Frequent opportunist fungal infections and the resistance to available antifungal drugs promoted the development of new alternatives for treatment, like antifungal drug combinations.
AimsThis work aimed to detect the antifungal synergism between statins and azoles by means of an agar-well diffusion bioassay with Saccharomyces cerevisiae ATCC 32051 and Candida utilis Pr1–2 as test strains.
MethodsSynergistic antifungal effects were tested by simultaneously adding a sub inhibitory concentration (SIC) of statin (atorvastatin, lovastatin, pravastatin, rosuvastatin or simvastatin) plus a minimal inhibitory concentration (MIC) of azole (clotrimazole, fluconazole, itraconazole, ketoconazole or miconazole) to yeast-embedded YNB agar plates, and a positive result corresponded to a yeast growth inhibition halo higher than that produced by the MIC of the azole alone. Yeast cell ergosterol quantification by RP-HPLC was used to confirm statin–azole synergism, and ergosterol rescue bioassays were performed for evaluating statin-induced ergosterol synthesis blockage.
ResultsGrowth inhibition was significantly increased when clotrimazole, fluconazole, itraconazole, ketoconazole and miconazole were combined with atorvastatin, lovastatin, rosuvastatin and simvastatin. Highest growth inhibition increments were observed on S. cerevisiae (77.5%) and C. utilis (43.2%) with a SIC of simvastatin plus a MIC of miconazole, i.e. 4+2.4μg/ml or 20+4.8μg/ml, respectively. Pravastatin showed almost no significant effects (0–7.6% inhibition increase). Highest interaction ratios between antifungal agents corresponded to simvastatin–miconazole combinations and were indicative of synergism. Synergism was also confirmed by the increased reduction in cellular ergosterol levels (S. cerevisiae, 40% and C. utilis, 22%). Statin-induced ergosterol synthesis blockage was corroborated by means of ergosterol rescue bioassays, pravastatin being the most easily abolished inhibition whilst rosuvastatin being the most ergosterol-refractory.
ConclusionsSelected statin–azole combinations might be viable alternatives for the therapeutic management of mycosis at lower administration doses or with a higher efficiency.
La frecuencia de micosis oportunistas y la resistencia a los antimicóticos convencionales han fomentado la búsqueda de nuevas alternativas terapéuticas, como las combinaciones de antimicóticos.
ObjetivosEl presente estudio trató de detectar el sinergismo antifúngico entre las estatinas y los azólicos mediante un bioanálisis de difusión en pocillos de agar, utilizando Saccharomyces cerevisiae (S. cerevisiae) ATCC 32051 y Candida utilis (C. utilis) PR1-2 como cepas de control.
MétodosLos efectos antifúngicos sinérgicos se examinaron mediante la adición simultánea de una concentración sub-inhibitoria (CSI) de estatina (atorvastatina, lovastatina, pravastatina, rosuvastatina o simvastatina) más una concentración mínima inhibitoria (CMI) de un azólico (clotrimazol, fluconazol, itraconazol, ketoconazol o miconazol) a placas de agar YNB con las levaduras sembradas por inclusión. Un resultado positivo correspondió a un diámetro del halo de inhibición del crecimiento de la levadura mayor que el producido por la CMI del azólico exclusivo. Para confirmar el sinergismo estatina-azólico, se cuantificó el ergosterol de la membrana celular de las levaduras con cromatografía líquida de alto rendimiento (HPLC-RP). Para valorar la inhibición de la síntesis de ergosterol inducida por estatinas, se emplearon bioanálisis de rescate de ergosterol.
ResultadosLa inhibición del crecimiento aumentó significativamente cuando se combinaron clotrimazol, fluconazol, itraconazol, ketoconazol y miconazol con atorvastatina, lovastatina, rosuvastatina y simvastatina. Los mayores incrementos de la inhibición del crecimiento se observaron en S. cerevisiae (77,5%) y C. utilis (43,2%) con una CSI de simvastatina y una CMI de miconazol de 4+2,4μg/ml o 20+4,8μg/ml, respectivamente. Para pravastatina apenas se identificaron efectos significativos (incremento de la inhibición del 0-7,6%). Los mayores cocientes de interacción correspondieron a la combinación de simvastatina y miconazol y fueron indicativos de sinergismo. Este también se confirmó por la mayor disminución de los niveles celulares de ergosterol (S. cerevisiae, 40% y C. utilis, 22%). La inhibición de la síntesis de ergosterol inducida por estatinas se corroboró mediante bioanálisis de rescate de ergosterol, donde la inhibición por pravastatina se abolió con facilidad, mientras que la de rosuvastatina fue la más refractaria.
ConclusionesLas combinaciones seleccionadas de estatinas y azólicos podrían ser alternativas viables para el manejo terapéutico de las micosis, en dosis más bajas o con una mayor eficiencia.
The incidence of systemic mycoses has dramatically increased over last years, a fact particularly favoured by the raising prevalence of acquired immune deficiency syndrome (AIDS) and the unrestrained use of immunocompromising drugs.9 Additionally, a number of different predisposing factors have been associated with the prevalence of infections caused by yeasts, even for immunocompetent patients.21,25 As already emphasized, the administration of azoles for treating fungal infections has encountered certain limitations, such as their low water solubility, low bioavailability, and frequent side effects consequent on the requirement of high doses and/or long-term administration.21,42
Rare fungal species of low pathogenic potential, or even species never described before as a cause of disease, are being more commonly detected in hospital settings as etiological agents of infections.39 Numerous cases of superficial or mild systemic infections caused by Saccharomyces cerevisiae, a yeast “generally regarded as safe” (GRAS) for industrial applications, have been up today reported, particularly in immunocompromised patients. So, safety status traditionally adjudicated to this microorganism has been upgraded to Biosafety level one in Europe.20,26 In this way, although not as virulent as C. albicans, the classical identification of S. cerevisiae as a non-pathogenic yeast has changed to opportunistic pathogen. As a further complication, the resistance of S. cerevisiae to certain antifungal agents has been also repeatedly observed.2,26,36,38
On the other hand, most of the reported candidosis have Candida albicans as the causative agent.4 However, although less frequently, the emergence of other species of the genus has also been described over last decades.28,39 Among those unconventional opportunistic pathogen Candida species, C. utilis, a yeast commonly used with biotechnological purposes such as single-cell protein production, has been reported in opportunistic fungemia.1,6,17
Considering the increased incidence of opportunist fungal infections and the development of fungal drug resistances, a great deal of attention has been focused on the investigation of new alternatives for treatment.9,14 Combination of antifungal agents with other drugs in order to improve the efficacy and/or decrease the toxicity has been described early as one of the possibilities.21,29,33 In this context, studies on the interaction between azoles and statins have gained renewed attention.10,11,27,32,37
Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the key enzyme which catalyzes the rate limiting step of sterols biosynthesis.24 This inhibition affects fungal propagation by decreasing ergosterol levels and its precursors. Additionally, their ability to block the synthesis of intermediate products in the mevalonate pathway critically influences cellular functions, thus being regarded as apoptosis-inducing agents.30,41 Yeast mitochondrial dysfunction and respiratory deficit have been also associated to statins.40 On the other hand, the toxicity of azoles against fungi results from the inhibition of the cytochrome P450-dependent C-14 lanosterol α-demethylase.18
An advantage of the synergistic interaction between these two kinds of drugs would be the low hydrophobicity and toxicity of statins for humans, as compared with the azole-family drugs. In this study, the in vitro activity of five azoles (clotrimazole, fluconazole, itraconazole, ketoconazole, miconazole) and five statins (atorvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin), either alone or in combinations, was tested against the low-virulent opportunistic pathogen yeasts S. cerevisiae and C. utilis by means of an agar-well diffusion bioassay in order to detect possible synergistic effects and to identify the most promising combinations.
Materials and methodsAntifungal agentsClotrimazole, fluconazole, ketoconazole and miconazole nitrate were purchased from Parafarm Laboratories (Argentina), and itraconazole was purchased from Sigma Chemical Co. (St Louis, MO, USA). Drugs were obtained as powders and stock solutions were prepared at a concentration dependent on the potency of each tested drug. Commercial statins (10mg per tablet) of: Liparex® (atorvastatin), Mevlor® (lovastatin), Pravacol® (pravastatin), Crestor® (rosuvastatin) and Tanavat® (simvastatin) were used to prepare standard-stock solutions. In order to obtain the active β-hydroxyacid form of statins, commercially provided in the lactone inactive form, a preliminary conversion was carried out as previously described.8,31 Subsequently, purified statins in the β-hydroxyacid form were extracted with HPLC-grade ethyl acetate and quantification by reversed-phase HPLC (RP-HPLC) was performed as previously described,8 in order to confirm final concentration.
Yeast strainsThe strains S. cerevisiae ATCC 32051 (American Type Culture Collection), and Candida utilis Pr1–2 (PROIMI-MIRCEN Culture Collection, Tucumán, Argentina), were systematically used for bioassays.
Bioassay procedureAs indicated previously (see Introduction section), statins are, like azoles, able to inhibit fungal growth, although by a different way. To evaluate the effect of selected azoles and statins as well as their combinations on fungal growth, a bioassay was developed. For this in vitro test, the yeasts S. cerevisiae ATCC 32051 and C. utilis Pr1–2 were grown at 26°C for 16h in YEPD liquid medium, harvested by centrifugation (3000×g, 10min), washed twice and re-suspended in sterile saline solution. Plate bioassays were performed by seeding 25ml of sterile molten yeast nitrogen base agar (YNB w/o amino acids and (NH4)2SO4; Difco, Detroit, MI, USA), supplemented with 20g/l glucose and 0.6g/l (NH4)2SO4 with 400μl of yeast suspension (OD550nm=0.6). Inoculated medium was poured into a 15-cm-diameter glass Petri dish. After solidifying, 6-mm-diameter wells were made with the aid of a sterile cork borer and 25μl of each tested drug dilution or combination was added in triplicate into the agar wells.
To determine the statins MICs (minimal inhibitory concentrations) and SICs (sub inhibitory concentrations), concentrations between 0.04 and 0.4μg/ml were tested. Meanwhile, azoles were assayed at varying concentrations for MIC determination, as follows: for S. cerevisiae, ketoconazole 200–1000μM; miconazole 0.01–5μM and clotrimazole 60–150μM and for C. utilis, ketoconazole 200–1000μM; miconazole 0.1–60μM and clotrimazole 60–200μM.
Synergism between the five tested azoles and each of the selected statins was evaluated by combining a SIC of statins plus an azole MIC. MIC was defined as the lower antifungal dilution at which a naked-eye detectable halo was formed by antifungal drug concentric diffusion.3,21 Sub inhibitory concentration (SIC) was arbitrarily defined as the inhibitor dilution immediately below the MIC, at which no halo was detected. Controls were included in order to verify the lack of solvent inhibitory effects on yeast growth. Plates were incubated at 26°C for 16h and inhibition halo diameters were subsequently scored.
Bioassay data analysisA statin–azole combination (a SIC plus a MIC, respectively) was qualitatively regarded as synergistic when the diameter of the formed halo was higher with respect to that produced by the MIC of the azole. The synergistic effects observed were confirmed calculating the interaction ratio, IR=Io/Ie. Ie is the expected (theoretical) growth inhibition percentage for a given interaction between two agents, and could be calculated from the Abbott formula: Ie=X+Y−(XY/100) where X and Y are the inhibition percentages due to the individual agents’ effect. Considering Io as the observed real inhibition percentage, IR would reflect the nature of the interaction between antifungal drugs. IR values between 0.5 and 1.5 correspond to additive interactions, IR<0.5 indicates antagonism and IR>1.5 denotes synergism.13 Bioassays were run in triplicate and mean and standard deviation values were calculated (Microcal OriginTM version 6.0).
Sterols preliminary evaluationTotal sterols content was assessed after saponification, as early reported by Breivik and Owades,7 with slight modifications. One millilitre of S. cerevisiae or C. utilis cell suspensions (OD550nm=0.6) was used to inoculate 200ml of YNB liquid medium containing SICs of miconazole and/or simvastatin. Growth controls without inhibitory agents were also performed. Cultures were incubated 16h at 26°C with shaking. Cells were harvested by centrifugation (3000×g, 15min) and washed twice with sterile saline solution. Two hundred milligrams of biomass wet weight were treated with 10ml of 30% (w/v) ethanolic KOH solution. After 3min of vortexing, cell suspension was transferred to screw-capped glass test tubes and incubated in a water bath at 80°C for 1h. Once the tubes were cooled to room temperature, 5ml of distilled water:n-hexane (1:3, v/v) mixture was added. After vortexing for 3min, the non-saponifiable fraction was extracted by centrifugation (10000×g, 10min) into the n-hexane layer. The obtained extracts were evaporated to dryness under vacuum and re-dissolved in 1ml of n-hexane. Ergosterol, purchased from Sigma Chemical Co., was used to prepare the standard-stock solution. Sterols were detected by thin layer chromatography (TLC) using silica gel 60F254 aluminium sheets (Merck, Darmstadt, Germany) and n-hexane: ethyl acetate (4:1, v/v) as the mobile phase. Plates were stained with p-anisaldehyde reagent and scanned for image processing and analysis by using the ImageJ program.
Ergosterol quantificationThe n-hexane extracts obtained as above described were evaporated to dryness under vacuum and re-dissolved in 1ml of methanol. Ergosterol content in the samples was determined by RP-HPLC using a Waters e2695 HPLC model with a Waters 2998 PDA detector (MA, USA) operating at 283nm (scanning tool in the range 200–600nm). A 150×4.6mm Phenomenex Gemini C18, 3-μm particle size column, with an integrated Phenomenex SecurityGuard C18 pre-column, was used. Analyses were performed under isocratic conditions. The mobile phase consisting of methanol:acetonitrile (100:2, v/v) was eluted at 1mL/min, and temperature was maintained at 30°C. Ergosterol from Sigma Chemical Co. was used as standard.
Ergosterol reversion bioassayTo evaluate the possibility to rescue statin-inhibited yeasts by sterol supplementation, a bioassay as described above was performed with both S. cerevisiae and C. utilis, in the presence of statins plus ergosterol. After solidifying YNB inoculated plates, agar wells were filled with 25μl of either a statin solution with a concentration equivalent to the MIC (see Results section) or, a solution containing a combination of statin (at MIC) plus variable concentrations of ergosterol (5–25mmol/l) dissolved in ethyl acetate. All assays were performed in triplicate and growth controls with either the solvent or the solvent plus ergosterol were included to verify the lack of yeast growth inhibitory effects. Plates were incubated at 26°C for 16h and subsequently, inhibition halo diameters were measured.
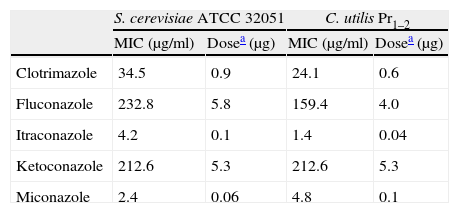
ResultsThe MICs and SICs of the azoles and statins were assessed using the herein proposed agar-well diffusion bioassay prior to testing their synergism. According to the azole MICs, C. utilis was more sensitive than S. cerevisiae to clotrimazole, fluconazole and itraconazole (Table 1). On the contrary, the MICs for atorvastatin, lovastatin, rosuvastatin and simvastatin were 40μg/ml against S. cerevisiae whilst 200μg/ml in the case of C. utilis, with corresponding doses of 1 and 5μg per well, respectively. Pravastatin showed the lowest yeast inhibition power, with a MIC of 200μg/ml against both yeasts. Based on these first results, the SICs of statins used for synergism tests were 4μg/ml for S. cerevisiae and 20μg/ml for C. utilis, whereas in the case of pravastatin, 20μg/ml was applied for both yeast strains.
Minimal inhibitory concentration (MIC) of azoles and the corresponding doses against S. cerevisiae ATCC 32051 and C. utilis Pr 1–2, as assessed by the agar-well diffusion bioassay.
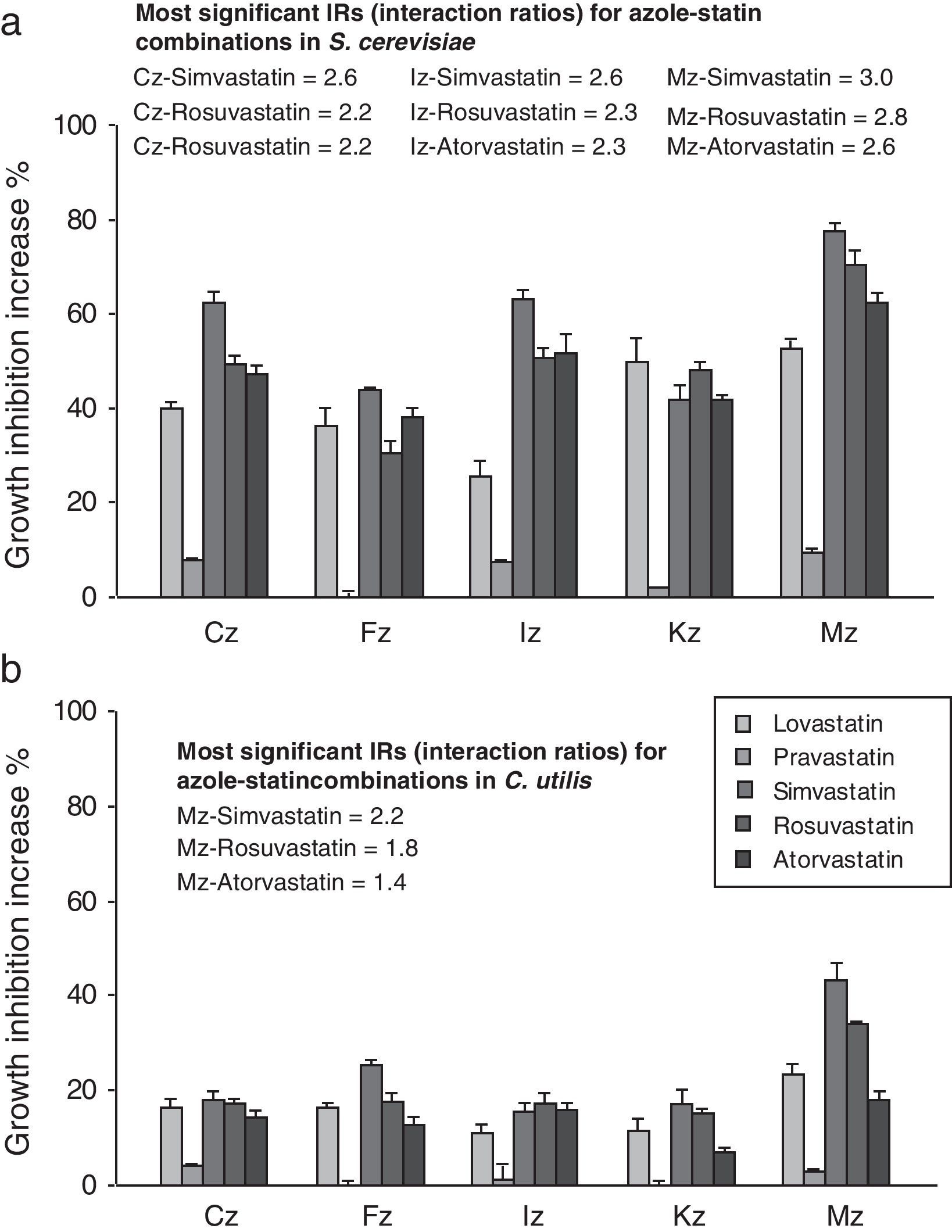
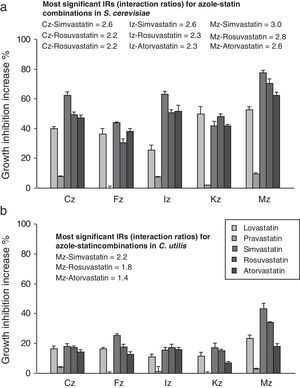
When azoles were applied in combination with atorvastatin, lovastatin, rosuvastatin and simvastatin, inhibition haloes became increased in comparison to the independent azole effect thus giving evidence of their synergism. The growth inhibition potentiation ranged between 25.3 and 77.5% for S. cerevisiae (Fig. 1a), and between 7.0 and 43.2% for C. utilis (Fig. 1b). Pravastatin exhibited the mildest synergism in both yeasts, with increment values around 0–7.6% for S. cerevisiae, and between 0 and 4% against C. utilis. The best combination according to the in vitro activity resulted from the association between the SIC of simvastatin and the MIC of miconazole, displaying a 77.5% inhibition enhancement against S. cerevisiae, and 43.2% against C. utilis (Fig. 1a and b).
Synergism between clotrimazole (CLT), fluconazole (FLC), itraconazole (ITC), ketoconazole (KTC) and miconazole (MCZ) at MIC, in combination with SICs of lovastatin, pravastatin, simvastatin, rosuvastatin and atorvastatin, as witnessed by the agar-well diffusion bioassay with S. cerevisiae ATCC 32051 (a) and C. utilis Pr 1–2 (b). Results are expressed as the percent increase in growth inhibition haloes in comparison to the individual azole inhibitory effects and interaction ratios (IRs) are also annotated. MIC, minimal inhibitory concentration; SIC, sub inhibitory concentration.
According to the IR criterion, the best combinations against S. cerevisiae were those between either atorvastatin, rosuvastatin or simvastatin at SIC with MICs of clotrimazole (IR, 2.2–2.6), itraconazole (IR, 2.3–2.6) or miconazole (IR, 2.6–3). Usually, the highest values corresponded to simvastatin associations. In the case of C. utilis, the most significant synergistic effects were observed for miconazole–rosuvastatin (IR, 1.8) and miconazole–simvastatin (IR, 2.2). For both yeasts, the highest IR values corresponded to miconazole–simvastatin associations (Fig. 1a and b).
In order to confirm the presence of a synergistic effect between statins and azoles and, to study the synergism influence on total cellular sterols content, the tested yeasts strains were grown in liquid culture media amended with either miconazole, or miconazole plus simvastatin. All of them were added at the SIC value previously assayed in liquid cultures (data not shown), which corresponded to 2.5μg/ml simvastatin plus 0.5μg/ml miconazole for S. cerevisiae, and 12.5μg/ml simvastatin plus 1.0μg/ml miconazole for C. utilis. The simultaneous use of azole and statin SIC values complied with the necessity of obtaining a suitable amount of yeast biomass for sterol extraction.
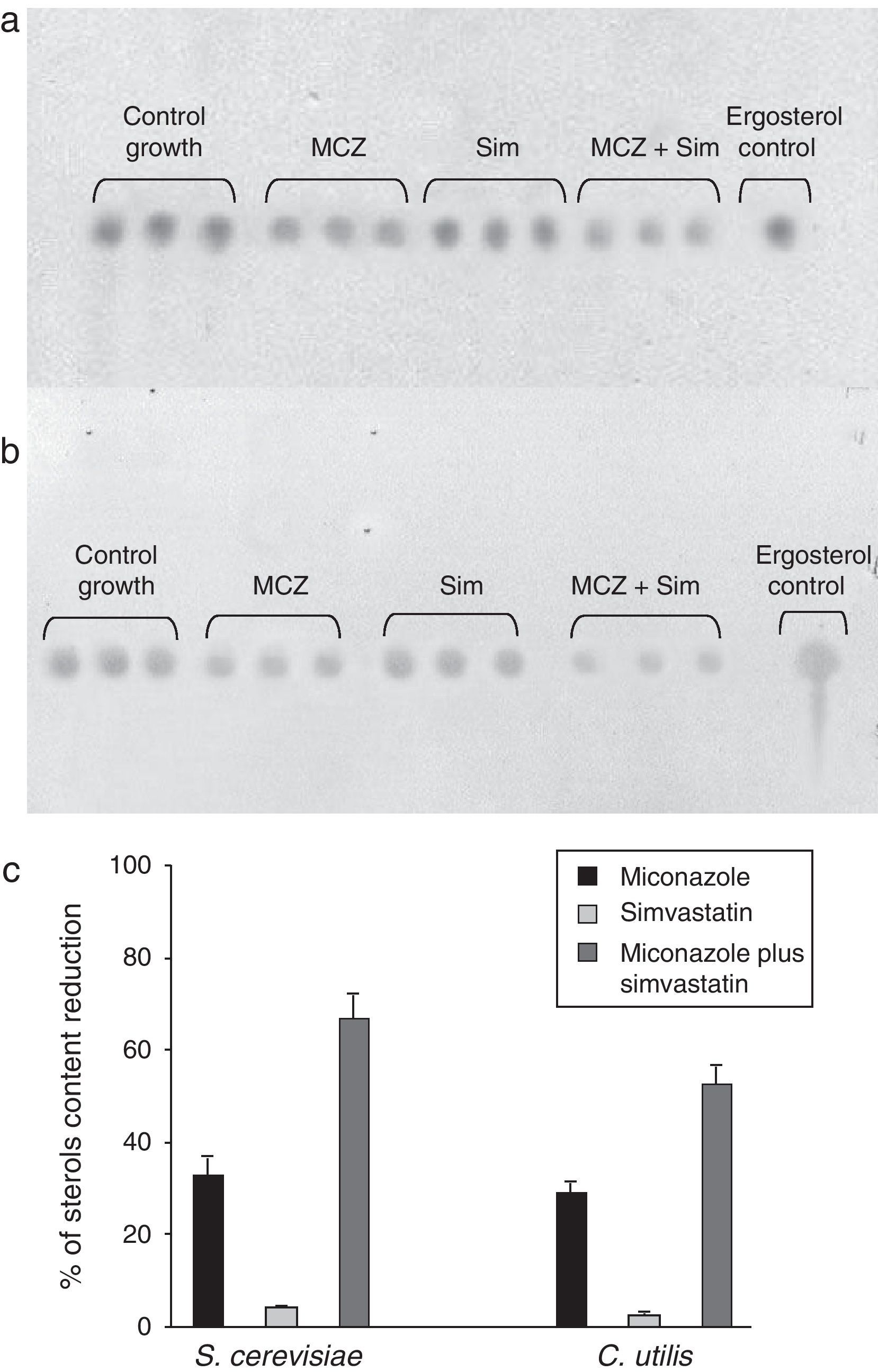
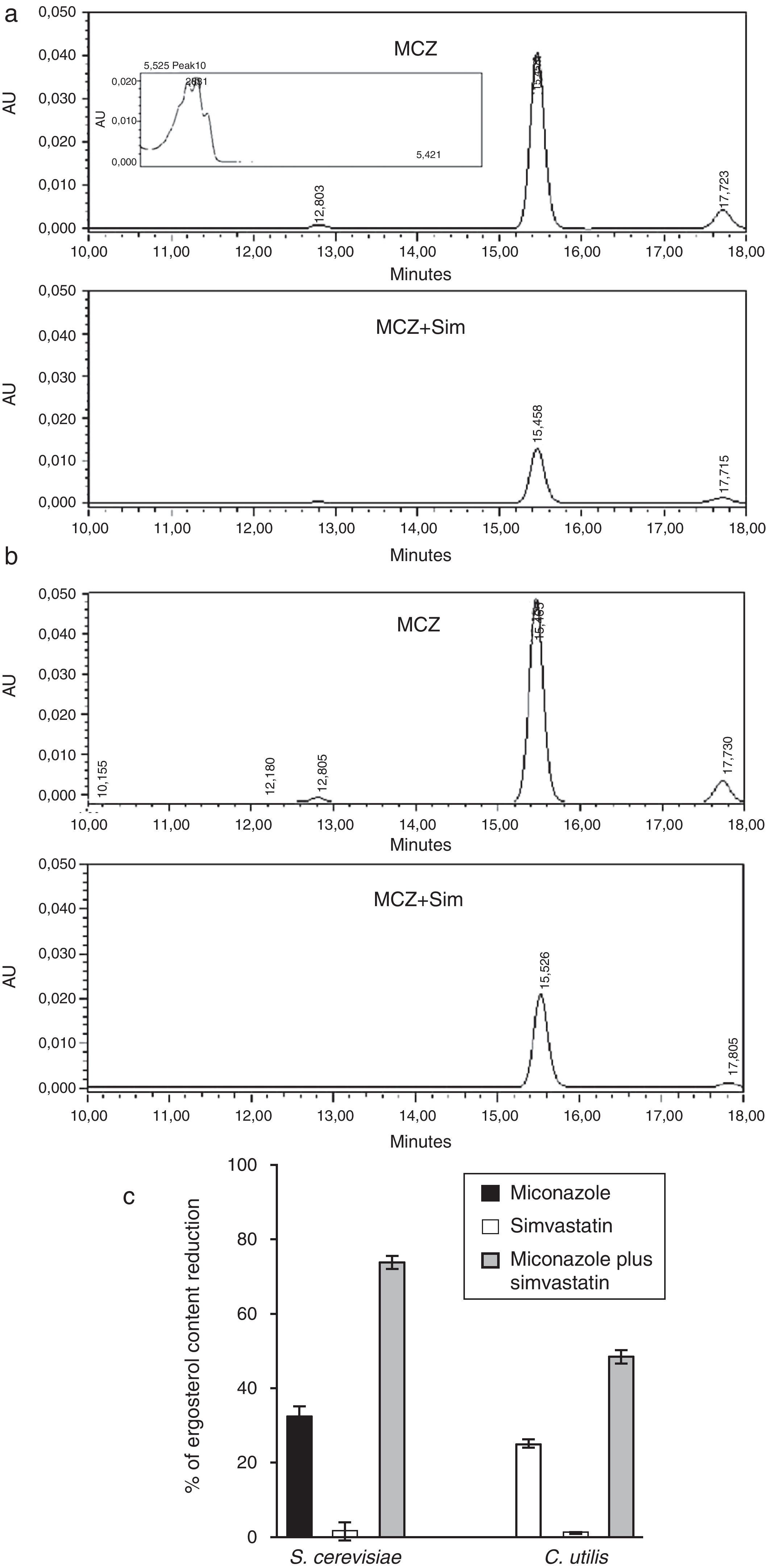
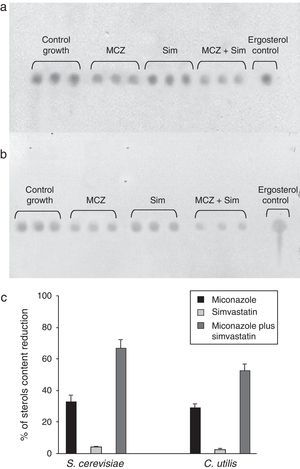
As it could be noted, the SICs of both miconazole and simvastatin were lower than those required on solid cultures (see Table 1 and results above), a fact not surprising considering the closest contact of yeasts and drugs, and the better diffusion rate under submerged culture conditions. Data concerning the sterol content reduction is displayed in Figs. 2 and 3. A preliminary indication was provided by TLC (Fig. 2), and these results were further confirmed by RP-HPLC analyses (Fig. 3) by using an ergosterol calibration curve (0–500μg/ml; R2=0.994).
Effects of miconazole (MCZ), simvastatin (Sim) and their synergic combination (MCZ+Sim) on the cellular sterols content of S. cerevisiae ATCC 32051 (a) and C. utilis Pr 1–2 (b) after growth in liquid cultures, as detected by TLC. (c) Graphical representation of TLC data, as analyzed by means of the ImageJ program. Percentages of reduction are referred to the control growth with no MCZ or Sim. Results from independent triplicate culture/extractions are displayed.
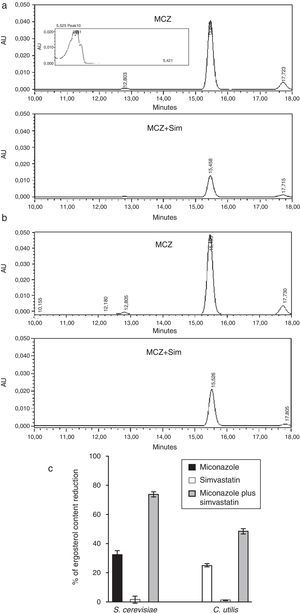
Influence of miconazole (MCZ), simvastatin (Sim) and their synergic combination (MCZ+Sim) on the cellular ergosterol content of S. cerevisiae ATCC 32051 (a) and C. utilis Pr 1–2 (b) after growth in liquid cultures, according to RP-HPLC. (c) Graphical representation of RP-HPLC data. Percentages of reduction are referred to the control growth with no MCZ or Sim (7.68 and 12.71mg ergosterol/g biomass wet weight for S. cerevisiae or C. utilis, respectively). Results correspond to independent triplicate culture/extractions. AU: absorbance units. Inset in (a) corresponds to UV–vis spectrum of ergosterol peak.
Analytical data revealed that the exposure of S. cerevisiae to simvastatin plus miconazole resulted in a 40%-higher ergosterol level reduction than the accumulated miconazole and simvastatin individual effects (Fig. 3a and c). Likewise, in the case of C. utilis, the same drugs association led to a 22%-enhanced ergosterol level drop as compared to the summation of simvastatin and miconazole separate effects (Fig. 3b and c).
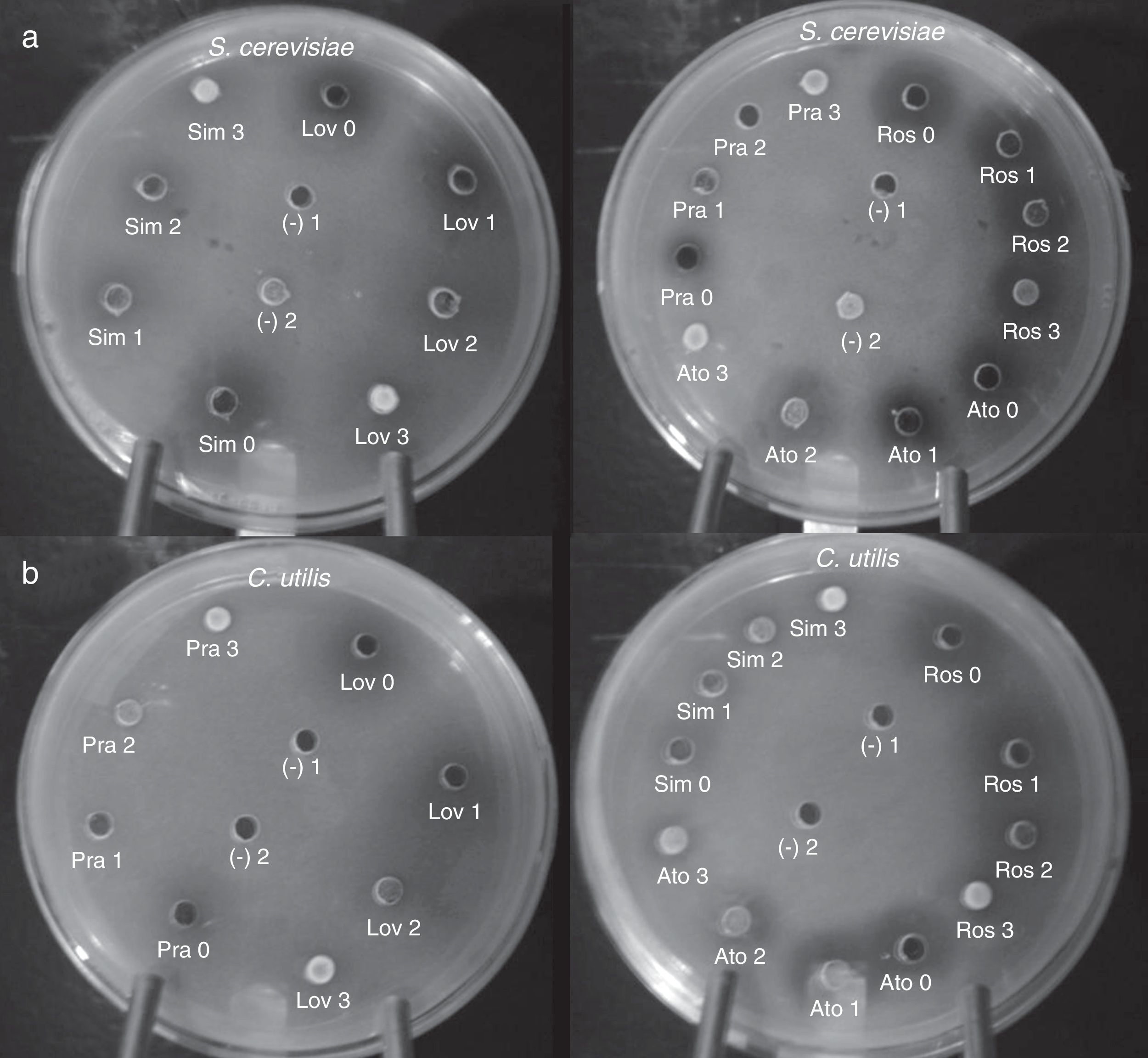
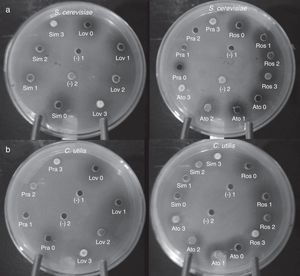
Finally, in order to confirm the implication of a blocked ergosterol biosynthesis in the growth inhibition of tested yeast cells, cultures were supplemented with increasing concentrations of ergosterol. In general, yeast cell growth could be progressively restored as ergosterol concentration increased (Fig. 4). In reversion tests, ergosterol could be incorporated in ethyl acetate solutions with no need of combination with Tween 80, as previously indicated.23 Ergosterol rescue could be detected at concentrations between 5 and 25mmol/l.
Ergosterol rescue of statin-induced growth inhibition in S. cerevisiae ATCC 32051 (a) and C. utilis Pr 1–2 (b). Wells were filled in with either solutions containing statins at MIC, or a combination of statin (at MIC) plus variable concentrations of ergosterol (1–3) dissolved in ethyl acetate (see Results section). Ato: atorvastatin, Lov: lovastatin, Pra: pravastatin, Ros: rosuvastatin, Sim: simvastatin. Ergosterol concentrations: 0, none, 1=5mmol/l, 2=10mmol/l, 3=25mmol/l. Controls: (–)1, ethyl acetate; (–)2, ethyl acetate-dissolved ergosterol. MIC: minimal inhibitory concentration.
The obtained results confirmed the rescue of statin-induced growth inhibition by means of ergosterol supplementation, both in S. cerevisiae and C. utilis. However, differences in ergosterol reversion could be observed among tested statins, pravastatin being the easiest inhibition to be abolished and rosuvastatin being the most ergosterol–refractory inhibition in both yeasts (Fig. 4). It should be also noted that, despite inhibition could be reverted to a higher or lesser extent, particularly for some statins, yeast growth could not be completely restored.
DiscussionThis work represents a practical approach for testing the synergistic antifungal interaction between several statins and azoles by means of the use of a straightforward agar-well diffusion bioassay. According to this methodology, the effects of five statins (atorvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin) and five azoles (clotrimazole, fluconazole, itraconazole, ketoconazole and miconazole) in twenty-five different antifungal combinations could be tested in a time-saving, simple, low-cost and reproducible bioassay.
Previous reports emphasized the relevance of studying the in vitro sensitivity to antifungals in order to obtain relatively reliable results to select the most appropriate drug for fungal treatment. To this end, continuous trials to normalize and standardize in vitro tests have been performed.9
Up to date studies published about the effect of statins, azoles or their combinations against yeasts were mostly performed in liquid cultures.11,13,21–23,27,32 Only Chin et al.11 mentioned in their work a preliminary test carried out on solid medium to determine antifungal activity of simvastatin, lovastatin, fluvastatin and pravastatin, but only fluvastatin was active under those conditions. That may have been due to the use of inactive-statin lactone forms, whose activation via hydrolysis is probably not possible under solid culture conditions. More recent work also briefly mentioned the growth inhibition effects of atorvastatin and simvastatin on C. glabrata during growth on solidified minimal media.40 However, statin–azole combined effects do not seem to have been previously assessed according to the herein proposed methodology.
The statin MIC values obtained in the present work would be in the order of those elsewhere reported by other researchers. The higher pravastatin concentrations eventually found may be related to the high hydrophilic nature of this statin, the most polar between natural statins. Lovastatin values ranging from 5 up to 200μg/ml have been already described for testing inhibitory effects and sterols levels modifications on yeasts.3,12,16,21 Macreadie et al.23 found MIC values higher than 40μg/ml for simvastatin and 100μg/ml for atorvastatin against Candida strains. Also for fluvastatin, a potent synthetic statin, complete inhibition of C. albicans at physiological pH would require around 50μg/ml,32 whilst MIC values above 128μg/ml were described for different Candida species and Cryptococcus neoformans.11 Distinct sensitivity to statins was also described by Lukács et al. in Mucor circinelloides,22 with MIC values in the order of those herein reported and being fluvastatin much more effective than lovastatin and simvastatin.
Controversy is frequently observed when comparing statins susceptibility data. That may be due to differences in the applied methodology and the strains involved.22 Divergence in susceptibility among fungal genera and species has been already demonstrated, although the underlying molecular background for this distinctive behaviour has not been discovered yet. A possible explanation may be a difference in HMG-CoA reductase gene copy numbers. Lukács et al. have elegantly demonstrated a decreased sensitivity to statins in M. circinelloides when the hmgR gene dose became increased by transformation.22 Likewise, differences on the effectiveness among statins have been correlated to structural divergences, which may influence the interaction with the catalytic site of HMG-CoA reductase.13
As also pointed out for azolic antifungals, variables such as culture medium, pH, inoculum size or reading criteria provide a wide range of sensitivities depending on the method used or the laboratory involved.9 For comparison purposes, the MIC definition may also account for differences. Some works, normally referring to liquid cultures, described MIC as the drug dose at which growth became reduced by 50%, as witnessed by optical density measurements.32,40 Nevertheless, there are authors that defined MIC as herein applied, i.e. the minimal concentration at which no visible growth occurred.3,21
By means of a different mechanism, statins by inhibiting HMG-CoA reductase, and azoles by blocking sterol C-14 α-demethylation, both interfere with sterol biosynthesis. These effects were herein confirmed by sterols qualitative evaluation and ergosterol quantification. Nevertheless, in comparison, azoles exhibit a certain degree of toxicity that has been already demonstrated.5,19 In this context, the possibility to evaluate the combined antifungal effects of statins with azoles may provide relevant information for alternative treatments.
As previously speculated, the statin-driven increase in cellular permeability to exogenous sterols may additionally augment the permeability, susceptibility or both, to antifungal azoles.21 Changes in the lipid structure and plasma membrane dynamics in lovastatin-treated Candida cells have been recently confirmed.16 As a further hypothesis for the enhancing effect observed, the ability of statins to reduce sterols esterification may lead to the accumulation of unsterified C-14 methyl sterols consequent on the azoles action, and the impossibility to accommodate them in cellular membranes would then inhibit yeast growth.21
Agar-well diffusion bioassays demonstrated that SICs of statins significantly potentiated the activity of azoles against yeast growth, except for pravastatin. Similarly, Nash et al. found no synergism between pravastatin and fluconazole against C. albicans isolates.27 The existing synergism between tested sterol biosynthetic inhibitors (SBIs), which would exert an attractive multiplying effect,12 might be applied for instance, to reduce the required doses of azoles for fungal treatments or eventually, for increasing their potency in the case of refractory infections.
Yeast strains herein tested have been already considered as low-virulent opportunistic pathogens.26,34 However, the bioassay herein developed could be also useful for testing sensitivity to new azole–statin combinations of more invasive or virulent pathogen fungal isolates. This work thus describes a technical tool with potential application for clinical purposes. Emergent resistance to conventional antifungal treatments has also reinforced the idea of continuing with sensitivity tests not only against new triazolic derivatives but also any other antifungal agent.9
It is currently known that statins alone are prescribed and administered despite already known eventual side effects such as rhabdomyolisis (0.3 fatal cases per 100,000 person-years). As it occurs with many other currently administered drugs, the knowledge on their side effects is under permanent investigation, and the results on this aspect are taken into consideration in order to evaluate the risk–benefit relationship of the drug when a highly refractory disease has to be attacked. In the case of statins, although the known side effects, they are widely recognized as the best and most effective drugs for treating unmanageable hypercholesterolemia when other resources fail.15
With respect to S. cerevisiae, it has been also described as an attractive experimental system for studying azole toxicity mechanisms.19 In the case of C. utilis, infections caused by non-albicans Candida species are progressively increasing and usually the cases are resistant to common antifungals, which emphasizes the need for novel therapies for their effective management.35 In addition, the use of antifungal azoles for prophylaxis or empirical therapy was shown to promote a shift to non-albicans Candida infections in immunocompromised or cancer patients.16 From another point of view, yeasts isolates currently in use for industrial or biotechnological processes may be potentially pathogenic.20,26,34 It would be therefore suitable to count with standardized and straightforward methodology for testing their sensitivity to currently available antifungal drugs and combinations.
Concerning ergosterol recovery from statin-induced inhibition, present results were in agreement with previous observations with reference to the sterol dose-dependent mode of action.23 Complete growth recovery could not always be achieved, even at the highest ergosterol concentrations. A slow sterol uptake as well as the deleterious statin-induced depletion of many other endproducts from the mevalonate pathway may account for the rescue fail by the sole ergosterol supplementation.40
In conclusion, the proposed agar-well diffusion bioassay showed to be a rapid, simple, low-cost and reliable approach to test antifungal activity of different statin–azole associations as compared to assays in liquid media. Synergism could be assessed with two different yeast species giving an idea of the not circumscribed potentiality of this evaluation tool.
The co-administration of statins may be useful not only for increasing the efficacy of the antifungal treatment or for decreasing the required doses of toxic azoles, but also for eventually obtaining desirable side effects such as tumour-abating properties.16,32,41 In the light of the obtained results, investigations to determine the applicability of these antifungals combinations to in vivo systems would deserve particular attention.
Conflict of interestThe authors declare no conflict of interest.
Authors gratefully acknowledge financial support from ANPCYT (PICT 38164/05), CONICET (PIP 6202) and UNT (CIUNT D-415).