OVA-sensitized mice (both p<0.001). Also, OVA-specific IgG1 and OVA-specific IgG2a levels in the alpha-TCP treatment of OVA-sensitized mice were significantly decreased compared to OVA group (both p<0.05) (Supplemental Fig. S1C and 1D).
Effects of α-TCP on the expressions of IL-4, 5, 13, and IFNγ mRNAsWe further found a significant increase in IL-4, IL-5, IL-13, and IFNγ mRNAs expression in OVA-treated nasal mucosa versus to control group (Supplemental Fig. S2). However, the expressions of IL-4, IL-5, and IL-13 mRNAs were significantly reduced in the alpha-TCP treatment of OVA-sensitized mice than in the OVA group (Supplemental Fig. S2). IFNγ mRNA in alpha-TCP treatment of OVA-sensitized mice showed even higher expression compared to OVA-treated mice.
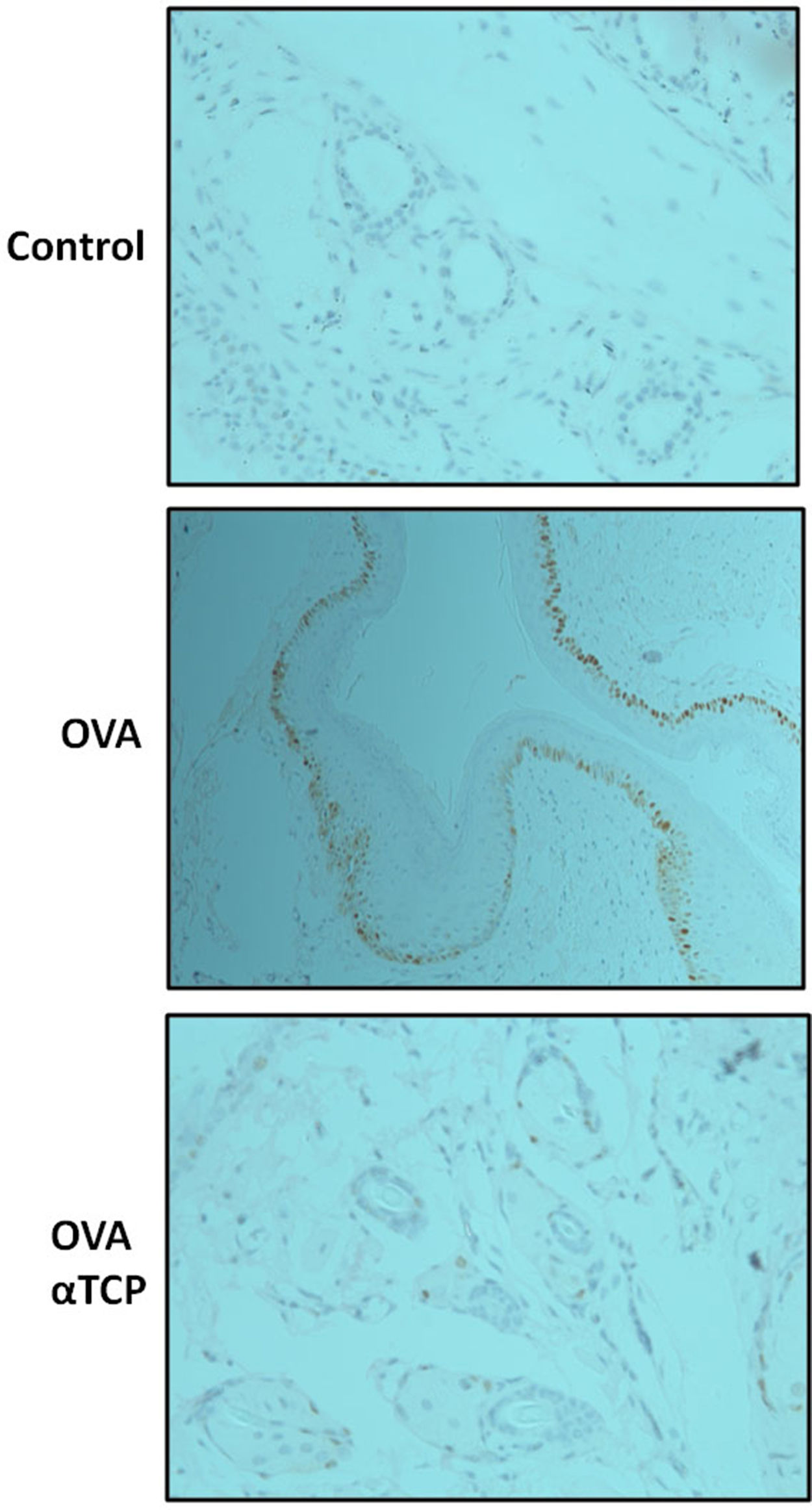
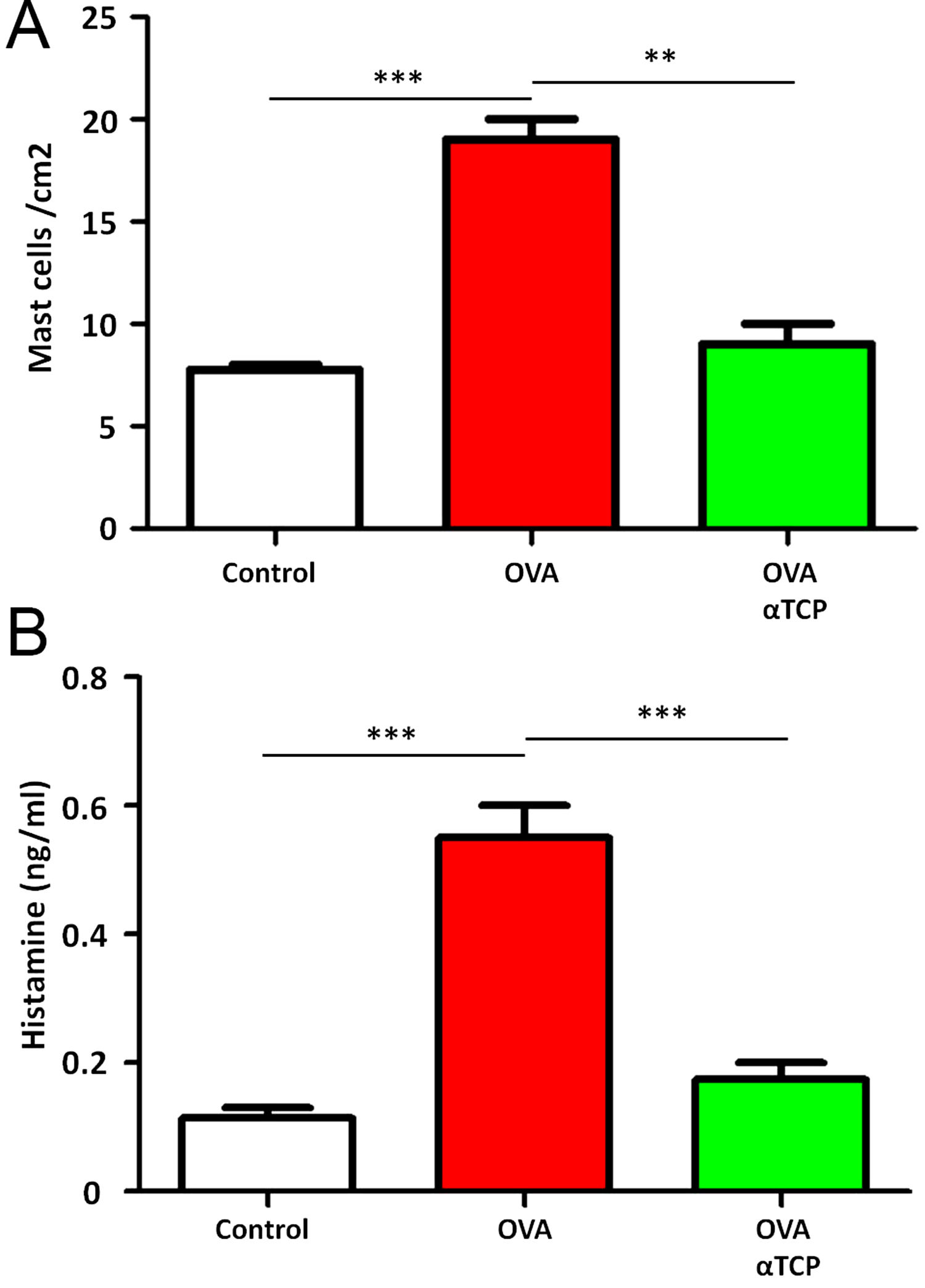
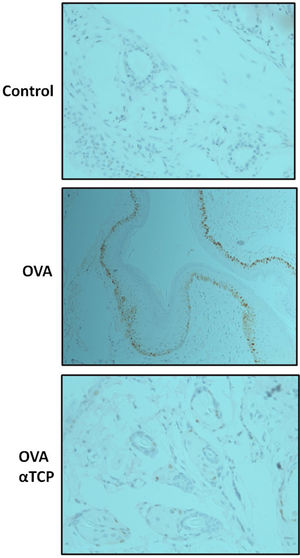
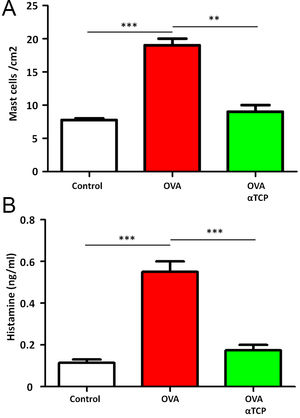
Subepithelial distribution of tryptase positive mast cells was reduced after α-TCP treatmentMast cells play a central role in the immune mechanisms of allergic rhinitis.9 There was an increase in the number of tryptase positive mast cells with subepithelial and deeper distribution in nasal mucosa of the OVA group (Fig. 4). Subepithelial and deeper distribution of tryptase positive mast cells in alpha-TCP treatment of OVA-sensitized mice demonstrated statistically reduced in nasal mucosa when compared to OVA-treated mice (Fig. 4). Toluidine blue-positive mast cells in the nasal mucosa sections from OVA (n=5) and α-TCP mice (n=5) were counted under the microscope (Fig. 5A). Single-cell suspensions of the nose (1×106) from OVA and α-TCP mice were lysed for total histamine (n=5 for each group); the results demonstrated that histamine release was reduced after α-TCP treatment (Fig. 5B).
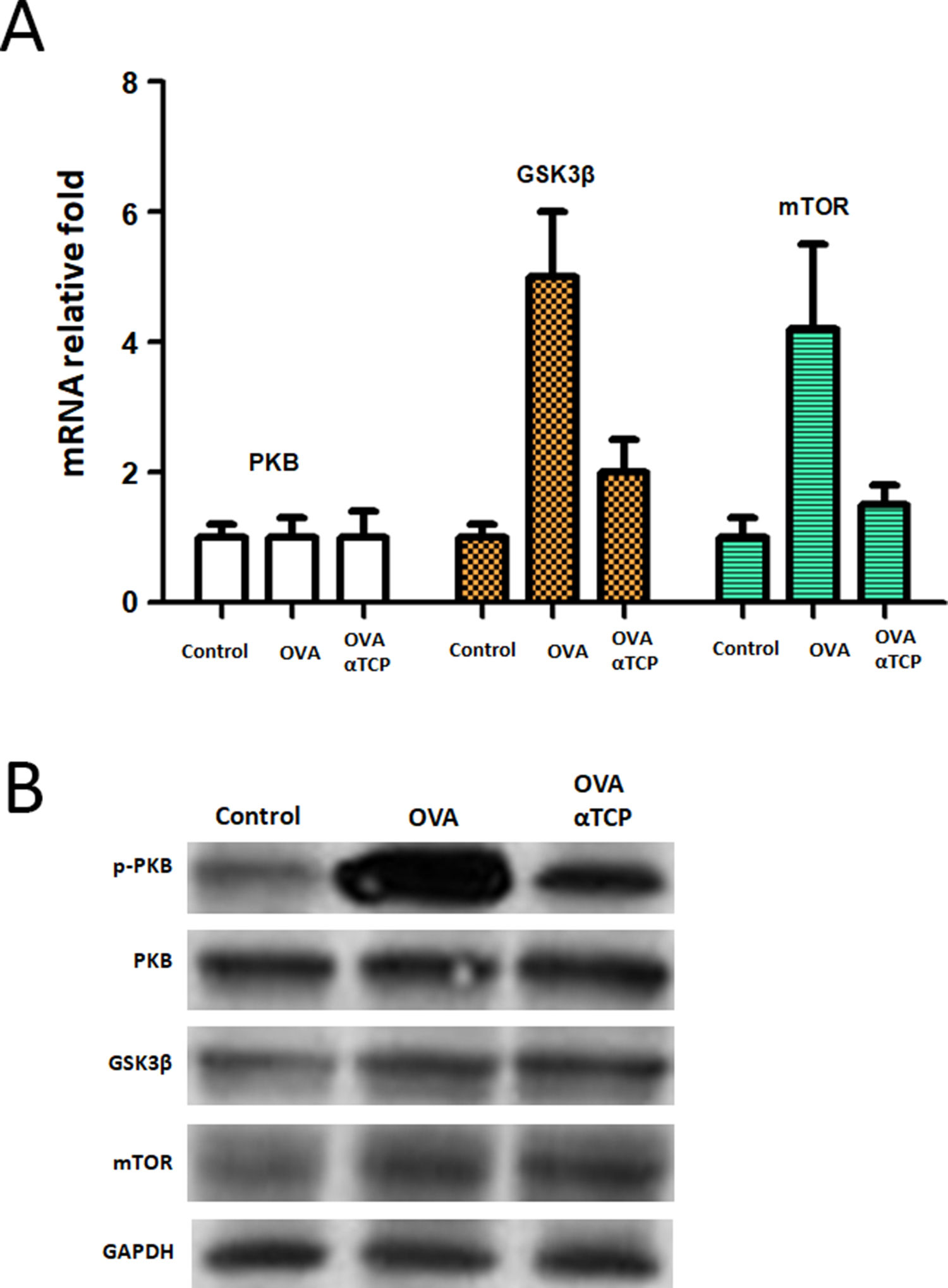
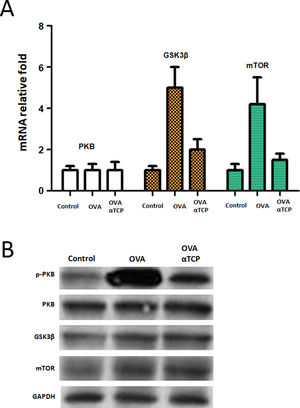
We further examined the mRNA level of PKB expression and PKB targets GSK3β and mTOR expression. Real-time PCR results showed that PKB downstream genes GSK3β and mTOR were significantly down regulated in mast cells isolated from alpha-TCP treatment of OVA-sensitized mice compared to those of the OVA group (Fig. 6). Western-blot demonstrated that p-PKB protein was inhibited in alpha-TCP treatment of OVA-sensitized mice compared to the OVA group (Fig. 6), further GSK3β and mTOR protein levels were also down regulated after α-TCP. However, both PKB mRNA and protein were not significantly changed between alpha-TCP treatment of OVA-sensitized mice and the OVA group.
mRNA and protein levels of PKB, GSK3β and mTOR. n=10 each group. Data are representative of at least two independent experiments. n.s. not significant; *: p<0.05; **: p<0.01; ***: p<0.001. Error bar values represent SEM. For comparison between two groups, Student’s two-tailed t test was used. Western-blot to detect protein levels of p-PKB, PKB, GSK3b and mTOR.
Alpha-tocopherol has antioxidant, gene regulatory, and anti-inflammatory properties.10,11 For example, α-TCP could attenuate LPS, IL-1, and TNFα-induced activation of alveolar macrophages in rat.12 Furthermore, the treatment of mice with α-TCP inhibited lung leukocyte accumulation and injury.11 α-TCP has redox properties further. In allergy, oxidant/antioxidant imbalances cause an oxidative stress that elicits the eventual pathophysiological effects. Other groups demonstrated that ү-tocopherol inhibits inflammation tissue injury by blocking reactive nitrogen species.13,14 J.G. Wagner et al.15 reported that ү-TCP could suppress lung inflammation induced by different allergens. Specifically, they further showed attenuation by ү-TCP of inflammatory cell recruitment and over production of mucus that characterizes allergic rhinoconjunctivitis. In this study we examined the inhibitory function of α-TCP in a mouse model of allergic rhinitis. Our results demonstrated that nasal administration of alpha-TCP before nasal challenge in OVA-sensitized mice suppressed the development of nasal symptoms. The numbers of nasal eosinophils and mast cells were reduced, and gene expressions of Th2 cytokines were suppressed and the Th1 cytokine was up regulated, total IgE, specific IgE and specific IgGs were reduced, and PKB pathway in mast cells was inhibited in alpha-TCP-treated mice compared with the OVA-treated control mice. Our findings demonstrated the importance to discriminate first stage response (by mast cells) and second stage response (by eosinophils and Th2 cells) during inflammatory response of nasal mucosa. However, it remains insufficient while critical to further examine the effect of αTCP on these two types of responses.
Mast cells participate in the immediate-phase allergic reaction as effector cells, especially IgE production and IgE-IgE receptor interaction.9 Nasal mast cells and T cells in allergic rhinitis are important sources of IL-4 and IL-13, and can also induce IgE synthesis.9 The action of histamine is important on the subepithelial blood vessels, causing vasodilatation, hyperemia, and mucosal edema. The inflammatory response in the nasal mucosa includes two stages. The first stage is an immediate IgE-mediated mast cell response; the second stage is a late phase response characterized by the recruitment of eosinophils and Th2 cells.16 Human mast cells were classified by the type of neutral proteases they express.17 There are two types of mast cells, including mast cells that contain only tryptase, and other mast cells that contain chymase, cathepsin G and carboxypeptidase. Mast cells are discovered to be abundantly accumulated in nasal mucosa epithelial compartment of AR patients.18 In our study, we found that the subepithelial distribution of tryptase positive mast cells was reduced after α-TCP treatment. More importantly, the PI3K-PKB pathway was suppressed by α-TCP in mast cells. PI3K leads to the production of 3-phosphorylated inositol lipids, which cause the activation of protein kinase B that regulates mTOR and glycogen synthase kinase-3 activity. PI3K-PKB-mTOR signaling is already known to regulate myeloid dendritic cells development in humans.19,20 Therefore, these results demonstrated that α-TCP-mediated suppression of PI3K-PKB activity in mast cells is a potential mechanism of anti-allergic function of α-TCP. Our data further indicated the involvement of the PI3K-PKB pathway in the effect of αTCP on mast cell-mediated inflammation. Because the PI3K-PKB pathway is critical for cell growth and survival, the authors strongly suggested to speculate on the relation between reduced mast cell number and reduced inflammation/allergy by αTCP.
There are some limitations in this study. First, gene-engineered animal model of PI3K-PKB knockout mice should be used to confirm the requirement of PI3K-PKB activation in AR development. The anti-allergic function of α-TCP should be validated using PI3K-PKB knockout mice. In addition, the effect of αTCP on each of mast cells and eosinophils deserves further investigation. Whether the PI3K-PKB pathway is involved in the effect of αTCP on eosinophils is also a future research direction.
Taken all together, the suppression of mast cell PI3K-PKB by α-TCP may contribute to anti-allergic function of α-TCP in animal allergic rhinitis. The findings provide potential novel strategies for therapeutic intervention. PI3K-PKB inhibitors may be considered an effective symptomatic treatment for AR patients.
Conflicts of interestThe authors declare that they have no conflict of interest.
Ethics statementThe study was approved by the research committee of ZhangJiaGang Hospital of Soochow University, Suzhou, China.
This study was supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK20161272). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.