The world has successfully overcome the menace of Coronavirus disease-19 (COVID-19) pandemic waves, nature has unleashed a new curveball in the years 2022 and 2023 with other highly infectious viral diseases. Understanding the origin and different transmission routes, evolution, the mechanism of their emergence, immune evasion, and vaccine research while dealing with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has advanced our scientific knowledge and public health infrastructure to address unknown future viral pathogens already underway. The current review briefly discussed the worldwide pandemics of Monkeypox (Mpox) and polio viral infection along with the origin theories of other global viral outbreaks post-COVID-19 era in 2022 and 2023 like Ebola, unexplained hepatitis in pediatric children, avian influenza, and Langya virus. The role of climate, biodiversity, zoonotic transmission, and trajectory of these viral infections. It also highlights the containment, preventive, and treatment strategies that are being developed.
El mundo superó exitosamente la amenaza de las olas de la pandemia de la enfermedad por coronavirus de 2019 (COVID-19), y la naturaleza desató un nuevo obstáculo en los años 2022 y 2023 con la aparición de otras enfermedades virales altamente infecciosas. La comprensión del origen y las diferentes rutas de transmisión, la evolución, el mecanismo de aparición, la evasión inmunológica, y la investigación vacunal, mientras se maneja el síndrome respiratorio agudo severo por coronavirus-2 (SARS-CoV-2) ha avanzado nuestro conocimiento científico y la infraestructura sanitaria para poder abordar patógenos virales futuros que ya están en camino. La revisión actual debate brevemente la pandemia mundial de viruela del simio (Mpox) y la infección viral de la polio junto con las teorías del origen de otros brotes virales globales tras la era COVID-19 en 2022 y 2023 tales como Ébola, hepatitis inexplicada en pacientes pediátricos, gripe aviar, y virus Langya, así como el rol del clima, la biodiversidad, la transmisión zoonótica, y la trayectoria de dichas infecciones virales. También subraya el confinamiento, las medidas preventivas y las estrategias terapéuticas que están siendo desarrolladas.
An unknown entity, be it anything, and if it is a virus, may be destructive to human civilization as evidenced during the Coronavirus disease-19 (COVID-19) pandemic. Amid the disastrous consequences of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the world is in continuous bombardment with other viral outbreaks which are spreading at an inexplicable and unprecedented speed. Since the detection of SARS-CoV-2 in China in December 2019, the virus has kept mutating and dominating broad geographical areas with different evolved variants in the last 30 months.1 COVID-19 revolutionized not only scientific advancements in terms of fastest developing mRNA vaccines, driving the world's most extensive vaccine campaigns, developing numerous diagnostics platforms, and collaborating efforts of more than 200-plus nations for the common goal but also impacted the scientific, socio-economic, and geo-political scenario of the world. Also, the worldwide relations and economic change, along with the change in attitude and conscious behavior of individuals towards nature and life in particular, rightfully created a new era of post-COVID. Though COVID-19 is no longer a pandemic, we are currently confronting 2 global emergencies, i.e., polio and Monkeypox (Mpox) viral disease.
This review will briefly focus on the recent global pandemic along with other known viral outbreaks of the year 2022 such as acute hepatitis of unknown etiology in pediatric children, vaccine-derived polio, H3N8 and H5N1 outbreaks, Crimean-Congo Hemorrhagic Fever (CCHF), and Langya outbreak. Additionally, origin theories as to how they emerged and spread globally as viral outbreaks which may potentially turn into a pandemic shortly in the future also discussed.
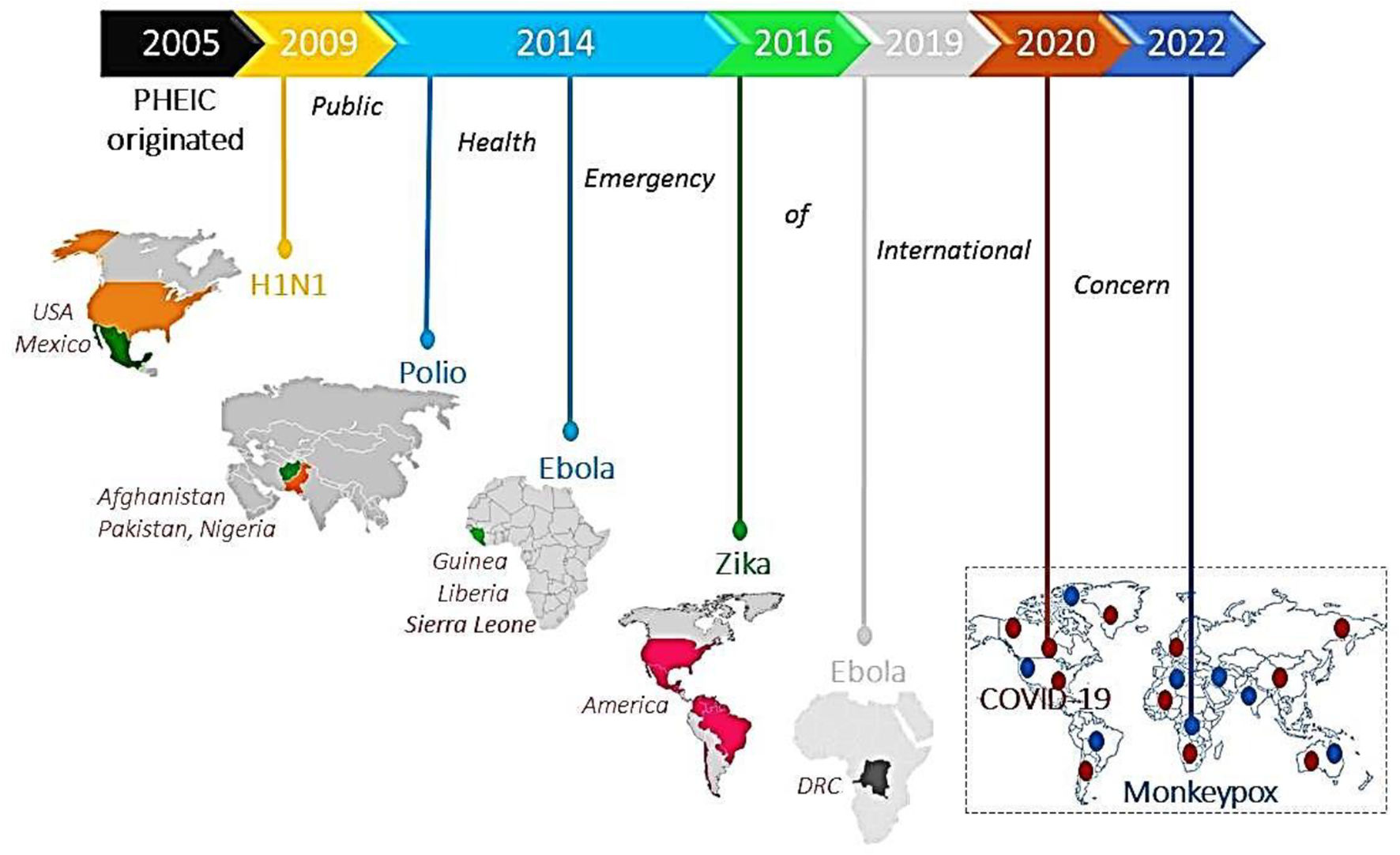
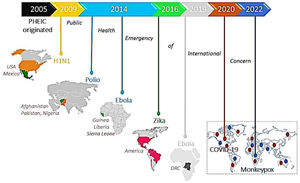
Public health emergency of international concern: Current global pandemicsThe World Health Organization (WHO) as an authority formally declares any event as a public health emergency of international concern (PHEIC) to focus attention and coordinate global efforts on high-risk public health problems with cross borders potentially affecting every dimension of life.2 It includes detection, verification, risk assessment, and response to the disease outbreak based on how unusual and impactful the event concerning public health, its risk of international spread, and travel or trade restrictions. To date, 7 PHEICs have been declared (Fig. 1).
Timeline showing 7 PHEICs declared by the WHO since 2005: The Influenza A (H1N1) pandemic emerged in Mexico and Northern America in 2009, followed by a declaration of poliovirus as PHEIC in 2014 affecting Pakistan, Afghanistan, and Nigeria. The same year, the Ebola virus outbreak started in Guinea, Liberia, and Sierra Leone and was declared PHEIC on August 8, 2014. The Zika virus outbreak was declared PHEIC on February 1, 2016, affecting Brazil, France, the United States of America, and El Salvador due to unusual clusters with complications of microcephaly and neurological disorders. The Ebola virus outbreak re-emerged in DRC and Uganda and was declared PHEIC on July 17, 2019. The COVID-19 pandemic emerged in China in 2019, and on January 30, 2020, it was declared as PHEIC. Recently, on July 23, 2022, WHO announced the Monkeypox virus (Mpox) outbreak as a PHEIC as it spread worldwide to 94 nations.
The COVID-19 pandemic caused by SARS-CoV-2 first emerged in December 2019 in Wuhan, China now spread globally, infecting around 773 819 856 cases with 7 010 568 reported deaths as of December 31, 2023,3 designated as the worst pandemic in history of humanity. Declared as PHEIC on January 30, 2020, and the pandemic since March 2020 has many contradictory theories about the emergence of the virus in humans either by the zoonotic jump from pangolins or snakes (natural spillover theory) or by the lab-related accidental release of the genetically engineered present version of SARS-CoV-2 (lab-leaked theory). Extensive research on bat coronavirus, recombination, and the capacity to evade immunity are other possibilities for the emergence of virulent novel omicron sub-variants. As no direct evidence is available for the pandemic origin, these theories are still inconclusive.
Completing 3 years of struggle with the COVID-19 pandemic, science has improved a lot in dealing with any future viral pandemic. Fortunately, we are now equipped with everything to deal with another ongoing pandemic of Mpox viral infection.
Monkeypox 2022 pandemic: Atypical and perplexing aspectsThe monkeypox virus (Mpox) was first identified in 1970 in the Democratic Republic of the Congo (DRC) crosses the African borders and reported 70 cases of Mpox infection linked to infected pet prairie dogs in the United States of America (USA) in 2003. In May 2022, the first Mpox case in a British resident, who came back to the UK from Nigeria was reported.4 Since then, an unprecedented elevation of cases being reported from around 115 nations forced the WHO to declare this as PHEIC on July 23, 2022. Till September 11, 2023, 90 439 confirmed Mpox cases including 157 deaths,5 were reported surpassing the previous outbreak records, which is a matter of great concern but the circulation of mild West African clade-IIb (Clade II, CFR<1%) is a major relief.6 Zoonotic transmission to humans is possible through prolonged close physical contact, respiratory droplets, direct contact with bodily fluids of an infected person, or lesions of Mpox-infected animals like rope/tree squirrels, dormice, rats, and monkeys where the virus persists for a long duration, still, a natural reservoir of Mpox is inconclusive.
The origin and the sudden surge of Mpox cases have speculations that the virus may have emerged in separate populations or was already circulating undetected outside Africa in humans or animals.7 Frequent traveling to and from the Mpox endemic countries of Africa in addition to a potentially volatile combination of zoonotic spill-over and anthropogenic drivers could be the other possibilities.8 Mpox virus may evolve or mutate to become adapted for human-to-human transmission. As shown by sequence analysis, these sequences have divergence by 50 SNPs from 2018–19 Nigerian Mpox viruses, rendering the virus more transmissibility. The emergence of 7 SNPs is the first sign of continuous microevolution within the clade 2B cluster and 46 SNPs presented mutational bias with 26 (GA>AA) and 15 (TC>TT) replacements in Mpox genomes9 which may facilitate the cryptic transmission of Mpox. Coincidently, 218 (41%) of 528 Mpox cases are HIV patients with a high level of APOBEC3 expressions, which may be beneficial for Mpox-biased mutations.10 Shotgun met genomics suggested D290N, P722S, and M1741I point mutations in the surface glycoprotein B21 as crucial changes conferring immune evasion advantage to Mpox. As of now, no substantial evidence suggests the Mpox genome has mutated to the extent that can govern the present trajectory of a rising number of confirmed cases.
Surprisingly, a significant proportion of the Mpox confirmed cases have been identified within sexual networks, or STI clinics such as among gays, or men who have sex with men (MSM), pointing out sex as an alternative route resulting in disproportionate spreading of infection with gender biases towards males which is still debatable. Also, 98% of the Mpox cases were detected as gay or bisexual, and 41% were HIV positive still, it is uncertain if Mpox can be communicated through semen, urine, or vaginal secretions.11 This unanticipated transmission could be the result of the coincidental introduction and prolonged silent virus circulation into this community as unlinked clusters. Interspecies, i.e., human-to-animal transmission12 reported in a man who reported co-sleeping with a dog who tested positive for the hMpox-1 clade (lineage B.1) with 100% sequence homology. This is a matter of deep concern, therefore, necessitating an urgent concerted effort to prevent its global spread. Therefore, ecological, human, and viral factors are plausible factors for Mpox re-emergence and resurgence.
Although the pre-exposure administration of the smallpox vaccine is 85% effective in adults against Mpox infection, children, and the younger population become vulnerable to Mpox due to the decline in the smallpox vaccination program post-smallpox eradication (1980). Available Jynneos and ACAM2000 vaccines are effective against both smallpox and Mpox infection,13 however, vaccinia IgG and tecovirimat remain alternative post-exposure prophylaxis.
The key containment measures include surveillance, expanding testing, genomics research, contact tracing, vigilance, creating public awareness, and policymakers implementing the guidelines to curb the inexplicable and unprecedented spread of monkeypox.14 As a precautionary measure, vaccinating the higher-risk group through the ring vaccination strategy.15 The prevention strategy employs testing of all the international travelers who returned from the Mpox endemic countries by RT-PCR and those found positive should be isolated to avoid community spreading. Therefore, a collaborative effort to develop a sustainable and equitable global plan for One Health approach, supporting research, laboratory, drug, and vaccines at affordable prices is an urgent requirement.
The emergence of vaccine-derived polio is a concernPoliovirus is a highly contagious disease infecting children under 5 years of age, causing permanent paralysis in 0.5% of infections and death in 2%–10% of paralyzed patients. With a concerted effort of WHO, original wild poliovirus 2 (WPV-2) and WPV-3 were declared eradicated in Sep 2015 and Dec 2019, respectively.16 Since then, WPV-1 and circulating vaccine-derived poliovirus (cVDPV) are contributing to the rise of polio cases with cVDPV2 predominance. Polio was declared a PHEIC on May 5, 2014 due to the enhanced circulation and international spread of wild poliovirus17 where of the total 74 cases, 59 have been reported from Pakistan as wild polioviruses are now only circulating in Afghanistan and Pakistan.
The identification of recent cases of genetically linked versions of Sabin-like type 2 poliovirus in the UK from the sewage water bells an alarm of the outbreak18 with the hypothesis of the importation of polio-infected or live-vaccinated persons into London and transmission to other non-immunized persons which eventually shed viral traces in their stool and end up in sewage wastewater. As of April 15, 7 positive cases of cVDPV3 have been confirmed. The first case of VDPV3 from Jerusalem City, Israel was an unvaccinated child with acute flaccid paralysis (AFP). Of the total 7, only 1 had incomplete polio immunization, while the other 6 were unvaccinated.19 Similarly, the USA reported cVDPV2 (>5 nucleotide changes in the viral sequences) isolates in the environmental samples and found them genetically associated with that from the UK and Israel despite using injectable IPV. Wastewater surveillance for polio is important in finding the extent of transmission. On July 8, a single non-vaccinated child case of cVDPV2 with AFP from southern Algeria was reported which is genetically linked to a virus previously isolated in Kano, Nigeria.10 Indonesia reported 6 cases of cVDPV2 from October 2022 to December 2023.20 The sub-optimal vaccination coverage of bOPV and IPV1 increases the risk of transmission. Subsequently, 1 case of VDPV2 was reported from Sudan, Burundi, and Tanzania, respectively, and 2 cases from Kenya in 202321–24 (Table 1). A case of wild poliovirus type 1 (WPV1) with AFP detected in Malawi in 202225 is genetically similar to a 2020 Pakistan (Sindh) sequence. Another WPV1 case with AFP from Mozambique26 was found to be genetically linked to Pakistan (2019) and Malawi (2022) strains, despite receiving 3 doses of OPV. The detection of WPV1 outside the 2 endemic countries demonstrates the continuous risk of the international spread of the disease. Therefore, polio-free countries remain to be vigilant to avoid the risk of polio re-emergence until every corner of the world is free of polio.
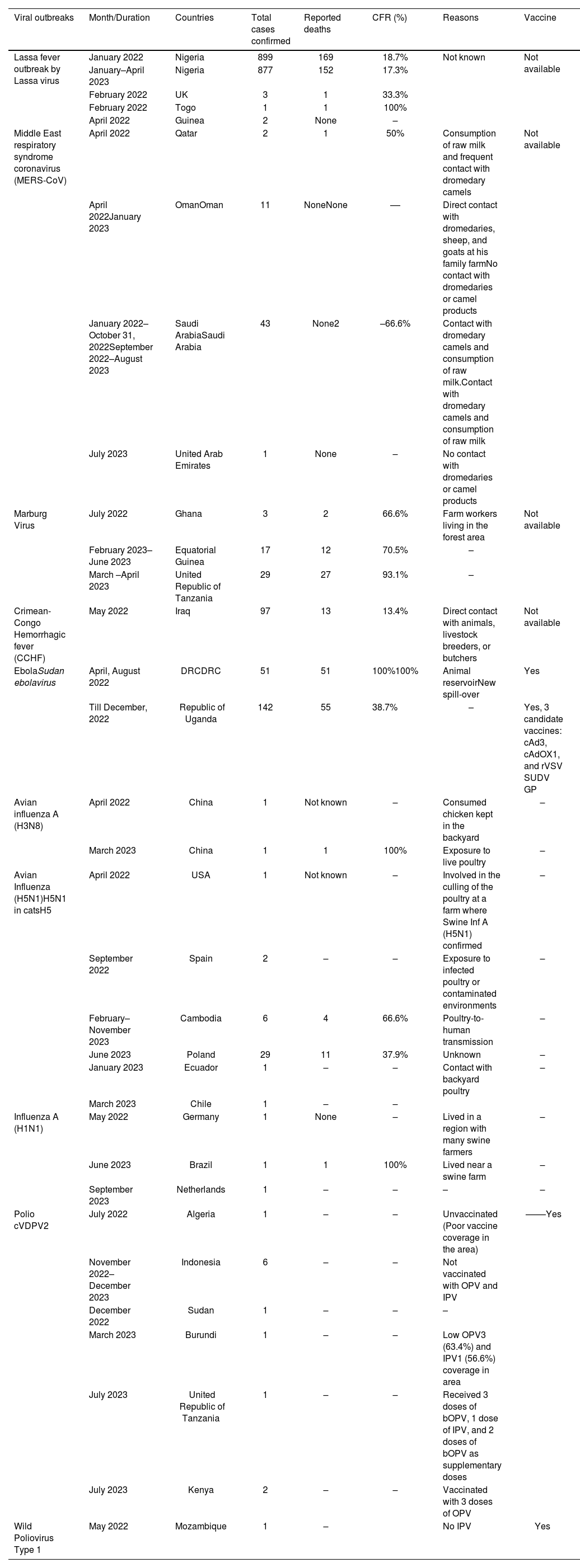
Viral disease outbreaks during 2022–2023.
| Viral outbreaks | Month/Duration | Countries | Total cases confirmed | Reported deaths | CFR (%) | Reasons | Vaccine |
|---|---|---|---|---|---|---|---|
| Lassa fever outbreak by Lassa virus | January 2022 | Nigeria | 899 | 169 | 18.7% | Not known | Not available |
| January–April 2023 | Nigeria | 877 | 152 | 17.3% | |||
| February 2022 | UK | 3 | 1 | 33.3% | |||
| February 2022 | Togo | 1 | 1 | 100% | |||
| April 2022 | Guinea | 2 | None | – | |||
| Middle East respiratory syndrome coronavirus (MERS-CoV) | April 2022 | Qatar | 2 | 1 | 50% | Consumption of raw milk and frequent contact with dromedary camels | Not available |
| April 2022January 2023 | OmanOman | 11 | NoneNone | –– | Direct contact with dromedaries, sheep, and goats at his family farmNo contact with dromedaries or camel products | ||
| January 2022–October 31, 2022September 2022–August 2023 | Saudi ArabiaSaudi Arabia | 43 | None2 | –66.6% | Contact with dromedary camels and consumption of raw milk.Contact with dromedary camels and consumption of raw milk | ||
| July 2023 | United Arab Emirates | 1 | None | – | No contact with dromedaries or camel products | ||
| Marburg Virus | July 2022 | Ghana | 3 | 2 | 66.6% | Farm workers living in the forest area | Not available |
| February 2023–June 2023 | Equatorial Guinea | 17 | 12 | 70.5% | – | ||
| March –April 2023 | United Republic of Tanzania | 29 | 27 | 93.1% | – | ||
| Crimean-Congo Hemorrhagic fever (CCHF) | May 2022 | Iraq | 97 | 13 | 13.4% | Direct contact with animals, livestock breeders, or butchers | Not available |
| EbolaSudan ebolavirus | April, August 2022 | DRCDRC | 51 | 51 | 100%100% | Animal reservoirNew spill-over | Yes |
| Till December, 2022 | Republic of Uganda | 142 | 55 | 38.7% | – | Yes, 3 candidate vaccines: cAd3, cAdOX1, and rVSV SUDV GP | |
| Avian influenza A (H3N8) | April 2022 | China | 1 | Not known | – | Consumed chicken kept in the backyard | – |
| March 2023 | China | 1 | 1 | 100% | Exposure to live poultry | – | |
| Avian Influenza (H5N1)H5N1 in catsH5 | April 2022 | USA | 1 | Not known | – | Involved in the culling of the poultry at a farm where Swine Inf A (H5N1) confirmed | – |
| September 2022 | Spain | 2 | – | – | Exposure to infected poultry or contaminated environments | – | |
| February–November 2023 | Cambodia | 6 | 4 | 66.6% | Poultry-to-human transmission | – | |
| June 2023 | Poland | 29 | 11 | 37.9% | Unknown | – | |
| January 2023 | Ecuador | 1 | – | – | Contact with backyard poultry | – | |
| March 2023 | Chile | 1 | – | – | |||
| Influenza A (H1N1) | May 2022 | Germany | 1 | None | – | Lived in a region with many swine farmers | – |
| June 2023 | Brazil | 1 | 1 | 100% | Lived near a swine farm | – | |
| September 2023 | Netherlands | 1 | – | – | – | – | |
| Polio cVDPV2 | July 2022 | Algeria | 1 | – | – | Unvaccinated (Poor vaccine coverage in the area) | ––––Yes |
| November 2022–December 2023 | Indonesia | 6 | – | – | Not vaccinated with OPV and IPV | ||
| December 2022 | Sudan | 1 | – | – | – | ||
| March 2023 | Burundi | 1 | – | – | Low OPV3 (63.4%) and IPV1 (56.6%) coverage in area | ||
| July 2023 | United Republic of Tanzania | 1 | – | – | Received 3 doses of bOPV, 1 dose of IPV, and 2 doses of bOPV as supplementary doses | ||
| July 2023 | Kenya | 2 | – | – | Vaccinated with 3 doses of OPV | ||
| Wild Poliovirus Type 1 | May 2022 | Mozambique | 1 | – | No IPV | Yes |
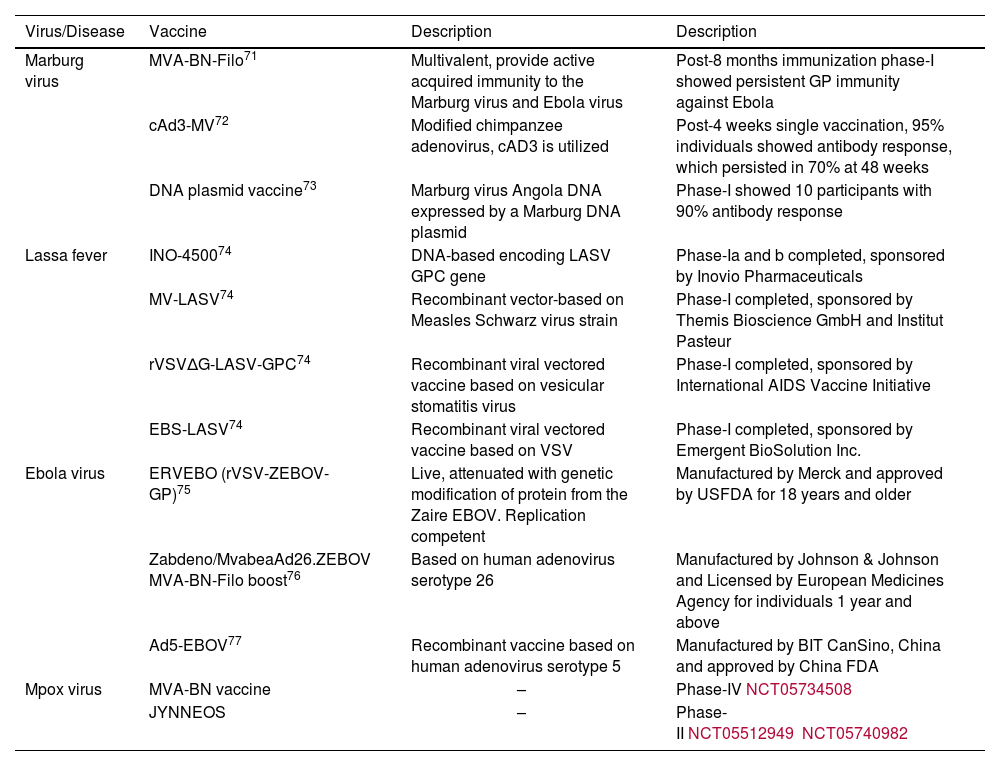
Details of vaccines and advancements are mentioned in Table 2.
| Virus/Disease | Vaccine | Description | Description |
|---|---|---|---|
| Marburg virus | MVA-BN-Filo71 | Multivalent, provide active acquired immunity to the Marburg virus and Ebola virus | Post-8 months immunization phase-I showed persistent GP immunity against Ebola |
| cAd3-MV72 | Modified chimpanzee adenovirus, cAD3 is utilized | Post-4 weeks single vaccination, 95% individuals showed antibody response, which persisted in 70% at 48 weeks | |
| DNA plasmid vaccine73 | Marburg virus Angola DNA expressed by a Marburg DNA plasmid | Phase-I showed 10 participants with 90% antibody response | |
| Lassa fever | INO-450074 | DNA-based encoding LASV GPC gene | Phase-Ia and b completed, sponsored by Inovio Pharmaceuticals |
| MV-LASV74 | Recombinant vector-based on Measles Schwarz virus strain | Phase-I completed, sponsored by Themis Bioscience GmbH and Institut Pasteur | |
| rVSVΔG-LASV-GPC74 | Recombinant viral vectored vaccine based on vesicular stomatitis virus | Phase-I completed, sponsored by International AIDS Vaccine Initiative | |
| EBS-LASV74 | Recombinant viral vectored vaccine based on VSV | Phase-I completed, sponsored by Emergent BioSolution Inc. | |
| Ebola virus | ERVEBO (rVSV-ZEBOV-GP)75 | Live, attenuated with genetic modification of protein from the Zaire EBOV. Replication competent | Manufactured by Merck and approved by USFDA for 18 years and older |
| Zabdeno/MvabeaAd26.ZEBOV MVA-BN-Filo boost76 | Based on human adenovirus serotype 26 | Manufactured by Johnson & Johnson and Licensed by European Medicines Agency for individuals 1 year and above | |
| Ad5-EBOV77 | Recombinant vaccine based on human adenovirus serotype 5 | Manufactured by BIT CanSino, China and approved by China FDA | |
| Mpox virus | MVA-BN vaccine | – | Phase-IVNCT05734508 |
| JYNNEOS | – | Phase-IINCT05512949NCT05740982 |
Significant increase in air travel and land-border crossing, worldwide disruption of the childhood polio vaccination drive, and excessive use of Albert Sabin's oral poliovirus vaccine tends to transform into VDPV and regain the potential to paralyze if a minimum of 6 nucleotide changes occur in the VP1, shedding of the mutated virus in their stool and unfortunate spreading in the community due to poor drainage systems are the factor for the increase in the resurgence of polio infection. The risk of international spread of cVDPV2 is high due to persisting suboptimal immunity, surveillance gaps, and decreased immunization. Large-scale population movements, therefore strengthening the environmental, clinical, and laboratory surveillance for polio, sewage sampling, and testing of enterovirus-positive stool samples for acute flaccid myelitis, and extended surveillance to figure out the focal point is essential for understanding the transmission extent and dynamics. In addition, country-wide catch-up vaccination campaigns with trivalent (OPV), and supplementary booster-inactivated polio vaccine campaigns, in consideration of the anti-vaccination sentiment are also a concern.
Potential 2022 viral outbreaks: Needs global attentionSevere acute hepatitis of unknown etiology in children was a unique outbreak infected young children above 16 years, presented with acute hepatitis of unknown etiology (non-hepatitis A–E) with elevated levels of AST/ALT (>500 IU/L). In 2022, till July, 1010 probable cases with 22 deaths were reported globally from 35 nations of which 46 cases have undergone liver transplants. One-third of the cases were from the USA followed by 27% from the UK. Adenovirus was predominant (n=209) precisely with adenovirus type 41 (n=31) followed by SARS-CoV-2. An analysis from the USA showed 32 of the 123 children (26%) with unexplained hepatitis had a history of COVID-19 before their liver illness and the majority were also infected with adenoviruses.27 Possible reasons for this surge are the presence of adeno-associated virus 2 (AAV2) in a dormant state, a variant of the HLA-DRB1*04:01 gene, and concurrent overlapping of current pediatric hepatitis outbreaks with the COVID-19 pandemic leading to SARS-CoV-2 induced damage triggering aberrant immune responses.
Hand, foot, and mouth sisease (HFMD) is a highly contagious infection of children (≤5 years) which may symptomatically progress to severe meningitis and encephalitis. It is caused by coxsackievirus A-16 (CVA16), A6 (CVA6), and enterovirus 71 (EV71), and spreads through direct contact, or by a fecal–oral route along with bodily secretions. Since the HFMD discovery in 1956, it has had global outreach with recently reported outbreaks in Malaysia in June 2022, where around 95 924 HFMD cases of which 90%, i.e., 86 230 cases were among children (≤6 years).28 The lifting of COVID-19 pandemic restrictions resulted in the upsurge of cases and community transmission by EV71, CA6, and CA16 in nurseries, kindergartens, and preschools.29 Previous studies with 31 cases from the 2017 HFMD outbreak in India showed 74.2% of cases were less than 5 years old with the majority (75%) of the cases associated with the Coxsackie-A6 followed by Coxsackie-A16.30 In 2022, during April–May, Kerala and Odisha provinces of India reported 82 children (<5 years) and 26 children (1–9 years), respectively with HFMD infection caused by Coxsackievirus CA6 and A16 strains co-circulating in the populations.
Preventive measures include continued surveillance, molecular serotyping, timely reporting, isolation with a balanced diet, good hygiene, better ventilation, and adequate hydration.31 Infection with CVA16 usually resolves within 7–10 days without medical treatment; however, EV71 infection has shown a higher incidence of neurologic involvement and fatality. Inactivated EV71 vaccines licensed by the Chinese FDA are available but effective against only EV71 infections. Therefore, intravenous immunoglobulins (IVIGs) reduce EV71-related encephalitis as an alternative.
Langya henipavirus (LayV) is a newly discovered virus from Langya town in the Shandong province of China,32 which has a close phylogenetic association with Mojiang henipavirus (rat-borne virus), isolated in 2012.33,34 Detection of LayV antibodies in a few goats and dogs living in the villages of infected patients and LayV viral RNA in 71 (27%) of the 262 sampled shrews suggesting animal origin and probably shrews as a natural reservoir for the virus. So far, only 35 unlinked cases (primarily farmers) have been reported between April 2018 and August 2021 during surveillance of people with unusual sicknesses; having no common exposure history of which the virus was noticed in only 26 cases. No human-to-human transmission was reported from contact tracing of infected individuals. Recently during sentinel surveillance of febrile patients in China, in August 2022, 1 patient was found positive for LayV through metagenomic analysis.35 Virus existence in shrews and spillovers to human needs research to avoid future pandemics. As no human deaths have been reported so far, sporadic cases of LayV infection in the human population are the only concern.
Marburg virus is known to cause Marburg virus disease (MVD), a severe hemorrhagic fever that is associated with a high CFR of 24%–88%. It spreads between humans via direct contact with the infected people's blood, secretions, and bodily fluids. The prime cause of human MVD infection is prolonged exposure to mines inhabited by the Egyptian fruit bat Rousettus aegyptiacus as a probable natural host.36 In July 2022, Ghana declared its first MVD outbreak in the South Ashanti region with 3 reported cases of which 2 died (CFR=67%). The first index case has a travel history to the Western region although both were farm workers living in a forest environment from different locations,37 the exact source of the infection is unknown (Table 1). However, on September 16, 2022, the MVD outbreak was declared to end. In 2023, Equatorial Guinea and the United Republic of Tanzania declared the MVD outbreak in February and March 2023, respectively. In June 2023, Guinea declared the end of the MVD outbreak with a total of 17 confirmed cases of which 12 cases died, whereas Tanzania reported 29 cases with 27 deaths during this outbreak.38,39 The unavailability of specific treatment and clinical similarity with infectious diseases like malaria, dengue, and other viral hemorrhagic fever is the concern.
Crimean-Congo hemorrhagic fever (CCHF) is a tick-borne viral disease mainly transmitted to humans by bites of infected ticks (Hylomma species) and by direct contact with blood or tissues from infected humans and livestock. CCHF is endemic in the Middle East; African and Asian countries. In 2022, till May 22, Iraq reported 97 confirmed CCHF cases including 13 deaths (CFR 13%).40 Among confirmed cases, 62% were male (livestock breeders or butchers) who have subsistent livestock farming and have direct contact with animals, and probably the reason for this outbreak (Table 1). As no vaccine is available, therefore, an epidemiological and entomological investigation for contact tracing and collecting and classifying the tick vectors from the site is vital as the preventive and control measure.
Middle East respiratory syndrome coronavirus (MERS-CoV) identified in 2012 in Saudi Arabia, is a zoonotic virus with dromedary camels as natural hosts, responsible for MERS disease that spreads from direct or indirect contact with the natural host or human to human.41 Close contact with dromedary camels and intake of camel's raw milk may spread this virus. In 2015, the WHO reported RNA or antibodies in 1493 patients from the 26 affected countries predominantly from Arabian Peninsula or Middle-East countries where MERS-CoV is circulating in dromedaries. In May 2015, the Republic of Korea reported 185 laboratory-confirmed cases and 38 deaths.42 In April 2022, a case of MERS-CoV from Oman reported direct contact with animals (dromedaries, goats, and sheep, at his family farm).43 As of October 2023, 2608 laboratory-confirmed MERS-CoV disease cases including 938 associated deaths (CFR=36%) were reported globally.44 The majority of the cases were reported from Saudi Arabia, with 2199 cases and 857 related deaths. Till October 2023, UAE, Oman, and Qatar reported 94, 26, and 28 confirmed cases with 12, 7, and 7 deaths, respectively, to the WHO. Early identification and isolation of cases in the healthcare setting and public health awareness can prevent human-to-human transmission of MERS-CoV.
The Ebola virus is infamously known for the 2014 worldwide outbreak in the Western African countries where 28 616 infected cases and 11 310 deaths (CFR=40%) were reported followed by another major outbreak in 2019, where DRC notified a total of 3470 cases with 2280 deaths.45 However, following the ring-fencing vaccination strategy, the outbreak was declared to end on June 25, 2020. In 2022, till July 3, 5 Ebola-confirmed cases with 100% mortality were reported from the Equateur province of the DRC, as all 4 secondary cases had an epidemiological link to the index case (Table 1). Sequencing suggested the new spillover from the animal population as the primary source. On August 21, DRC again declared a new lab-confirmed Ebola case in the North Kivu province. Sequencing confirmed the genetic linking with the 2018–2020 outbreak in North Kivu, Ituri (Ebola Zaire strain), and not a new spillover event.46 Ring-fencing vaccination strategy, along with community engagement, helps in controlling the Ebola outbreaks. Uganda also declared an outbreak of Ebola disease caused by Sudan ebolavirus in September, 2022 and declared the end of the Ebola disease outbreak on January 11, 2023 with a total of 142 confirmed cases with 55 confirmed deaths.47 Before this,4 outbreaks in 2000, 2011, and 2 in 2012 were also reported as SVD is enzootic and present in animal reservoirs in the region.
Lassa fever in Nigeria, the UK, Togo, and Guinea was caused by the Lassa virus, first identified in 1969.48 It is an acute viral hemorrhagic illness, known to be endemic in West African countries. It is transmitted to humans via contact with food or household items contaminated with urine or faces from the main reservoir of Mastomys rats. In 2022, till August, 899 laboratory-confirmed Lassa fever cases with 169 deaths (CFR=18.8%) have been reported in Nigeria49 (Table 1). The UK and Togo reported 3 and 1 cases, respectively, with 1 death each. However, in 2021, the Lassa fever outbreak in Guinea reported 7 deaths among the 8 reported cases with 88% CFR. In 2023, Nigeria reported another outbreak with 877 confirmed cases with 152 reported deaths till April 2023.50 As no licensed vaccine is available for Lassa fever; the antiviral drug ribavirin has been used as a therapeutic agent.
Avian influenza A in China and the USAAvian A(H3N8) influenza viruses are widely known to circulate in animals and represent one of the most frequently found subtypes in wild birds. In April 2022, Henan Province of China reported the first and only confirmed human case with an avian influenza A(H3N8) virus,51 as the case had consumed chickens that were kept in the backyard. Cross-species transmission events of avian A (H3N8) influenza viruses have also been reported for various mammal species like horses and dogs but for humans the risk is low. Avian influenza A (H5N1) is considered highly pathogenic Avian influenza (HPAI). A human case of H5N1 was reported in January 2022 from the UK, where the case lived with a large number of domestically kept birds. Similarly, another case of human infection of Avian H5N1 was reported in Colorado State, USA in April, which was involved in the culling of poultry at a farm where the H5N1 virus was confirmed in the poultry.52 Subsequently, during 2023, 6 cases of H5N1 have been reported from Cambodia with 4 deaths,53 and 29 cases in cats with 11 deaths from Poland.54 Japan invested US$2 billion in a 100-day mission to boost vaccine research for 8 deadly pathogens, including coronaviruses, and monkeypox virus, using a range of technologies to produce diagnostic tests, treatments, and vaccines ready for large-scale production within the first 100 days of a pathogen with pandemic potential.55
Disease “X”.According to the WHO, Disease “X” is defined as the ‘knowledge that a serious international epidemic could be caused by a pathogen currently unknown to cause human disease’.56 This ominously named Disease “X” is possibly caused by a ‘Pathogen X', which may emerge from anywhere (unidentified spillover event) and result in outbreaks, therefore is on the list of high-priority diseases requiring research and development. Engineered pathogens may lead to a disastrous Disease “X” either through accidental lab release or by an act of bioterrorism therefore, the development of preventive or curative options, and identification of emerging infections, for transmission containment, through a surveillance system may help in the identification of the novel pathogen. As per the estimation, 631 000–827 000 viruses can infect humans.57,58 The risk of the emergence of these yet-to-be-recognized threats grows higher with the growing human population, erratic weather patterns, loss of biodiversity, desynchronizations of the life cycles of animals and plants, changing climates, and aggressive land use for human habitation and agriculture, all of which alter the interaction dynamics with the potential for new cross-over infection with succeeding human spillovers to influence future pandemics.59,60
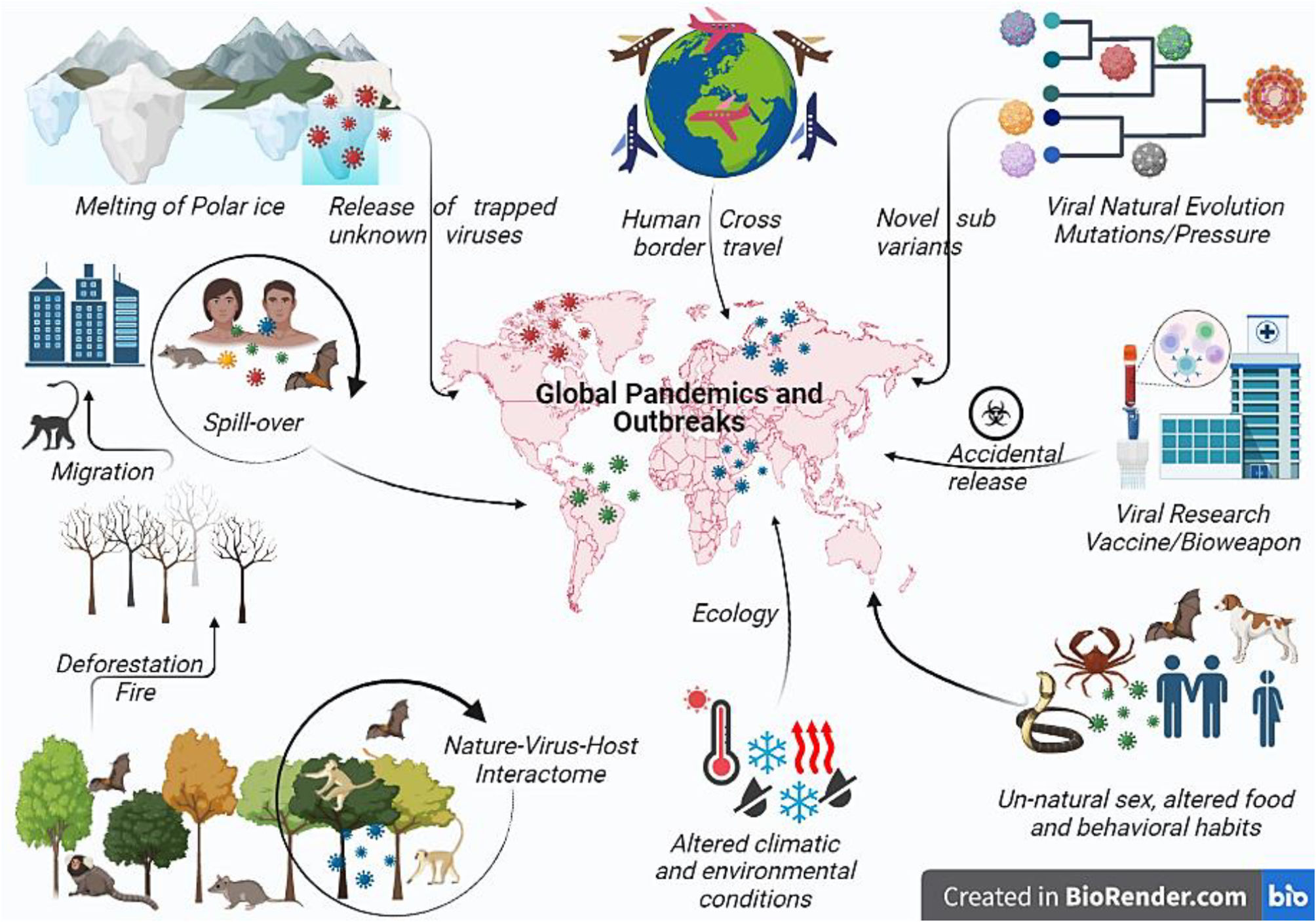
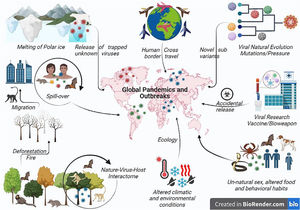
Potential cause of viral pandemics: How and whyThe main factors contributing to the emergence of infectious disease are viral, human, and ecological, where human factors decide the trajectory and influence of any disease. Manmade situations like cross-border conflict, greed to become the global power, fear of future biological war, human unnatural sexual behavior (Mpox), traditional funeral rites (Ebola outbreak), hunting, pasture practices, expanding agriculture,61 deforestation, land development, cross-boundary trade, and unmanned international trade of animal and animal products, modern research (SARS-CoV-2 and smallpox) with a possibility to be the reason for the next global pandemic favoring transmission. As the human population reaches to 8 billion mark, urbanization, globalization of commerce, including global food production, and human population movements are the most often factors driving disease emergence. Forests provide a defense against zoonosis; therefore, habitat loss due to deforestation and rearing livestock on farms changed the distribution pattern of animals and people, allowing viruses to breed and mutate in the animals before jumping into the humans (Fig. 2).
Pictorial representation of various factors resulting in global viral pandemics. Environmental changes like global warming may increase global temperature releasing new viruses entrapped in the polar ice. Such novel viruses may emerge and result in a dreadful global pandemic. Change in the ecology or environmental conditions due to human activities such as deforestation or fire alter the nature–host–virus interactome and forces the animal to migrate to the urban area resulting in interspecies transmission or spill-over resulting in the spread or global outreach of newer or recombinant viral pathogen pandemic. Viruses also undergoing continuous evolution for fitness or under vaccine pressure evolve to evade the immune system and to form more virulent strains resulting in widespread pandemics such as SARS-CoV-2 Omicron sub-variant. Cross-country travel and viral research may result in the dissemination of the virus across the border either by commuting from an endemic to a new or non-endemic country, by war or bioweapon, or through accidental leakage. Unusual transmission has been reported in MPX through unnatural sex or altered food habits or behavioral habits where different exotic animals have been eaten, which may contain viruses like bats. Also export or import of such infected food creates the possibility of future pandemics.
Global air travel networks and favorable environmental adaptations facilitate the virus spillage anywhere in the world, causing a pandemic. Zoonotic spillover is the mechanistic link between global environmental change and disease emergence. Viruses in wild animals are still evolving, and any variations in climate and land use will lead to changes in the viral distribution pattern. The phylogeographical model of the mammal virus network showed shifts for 3139 mammal species under climate alteration, and land use consequences for the year 2070. They predicted that species would accumulate in new combinations at high elevations, in biodiversity hotspots, and the areas of high human population density in Asia and Africa, resulting in cross-species transmission. Bats account for the most novel viral involvement that will facilitate future emergence in humans. High biodiversity and deforestation rate of 0.25%–0.4% per year increased the risk of zoonoses in DRC has been supported by increased MPX cases in the past years. Change in lifestyle and food habits, based on sustainability, equity, and fairness with harmony with animals and wildlife. Ecological factors are extreme climatic conditions extended to floods, droughts, global warming, and El Nino southern oscillations (ENSO).62 ENSO is a potent source of climatic variability, resulting in the NiV outbreak in 1997–98. Fruit bats were forced to invade new areas of fruit cultivation due to ENSO-driven draught, causing a scarcity of fruit trees. Additionally, global warming causes an increase in global temperature and glacier recessions resulting in high land environmental changes triggering insect population explosion as seen in Asian, Eastern, and Central African countries where the mosquito-borne infection is dengue and yellow fever by mosquito expansion. Climate change is anticipated to aggravate the alarming developments of zoonotic spillovers, where animal viruses begin to infect humans. Over half of the world's human infectious illnesses are credited to climate change.63 Between 1940 and 2004, 335 pathogens emerged, with 60% having a zoonotic source, of which 71% originate from wildlife.64,65 Any safety lapses (virus leaks from the lab) will be a significant concern for the select agents like Nipah and Marburg virus etc. due to their potential to pose a severe threat to public health.
Vaccine availability and strategies against deadly virusesFood and Drug Administration (FDA) approved vaccines are not available for all the class-III and IV viral pathogens. However, research and development to develop novel and effective vaccines is a continuous process.
No vaccine is available for humans against the Marburg virus, but several potential vaccine candidates and strategies have been assessed.39 The vesicular stomatitis virus vector-based vaccine VSV-EBOV-MV protects against both Marburg and Ebola in pre-clinical research.66 Adenovirus-based Ad26.ZEBOV/MV and mRNA technology-based mRNA-1360 vaccines with promising results are being studied in clinical settings. In non-human primate (NHP) models, several vaccination approaches have been developed. The VSV-M vaccine produces the MV glycoprotein and MVABN-Filo contains Marburg and Ebola virus antigens are preventive vaccines against Marburg infection.67,68 Substantial progress has been made in the development of Lassa fever vaccine. Four vaccine candidates which are in different phases of clinical trials are INO-4500, MV-LASV, rVSVΔG-LASV-GPC, and EBS-LASV.69 Different platforms are available for the Ebola virus vaccine development such as mRNA and plasmid vaccines, viral vectored vaccines, protein-based and virus-like particle vaccines. Only one vaccine named ERVEBO approved against Ebola virus for patients above 18 years and older. Vaccines and treatment modalities available for Mpox are highlighted in the following section.70
Conclusion and future directionsAs we are still grappling with the COVID-19 pandemic, the current influenza outbreaks, the identification of the recent cases of mutated poliovirus in sewage water after 4 decades from the UK, the persistence of sporadic outbreaks of the Ebola virus in West and Central African countries, and the emergence of Marburg viral cases from Ghana in West Africa are some countable outbreaks we have to cope with on international and national levels. History proves that these microbes' contagious nature kept us in the confinement of our physical arrest. Zoonotic pathogens cause many infectious diseases leading to the pandemic due to increased proximity to animals, changing lifestyles, and global trade activities. Creating public health and moral awareness among the masses limits the further spread of infection and other associated social consequences of the diseases in the community.
So preparing for the future pandemic involves strengthening the entire chain of outbreak response, from identifying a pathogen through mass and ring vaccination. Monitor zoonosis, sequence globally, and strengthen manufacturing of vaccines to avoid vaccine inequality to stop the spread. Artificial intelligence and machine learning-based models by Bluedot and others like HealthMap, Metabiota, and PyRo have helped predict the outbreaks. So it is rightly said by Sir AS Fauchi78: It ain't over till it's over…..but it's never over.
Funding StatementThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributionPryanka Thakur: Conceptualization, Data curation, Formal analysis, Writing-original draft, Writing-Review & editing, Validation; Vikram Thakur: Conceptualization, Data curation, Formal analysis, Writing-original draft, Writing-Review & editing, Validation; Monika Sapra: Data curation, Formal analysis; Sonakshi Srivastava: Writing-Review & editing, Validation; Sanjay Kumar Singh Patel: Data curation, Formal analysis, Validation.
This work was supported by KU Research Professor Program of Konkuk University.