The diagnostic suspicion of an atypical lipomatous tumour (ALT) is difficult. The aim of this study is to delve into the most controversial diagnostic aspects of the subject.
Material and methodObservational, longitudinal and retrospective study of a series of 96 deep adipose tumours (75 lipomas and 21 TLA) from 2006 to 2016: demographic, clinical, imaging and pathological variables were analysed and compared, as well as other variables related to treatment and oncological outcomes of the patients. A descriptive analysis of the collected variables was performed for the statistical study. To evaluate the potential predictor variables of malignancy, a multivariate logistic regression analysis was performed, including those that were statistically significant in the univariate analysis.
ResultsOlder age at diagnosis, lower limb location and larger size were significantly more frequent in ALTs. MRI findings showed no statistically significant differences between the two groups. In multivariate analysis, the same clinical variables were confirmed as predictors of malignancy. In the ROC curve, an optimal cut-off point of 134.0mm was used as a predictor of malignancy.
ConclusionsAdvanced age, location in the lower limbs and larger size are risk factors for malignancy in the differential diagnosis of deep lipomas and atypical lipomatous tumours. No radiological variable on MRI reached significance as a predictor of malignancy in our series.
La sospecha diagnóstica de un tumor lipomatoso atípico (TLA) es difícil. El objetivo de este estudio es ahondar en los aspectos diagnósticos más controvertidos del tema.
Material y métodoEstudio observacional, longitudinal y retrospectivo de una serie de 96 tumores grasos profundos a la fascia (75 lipomas y 21 TLA) de 2006 a 2016: Se analizaron y compararon variables demográficas, clínicas, de imagen y patológicas, así como otras relacionadas con el tratamiento y los resultados oncológicos de los pacientes. Para el estudio estadístico se realizó un análisis descriptivo de las variables recopiladas. Para evaluar las potenciales variables predictoras de malignidad se realizó un análisis multivariante tipo regresión logística, incluyendo las que fueron estadísticamente significativas en el análisis univariado.
ResultadosUna edad mayor al diagnóstico, la localización en miembros inferiores y un mayor tamaño fue significativamente más frecuente en los TLA. Los hallazgos en la resonancia magnética (RM) no mostraron diferencias estadísticamente significativas entre ambos grupos. En el análisis multivariado, las mismas variables clínicas se confirmaron como factores predictores de malignidad. En la curva RO, se determinó como como factor predictor de malignidad un punto de corte óptimo de 134,0mm.
ConclusionesLa edad avanzada, la localización en miembros inferiores y un tamaño mayor son factores pronósticos de malignidad en el diagnóstico diferencial entre los lipomas profundos y el tumor lipomatoso atípico. Ninguna variable radiológica en la RM se alcanzó significación como factor predictor de malignidad en nuestra serie.
Lipomatous tumours are the most common soft tissue neoplasms and include a wide lesion spectrum, with highly variable biological behaviour. In 2020 the World Health Organization (WHO) classified them into three categories: benign, intermediate or locally aggressive, and malignant. Within adipose tumours of intermediate aggressiveness, it distinguished atypical lipomatous tumour (ALT), to refer to those located in the extremities or trunk, and well-differentiated liposarcoma (WDLS), for those located in cavities such as the mediastinum, retroperitoneum, abdomen or pelvis.1 Although both entities are identical in morphology, karyotype and biological behaviour, the prognosis of the latter is considered worse, as they are located in sites with a more complex surgical approach.1
The prognosis of a deep-seated lipoma to the fascia (DSL) and an ALT/WDLS is very different from a biological point of view. Although in the former the risk of local recurrence is very small and there is no risk of metastasis or malignancy, the latter can recur (7.5%–52%),2,3 metastasise to distant organs (0%–1.2%) and even de-differentiate (1.1%–8.3%).4
Diagnostic suspicion based on clinical and imaging data between a deep-seated lipoma (DSL) and an ALT is difficult, despite the existence of signs and magnetic resonance imaging (MRI) findings that suggest the distinction. Cellular morphology is also non-defining. In fact, cytological findings of multinucleated cells, pleomorphism and large atypical cells in tissue samples stained with Papanicolaou may be present in both entities.5 Only the demonstration of the presence of amplifications for the chromosomal region 12q13-15, which constantly affect MDM2 (100%) and frequently CDK4 (90%) in ALT, would ensure the diagnosis.6,7 However, detection using immunohistochemical techniques (IHC) has poorer sensitivity (68.4% for CDK4 and 89.5% for MDM2) than that provided by molecular techniques,7 and these are less accessible in laboratories. However, leaving aside the controversy surrounding the indication or not of a preoperative biopsy,6 the amplification of MDM2 by fluorescence in situ hybridisation (FISH), in the analysis of the resection specimen, is considered the “gold standard” in the differential diagnosis of a deep adipose tumour.3
Regarding treatment, DSLs are surgically removed with marginal margins when they are symptomatic, or there are diagnostic doubts, usually without a prior biopsy. Given a reasonably certain diagnosis in asymptomatic patients, observation is the preferred option. The treatment of ALT/WDLS is surgical, with the margin of resection and the need or not for radiotherapy being controversial. This would be decided in multidisciplinary teams, taking into account different factors.8,9
Although lipomatous tumours have been widely studied, there are relatively few studies that continue to focus on the predictors of malignancy in deep lesions of uncertain aggressiveness. The objective of our study was to present our experience in the most controversial diagnostic aspects of the topic in a large series and with one of the longest follow-up periods.
Material and methodA retrospective observational study of clinical and imaging variables potentially predictive of malignancy was carried out in a series of patients with deep adipose tumours of the limbs and trunk. Patients over 18 years of age, treated surgically from July 1, 2006 to December 31, 2016, in the same tertiary hospital centre with a pathological diagnosis of DSL or ALT, were included. All patients underwent limb-sparing surgical procedures and in all cases radiographs and MRI were performed prior to surgery. Once the pertinent imaging tests had been obtained, patients were evaluated at the Musculoskeletal Tumours Committee (CTME for its initials in Spanish), where it was decided whether or not it was necessary to biopsy the tumour. The decision on which patients required biopsy was at the discretion of the CTME. In general terms, no biopsy was indicated in cases with a radiological diagnosis of DSL or ALT. In contrast, in those cases in which there were other presumptive diagnoses other than the previous ones or suspicion of an aggressive lesion, the tumour was biopsied.
The definitive diagnosis of the lesion was obtained after histological analysis of the resection specimen by the Pathological Anatomy Service, for which conventional microscopy, IHC (CDK4 and MDM2) and cytogenetics (MDM2-FISH amplification) studies were performed.
Demographic, clinical and imaging variables were evaluated: age; sex; first symptom or sign leading to medical consultation; growth and location of the tumour (upper, lower limb or trunk); diagnosis in the radiological report; presence of fibrous septa and their thickness; size of the tumour (according to the largest diameter on MRI); location (intra or intermuscular); lesion contour (capsulated or not); type of fatty pattern (homogeneous or heterogeneous); perilesional oedema and contrast enhancement on MRI (presence or non-presence). It was recorded whether the patient had received radiotherapy and whether local recurrence (LR) or metastasis occurred during follow-up. Postoperative radiotherapy in ALT was agreed upon in the CTME based on the age, size, location and resection margins of the tumour. In general, it was indicated in those patients with marginal resection and without express contraindications for it, such as local complications of the surgical wound. Based on these criteria, 13 (61.9%) of the 21 patients in group B received said adjuvant treatment.
The median follow-up was 72.1 months (interquartile range [IQR]: 54.6), in patients in group A; and 93.3 months (IQR: 56.7) in those diagnosed in group B.
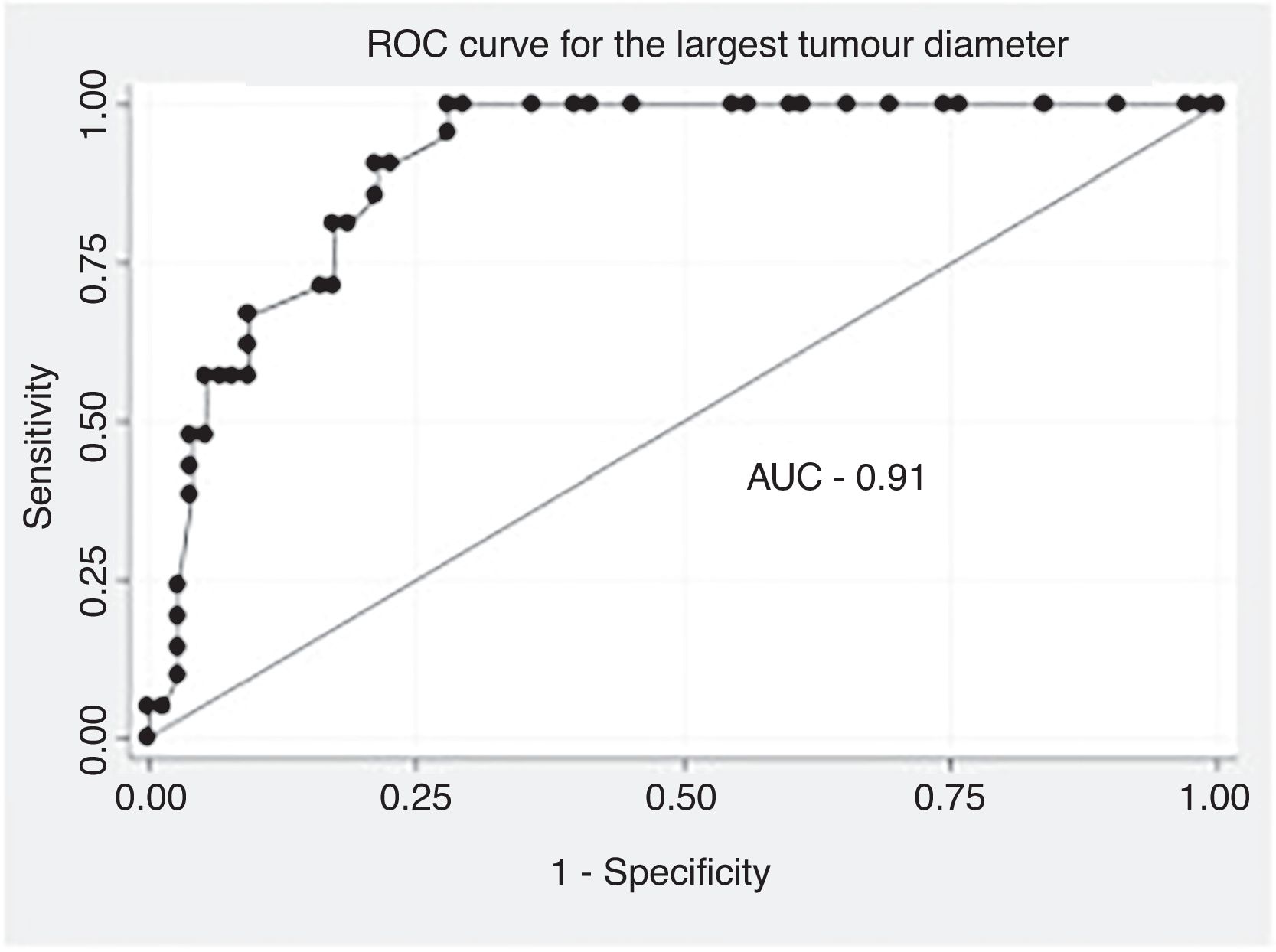
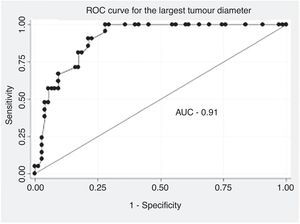
The data were analysed with SPSS statistical software, version 25.0 (IBM Corp., Armonk, NY, USA). The descriptive analysis of the categorical variables was expressed in the form of percentages and the quantitative variables in terms of median and IQR. The comparative analysis of the categorical variables was performed using the χ2 test or Fisher's exact test. When comparing quantitative and qualitative variables with two categories, the Mann–Whitney U test was used as a non-parametric test. For all the tests previously described, a value of p<.05 was considered significant. A multivariate analysis was performed according to a binary logistic regression model, including those variables that had reached statistical significance in the univariate analysis. The results were expressed in the form of odds ratio (OR), with their respective 95% confidence intervals (95% CI). The adequacy of the model was evaluated using the Hosmer and Lemeshow goodness-of-fit test, with a good fit considered the presence of a non-significant p value. An analysis of the receiver operating characteristic (ROC) curve and obtaining its respective area under the curve (AUC) was performed to locate the optimal cut-off point in terms of largest diameter of the tumour, for discrimination of the diagnosis of malignancy.
ResultsThe final sample included 96 patients, who were divided into two groups: group A (DSL, 75 cases) and group B (ALT, 21 cases). The clinical and imaging results are summarised in Tables 1 and 2, respectively. Table 3 shows the results of the microscopic analysis of the lesion. IHC for CK4 and MDM2 was negative in all patients in group A. In those in group B, it was positive for CDK4 in 15 (71.4%) and for MDM2 in 18 (85.7%). Genomic amplification of MDM2 by FISH technique was negative in all patients in group A and positive in all patients in group B (100%).
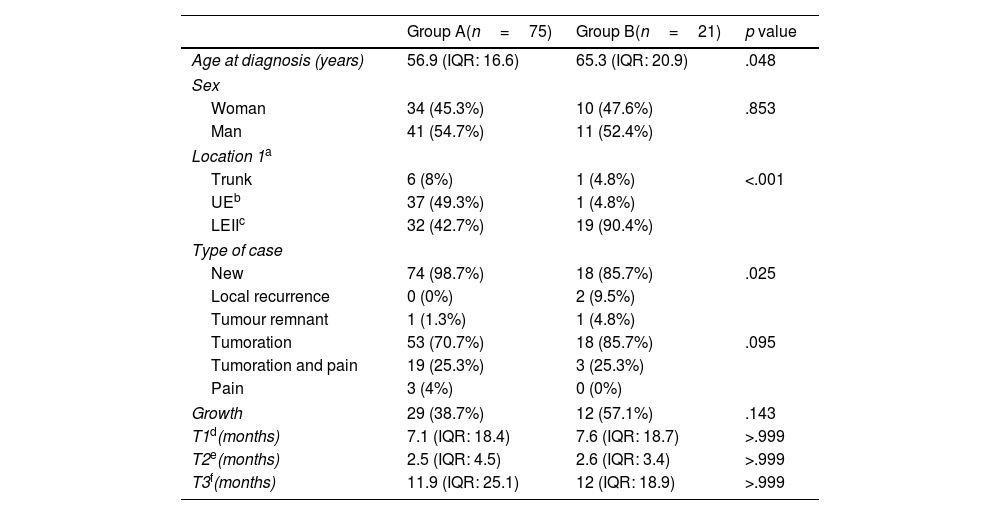
Main clinical and epidemiological characteristics of the study groups.
| Group A(n=75) | Group B(n=21) | p value | |
|---|---|---|---|
| Age at diagnosis (years) | 56.9 (IQR: 16.6) | 65.3 (IQR: 20.9) | .048 |
| Sex | |||
| Woman | 34 (45.3%) | 10 (47.6%) | .853 |
| Man | 41 (54.7%) | 11 (52.4%) | |
| Location 1a | |||
| Trunk | 6 (8%) | 1 (4.8%) | <.001 |
| UEb | 37 (49.3%) | 1 (4.8%) | |
| LEIIc | 32 (42.7%) | 19 (90.4%) | |
| Type of case | |||
| New | 74 (98.7%) | 18 (85.7%) | .025 |
| Local recurrence | 0 (0%) | 2 (9.5%) | |
| Tumour remnant | 1 (1.3%) | 1 (4.8%) | |
| Tumoration | 53 (70.7%) | 18 (85.7%) | .095 |
| Tumoration and pain | 19 (25.3%) | 3 (25.3%) | |
| Pain | 3 (4%) | 0 (0%) | |
| Growth | 29 (38.7%) | 12 (57.1%) | .143 |
| T1d(months) | 7.1 (IQR: 18.4) | 7.6 (IQR: 18.7) | >.999 |
| T2e(months) | 2.5 (IQR: 4.5) | 2.6 (IQR: 3.4) | >.999 |
| T3f(months) | 11.9 (IQR: 25.1) | 12 (IQR: 18.9) | >.999 |
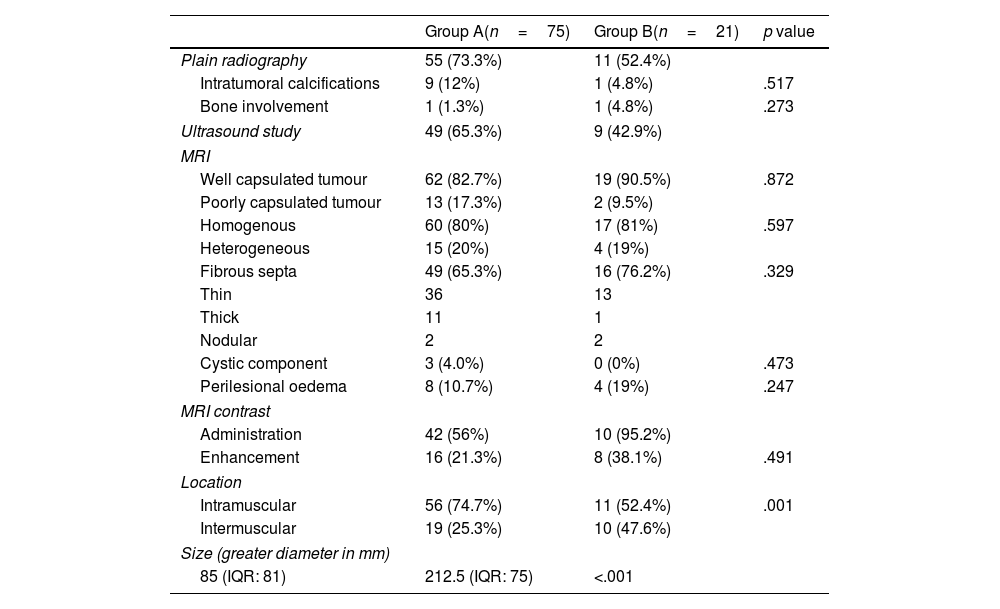
Main characteristics of the imaging tests used in the diagnostic process of fatty tumours for the different study groups.
| Group A(n=75) | Group B(n=21) | p value | |
|---|---|---|---|
| Plain radiography | 55 (73.3%) | 11 (52.4%) | |
| Intratumoral calcifications | 9 (12%) | 1 (4.8%) | .517 |
| Bone involvement | 1 (1.3%) | 1 (4.8%) | .273 |
| Ultrasound study | 49 (65.3%) | 9 (42.9%) | |
| MRI | |||
| Well capsulated tumour | 62 (82.7%) | 19 (90.5%) | .872 |
| Poorly capsulated tumour | 13 (17.3%) | 2 (9.5%) | |
| Homogenous | 60 (80%) | 17 (81%) | .597 |
| Heterogeneous | 15 (20%) | 4 (19%) | |
| Fibrous septa | 49 (65.3%) | 16 (76.2%) | .329 |
| Thin | 36 | 13 | |
| Thick | 11 | 1 | |
| Nodular | 2 | 2 | |
| Cystic component | 3 (4.0%) | 0 (0%) | .473 |
| Perilesional oedema | 8 (10.7%) | 4 (19%) | .247 |
| MRI contrast | |||
| Administration | 42 (56%) | 10 (95.2%) | |
| Enhancement | 16 (21.3%) | 8 (38.1%) | .491 |
| Location | |||
| Intramuscular | 56 (74.7%) | 11 (52.4%) | .001 |
| Intermuscular | 19 (25.3%) | 10 (47.6%) | |
| Size (greater diameter in mm) | |||
| 85 (IQR: 81) | 212.5 (IQR: 75) | <.001 | |
IQR: interquartile range; MRI: magnetic resonance imaging.
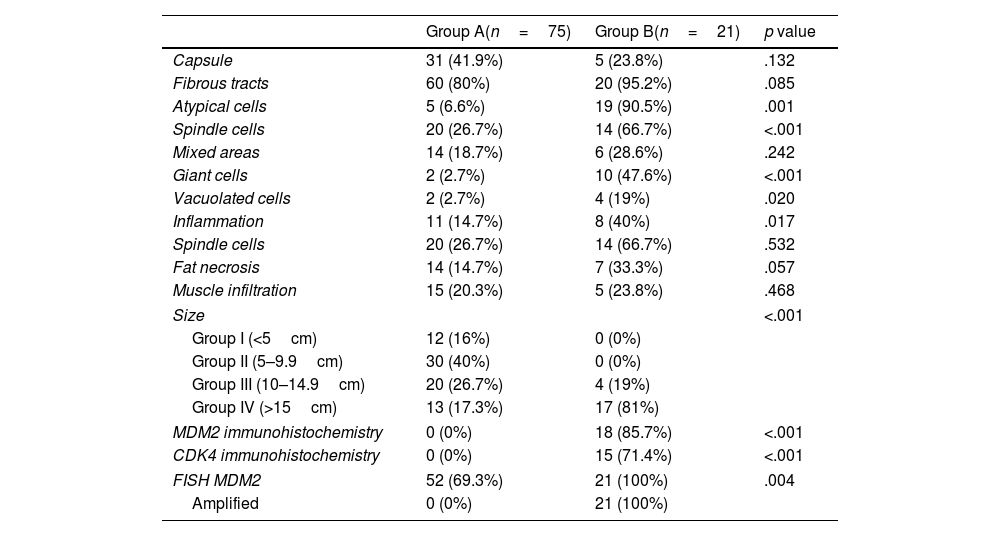
Main histopathological characteristics of the different Groups studied.
| Group A(n=75) | Group B(n=21) | p value | |
|---|---|---|---|
| Capsule | 31 (41.9%) | 5 (23.8%) | .132 |
| Fibrous tracts | 60 (80%) | 20 (95.2%) | .085 |
| Atypical cells | 5 (6.6%) | 19 (90.5%) | .001 |
| Spindle cells | 20 (26.7%) | 14 (66.7%) | <.001 |
| Mixed areas | 14 (18.7%) | 6 (28.6%) | .242 |
| Giant cells | 2 (2.7%) | 10 (47.6%) | <.001 |
| Vacuolated cells | 2 (2.7%) | 4 (19%) | .020 |
| Inflammation | 11 (14.7%) | 8 (40%) | .017 |
| Spindle cells | 20 (26.7%) | 14 (66.7%) | .532 |
| Fat necrosis | 14 (14.7%) | 7 (33.3%) | .057 |
| Muscle infiltration | 15 (20.3%) | 5 (23.8%) | .468 |
| Size | <.001 | ||
| Group I (<5cm) | 12 (16%) | 0 (0%) | |
| Group II (5–9.9cm) | 30 (40%) | 0 (0%) | |
| Group III (10–14.9cm) | 20 (26.7%) | 4 (19%) | |
| Group IV (>15cm) | 13 (17.3%) | 17 (81%) | |
| MDM2 immunohistochemistry | 0 (0%) | 18 (85.7%) | <.001 |
| CDK4 immunohistochemistry | 0 (0%) | 15 (71.4%) | <.001 |
| FISH MDM2 | 52 (69.3%) | 21 (100%) | .004 |
| Amplified | 0 (0%) | 21 (100%) | |
No patient in group A presented local recurrence during the follow-up time. In group B, six (28.6%) local recurrences were recorded, with a median time to LR of 13 (IQR: 80.3) months. Regarding the development of lung metastases, this did not occur in any patient in the series.
Malignancy prognostic factorsThe binary logistic regression analysis, considering age, the largest diameter of the tumour on MRI, body location (lower extremities [LEII] vs. another) and on MRI (intramuscular or intermuscular), showed that the model was significant (χ2=48.9; p<.001) and it was confirmed that age (OR 1.1 per year [95% CI 1.02–1.13]; p=.008), tumour size (OR 1.3 per cm [95% CI: 1.13–1.49]; p<.001) and location in the lower limbs (OR 6.6 [95% CI: 1.01–41.32]; p=.049), were shown as independent prognostic variables of malignancy. On the contrary, the interintramuscular location did not reach statistical significance (p=.128), being excluded from the model.
In the analysis of the ROC curve (Fig. 1), an optimal cut-off point for the diagnosis of ALT of 134.0mm was obtained with an AUC of .91 (95% CI: .85–.96), a sensitivity of 90.5% (95% CI: 71.1–97.3) and a specificity of 78.7% (95% CI: 68.1–86.4). For a sample prevalence of 21.9% of ALT, the positive (PPV) and negative (NPV) predictive values were 54.3% and 96.7%, respectively.
DiscussionThe suspected diagnosis of high-grade liposarcoma is relatively simple. In contrast, distinguishing between a DSL and an ALT is much more complex. With respect to demographic aspects, most authors agree that there is a statistically significant association between age and the diagnosis of malignancy, resulting in patients with a diagnosis of ALT having a median age higher than that of patients with diagnosis of lipoma.10–13 Our results in this regard do not differ from those of the majority of authors, with a significantly higher median age at diagnosis in the ALT group compared to DSL. Furthermore, in the binary logistic regression model, a greater risk of being diagnosed with low-grade liposarcoma compared to lipoma is observed as age increases, with an OR 1.1 (95% CI: 1.02–1.13). Regarding gender, some authors have suggested that male sex is significantly associated with the diagnosis of ALT.11,14 However, more authors have found no such differences.12,13,15 In our study no significant differences were observed either. Regarding the symptoms, there is agreement among the authors that the first sign or symptom for which the patient consulted was most frequently the presence of the lump or a palpable tumour.11,14
Regarding imaging, tumour size on MRI has been revealed in the different series as one of the most supported predictors of malignancy, with the diameter of the ALT being significantly greater than that of the lipomas, establishing different cut-off points according to different authors. Although the American Joint Committee on Cancer (AJCC)16 has suggested a cut-off point >8cm as a prognostic factor for malignancy in sarcomas, the characteristics of ALT have shown higher values in different series. Brisson et al.10 determined a median of 13cm in the lipoma group compared to that of ALT, which had a median of 18.4cm. In the case of Kransdorf et al.,11 the ALT had a median size almost double that of the lipoma group (24cm vs. 10cm). Knebel et al.13 observed that fatty tumours with a largest diameter greater than 130.0mm were more likely to be diagnosed as ALT than as lipomas (66.1% vs. 33.9%). These authors performed a ROC analysis to differentiate both entities with an AUC of .809 (95% CI: .73–.89), an optimal cut-off value of 130.0mm, a sensitivity of 80.9% and a specificity of 69.7%, which means that the probability that a fatty tumour is finally diagnosed with ALT will increase by 2.74 (OR) times (95% CI: 1.82–4.11, p<.001), if its largest diameter is greater than 130mm. These data are comparable to the results of the present study. Furthermore, in the multivariate analysis, size was independently associated with the diagnosis of ALT. Therefore, it would behave as a predictor of malignancy, with an OR of 1.3 (95% CI 1.13–1.49), p<.001.
When comparing the location of lipomas with that of ALT, in most studies, a more frequent distribution of the latter was observed in the lower limbs and gluteal region compared to that of lipomas.10–14 We observed similar results in the present study, although in our multivariate analysis the statistical power for location in the lower limbs as a predictor of malignancy was lower than in the case of age and lesion size.
The few series that evaluate the MRI margins of deep adipose lesions concur that those with non-capsulated or pseudoinfiltrative margins most frequently correspond to ALT.13,15 This was not the case in our study, although the results are probably due to interobserver variability, given that the MRIs came from different centres, where the experience in musculoskeletal oncological pathology of the intervening radiologists was not the same. Methodological variability also exists in the scientific literature in relation to the evaluation of the fat signal.10–13,15 In our series, homogeneous patterns were more frequent in all groups, obviating a volumetric division of the tumour area based on its fatty pattern and leaving interpretation to the subjective criteria of the radiologist. Evaluation by MRI with paramagnetic intravenous contrast is a technique that is generally used in the study of a partial shelf mass, but its use in this context is inconsistent.11
In our series, although uptake was more frequent in the ALT group, these findings did not reach statistical significance, unlike for other authors.13–15 Regarding perilesonal oedema on MRI, Jaovisidha et al.15 identified a higher frequency in the case of ALT (11.1%), compared to lipomas (3.4%), but without these differences reaching statistical significance. Something similar happened in our series.
Focusing on the presence of septa in MRI, which is a constant topic of discussion as a predictor of malignancy in the topic at hand, Kransdorf et al.11 found significant differences between DSL (6%) and ALT (96%), considering the presence of thick and nodular septa as findings of malignancy. Cheng et al.14 also described differences between the two groups, with the presence of fibrous septa in 14.4% of lipomas and 70.7% of ALT, although they did not study the impact of nodular septa in the differential diagnosis of the two entities. Jaovisidha et al.15 also indicated a greater presence of septa, in the case of ALT, 88.9% compared to lipomas 10.3%. However, they did not define differences in thickness or whether they were nodular or not, limiting themselves to describing whether they were present in any of their forms. For Knebel et al.,13 the presence of thick septa was considered a risk factor for suffering from ALT. In our study, we did not observe significant differences between the diagnosis of malignancy and the presence of fibrous septa (65.3% in the case of DSL and 76.2% in the ALT group; p=.329), although they were more common in the group of malignant tumours. The finding could be interpreted as a referral bias, since lipomas referred to an expert sarcoma centre are more likely to be those that present atypical or malignant features on imaging tests. We also did not observe a significant correlation when comparing whether the septa were thin, thick, or nodular.
The use of biopsy in the differential diagnosis of a DSL or ALT remains controversial; its indication will depend on the degree of clinical and radiological suspicion.17 Thavikulwat et al.18 found that the diagnostic yield of core needle biopsy (CNB) was lower for ALT compared to high-grade sarcomas. However, the identification of the MDM2 gene could make the procedure more sensitive and specific,19 especially if the samples are obtained by incisional biopsies20 and by amplification of MDM2 by FISH compared to the detection of MDM2 protein expression by immunohistochemical techniques.6 In our study, in the 29 patients in whom they were performed, always with a trocar, there was diagnostic coincidence after analysis of the resection specimen in 85.7% of the DSL and in 75% of the ALT. However, it seems advisable to biopsy only those cases in which the therapeutic approach could change, or when there are doubts regarding the degree of malignancy assumed.5
The biopsy could be skipped if the resection margins were the same, regardless of whether it was a DSL or an ALT. In this case, the patient would have to be informed of the possibility or not of requiring postoperative radiotherapy. Currently, the role of radiotherapy (RT) in the treatment of these tumours is controversial. Although some authors suggest that treatment with RT reduces local recurrence rates,8 others recommend against its widespread use in this group of patients, given that the current recurrence rates together with the infrequency of metastasis and dedifferentiation to high-grade sarcoma does not justify the toxicity of RT. In addition, it is possible that the response of ALT to RT is low due to its low-grade nature, reserving these treatments for those cases that recur9 or in complex locations at the discretion of the CTME.
As a further observation on local recurrence, our series obtained a rate of 28.6% (six patients), within the range of values published by other authors.2,3 Although it should be noted that of the six patients who recurred, three received radiotherapy treatment and in the other three it was deemed inappropriate, in accordance with the CTME criteria.
Regarding our histopathological results, both immunohistochemical and cytogenetic, in the analysis of the resection specimen, these do not differ from those of other authors.5–7
ConclusionsIn conclusion, it remains difficult to distinguish a DSL from an ALT based on clinical, imaging, and even histological data. ALT will be suspected clinically in elderly patients with a large adipose lesion located in the lower limbs. No radiological variable on MRI reached significance as a predictor of malignancy in our series.
Level of evidenceLevel of evidence IV.
FundingThe study was financed by a grant for research projects in Biomedicine, Health Management and Socio-health Care from the Regional Health Management of Castilla y León, Ministry of Health (Junta de Castilla y León) in 2016 to the project “Comparative study of lipomas and Well-differentiated liposarcomas/atypical lipomas’. Diagnostic and therapeutic guidelines for deep lipomatous tumours of the limbs”, whose main researcher was Dr. Francisco Miguel Izquierdo-García, with a grant of 3983 euros (file number GRS 1281/A/16).
Conflict of interestsThe authors have no conflict of interests to declare.
Our thanks to Carlos Pérez for his support in the statistical analysis. Also, to Dr. Yesica Soto for her logistical collaboration in the data collection phase, and to Rosario Turiel Vicente, pathological anatomy technician, for performing the immunohistochemistry and FISH techniques.