Corticosteroids play a vital role in enabling the body to tolerate stressful situations and regulate the inflammatory process. Excessive or persistent inflammation can lead to tissue damage and the development of diseases. Glucocorticoids are crucial in modulating the impact of inflammatory disorders, such as allergies, autoimmune diseases, and sepsis. These disorders can be life-threatening and benefit from glucocorticoid administration. Natural and synthetic glucocorticoids are utilized for the diagnosis and treatment of adrenal function disorders and various inflammatory, immune, and neoplastic conditions. The discovery of glucocorticoids is a testament to the ingenuity and collaboration of researchers. For over 70 years, glucocorticoids have revolutionized medicine and are widely employed in various medical conditions. This article provides a comprehensive review of the critical considerations for the clinical application of glucocorticoids, aiming to strike a balance between their benefits and potential risks. Understanding its history, chemical structure, pharmacology, and mechanisms of action provides the foundation for achieving proper use of this type of medication.
Los corticosteroides desempeñan un papel vital al permitir que el cuerpo tolere situaciones estresantes y regule el proceso inflamatorio. La inflamación excesiva o persistente puede ocasionar daño tisular y el desarrollo de enfermedades. Los glucocorticoides son cruciales para modular el impacto de trastornos inflamatorios, como alergias, enfermedades autoinmunes y sepsis. Estos trastornos pueden ser potencialmente mortales y se benefician de la administración de estos medicamentos. Los glucocorticoides naturales y sintéticos se utilizan para el diagnóstico y tratamiento de trastornos de la función adrenal y diversas condiciones inflamatorias, inmunológicas y neoplásicas. El descubrimiento de los glucocorticoides es un testimonio del ingenio y la colaboración de los investigadores. Desde hace más de 70 años los glucocorticoides han revolucionado la medicina y se emplean ampliamente en diversas condiciones médicas. Este artículo ofrece una revisión exhaustiva de las consideraciones más relevantes para su aplicación clínica, con el objetivo de encontrar un equilibrio entre sus beneficios y sus posibles riesgos. Comprender su historia, su estructura química, su farmacología y sus mecanismos de acción proporciona bases para lograr el adecuado uso de este tipo de medicamentos.
Corticosteroids enable the body to better tolerate stressful situations, such as exposure to harmful stimuli or changes in the environment. Inflammation is a reflex response to tissue injury, regardless of its origin (infection, mechanical damage, etc.). The hypothalamic–pituitary–adrenal (HPA) axis and glucocorticoids (GC) are essential to limit and resolve the inflammatory process. Controlled inflammation is beneficial, but excessive or persistent inflammation can cause tissue damage and contribute to the development of disease. Inflammatory disorders, such as allergies, autoimmune diseases, and sepsis, are life-threatening entities in which GC can modulate their impact.1
Natural and synthetic GC are employed in the diagnosis and treatment of adrenal function disorders, for example, in the diagnosis of endogenous Cushing's syndrome and as the replacement therapy for patients with adrenal insufficiency.2 Furthermore, glucocorticoids are commonly utilized, often at high doses, for the treatment of a variety of inflammatory, immune, and neoplastic conditions. They are recognized as highly effective and broad-spectrum anti-inflammatory agents but also have a wide range of adverse events.3,4 It has been estimated that up to 3% of the population uses them each year.5
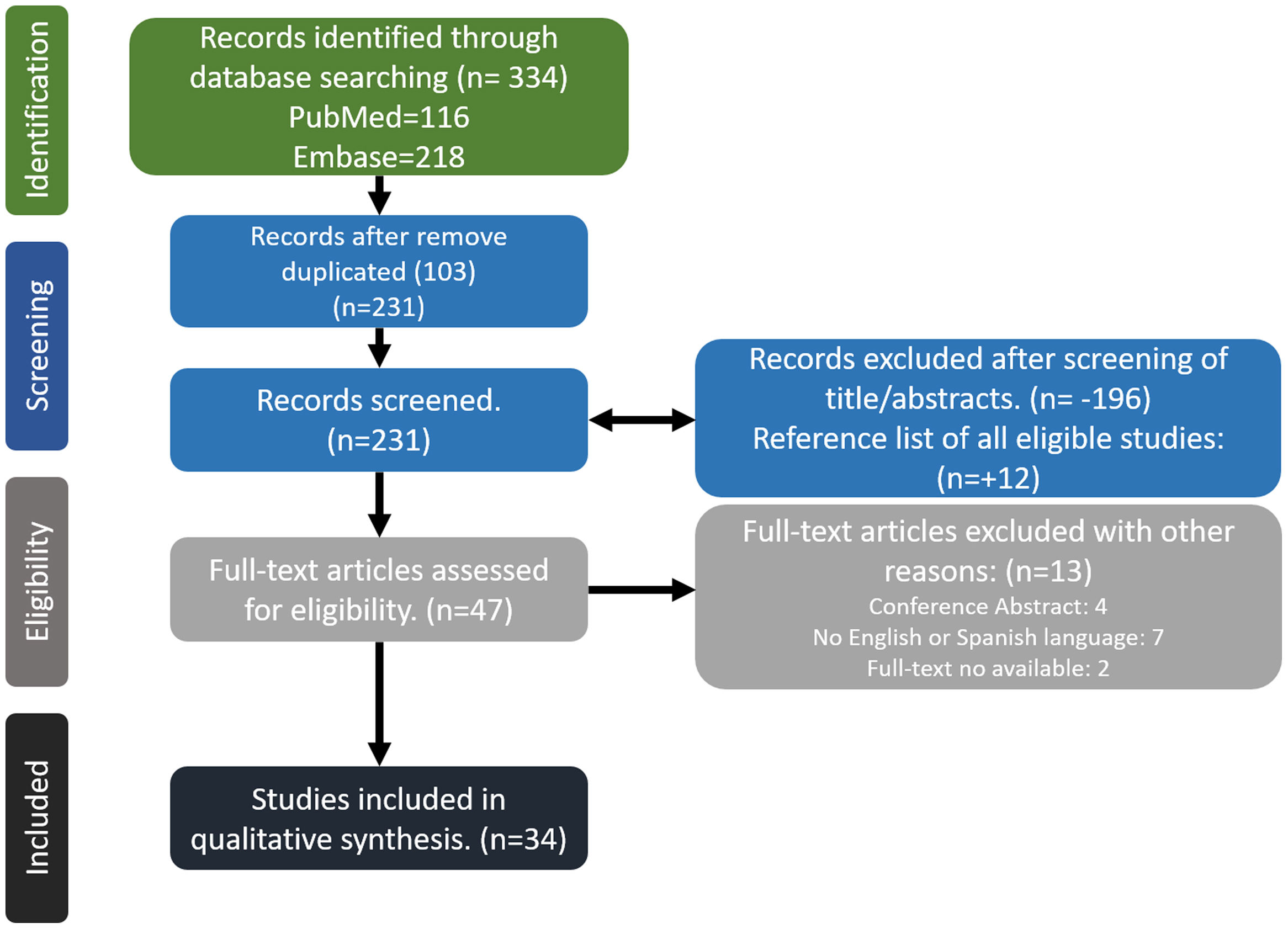
MethodsWe conducted a comprehensive search in MEDLINE/PubMed and EMBASE databases to identify relevant studies. The search period spanned from January 1986 to March 2023. Our search strategy employed a combination of free text terms and controlled vocabulary terms (MeSH and DeCS). The keywords used included “glucocorticoids” OR “corticosteroids” AND “pharmacology” OR “pharmacokinetics” OR “adverse event” OR “side effects” in various combinations. The search was limited to articles published in English or Spanish and focused on human subjects. Two independent reviewers assessed the titles and abstracts of the identified references, eliminating irrelevant studies during the initial screening phase. Full-text articles were obtained for potentially relevant studies, which were then evaluated by the reviewers independently for final inclusion in the review. Additionally, the reference lists of eligible studies were manually screened for potential inclusion of additional articles. Any discrepancies between the reviewers were resolved through discussion or consultation with a third reviewer, as needed. Due to the extensive volume of bibliographic references retrieved, we opted not to include a review of the gray literature in our analysis (Fig. 1).
HistoryThe discovery of glucocorticoids is a fascinating story of good science, perseverance, and luck. Endogenous GC, like cortisol in humans, plays a crucial role in regulating various physiological processes necessary for survival. In 1849, Thomas Addison, an English physician, observed a rare and fatal disease characterized by a “general state of languor, weakness, and intense fatigue, irritability in the stomach, and peculiar hyperpigmentation”.6 He was able to demonstrate through autopsies that this clinical picture occurred in patients whose adrenal gland was destroyed.6 Later works by Brown-Sequard showed that adrenalectomized animals quickly succumbed to even the slightest physiological stress, but improved when given extracts of the adrenal cortex.
On September 21, 1948, at the Mayo Clinic in Minnesota, “Compound E,” a synthetic version of a steroid isolated from animal adrenal glands, was used for the first time. A patient, Mrs. G., 29 years old, who suffered from severe rheumatoid arthritis, was admitted to the hospital due to marked inflammation in her joints and terrible pain that eventually left her bedridden. Despite feeling desperate and exhausted, she firmly refused to leave the hospital until she experienced an improvement in her condition. A clinician, Dr. Philip Hench, consulted with Edward Kendall (a chemist from the same organization), and together they contacted Merck laboratories to try Compound E for the first time. The day after, Mrs. G. did not feel better, but by the third day, she had marked improvement, and by the fourth day, her pain and stiffness had disappeared, and she was visiting other patients to show her progress. After the first week, she was almost asymptomatic and stated, “I have never felt better in my life”.7,8 In 1950, two years later, the Nobel Prize in Physiology or Medicine was awarded to Philip S. Hench, Edward Kendall, and Tadeus Reichstein for their seminal discoveries related to the hormones of the adrenal cortex, including their structure and biological effects. Their research transformed the diagnosis and treatment of multiple diseases.
The discovery of GC is not only a tribute to the researchers’ ingenuity and resourcefulness but also to their scientific collaboration across multiple disciplines. The synthesis and use of Compound E marked a turning point in the treatment of inflammatory conditions and revolutionized modern medicine. The use of glucocorticoids is now widespread and encompasses a broad range of medical conditions. Understanding the history of these drugs and their development provides a rich perspective on the advancements in medical science and inspires us to continue to push the boundaries of scientific inquiry.
Clinical pharmacology of glucocorticoidsTo fully comprehend the clinical use of GC in various medical scenarios, it is essential to understand the chemical structure of steroids, characterized by a common structure of multiple rings, which includes various molecules such as sex hormones and corticosteroids. Among the latter, corticosteroids are classified based on their glucocorticoid activity, anti-inflammatory effects, and mineralocorticoid potency. These factors respectively regulate carbohydrate metabolism, immune function, and hydroelectrolytic balance. The anti-inflammatory effects of corticosteroids are closely related to their activity on glucose metabolism, but not on sodium retention. Therefore, historically corticosteroids have been described as GC and mineralocorticoids.1,9 In humans, cortisol (hydrocortisone) and aldosterone are the main GC and mineralocorticoid, respectively. The chemical modification of endogenous steroids allowed for the production of synthetic GC, some of which have proven to be effective anti-inflammatory and immunosuppressive compounds with rapid effects. Hence, we will use the term GC to describe the use of these drugs.
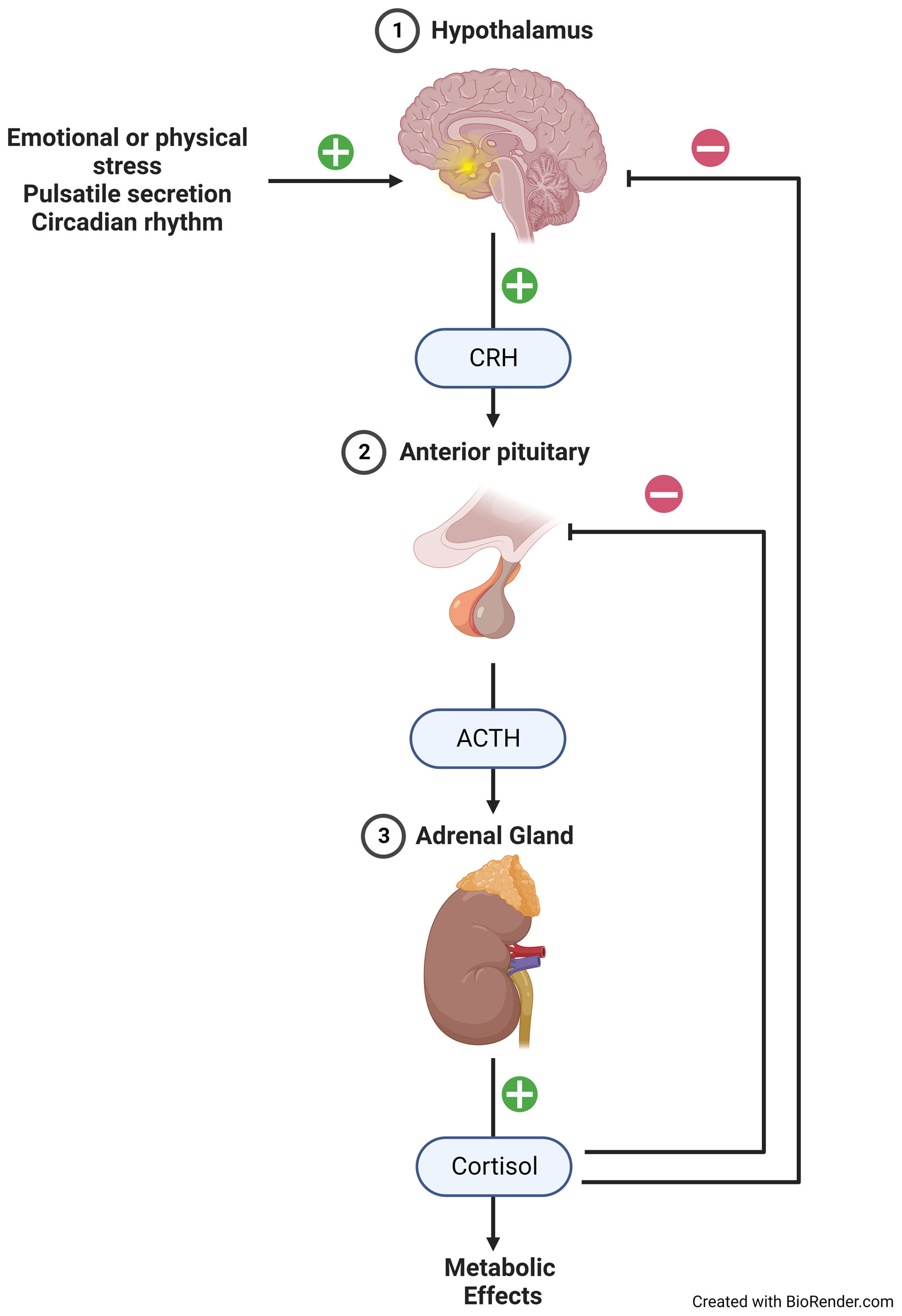
The precursor molecule of all steroid hormones is cholesterol, which is also a part of cell membranes, vitamin D, and some cell organelles. The basic ring of steroids is the cyclopentanoperhydrophenanthrene. This is necessary for the activity of GC and is present in all of them, whether natural or synthetic. Depending on the molecules that are added to the basic steroid ring, quantitative changes in anti-inflammatory and mineralocorticoid potency, as well as in the duration of action and metabolism, may occur.1,10 GC biosynthesis occurs in the mitochondria of the inner part of the adrenal cortex, the fasciculata-reticular zone. It involves a multiple enzymatic process known as steroidogenesis.11,12 This production is induced by the activation of HPA axis, which is a neural-endocrine “center” that coordinates physiological responses to external stimuli and includes a negative feedback circuit that controls endogenous GC production (Fig. 2).
The secretion rate of cortisol follows a complex circadian and ultradian rhythm, which is primarily regulated by pulses of adrenocorticotropic hormone (ACTH). These ACTH pulses exhibit diurnal variations and typically peak in the early morning and after meals. This intricate hormonal regulation of cortisol secretion is essential for the maintenance of physiological homeostasis. In plasma, cortisol binds to circulating proteins. Ninety percent is bound to corticosteroid-binding globulin, also called transcortin. About 5% remains weakly bound to albumin (considered free), and the remaining 5% is free and available to exert its effect on target cells.13 The affinity of the different GC for plasma proteins is variable. Synthetic GC, excluding prednisolone, exhibit a weak binding affinity two-thirds toward albumin, negligible binding to transcortin (<1%), and the remaining one-third of the drug circulates in a free form.10,13 Prednisolone binds reversibly to both albumin and transcortin. However, its protein binding is higher (around 80–90%) at lower concentrations. As concentrations increase, its protein binding becomes lower (about 60–70%) due to the saturation of transcortin, as albumin has lower affinity and transcortin has limited capacity. Thus, we can understand how a high dose of these drugs, with the consequent saturation of albumin and transcortin, will cause more of the drug available to exert its effect. Under this reasoning, in patients in whom severe hypoalbuminemia is documented, the dose could be adjusted with the theoretical aim of reducing adverse effects.14 Synthetic GC are subject to the same reduction, oxidation, hydroxylation, and conjugation reactions as endogenous steroids. They are metabolized mainly in the liver to inactive metabolites that are excreted by the kidneys; only small amounts of unmetabolized drug are excreted in the urine. It is known that there is an inverse correlation between GC elimination and age, degree of liver disease, and African American population.1,10,13,15 This means that a dose considered low could have a greater effect in these population groups. In contrast to the above, hyperthyroidism and nephrotic syndrome result in increased clearance.16 In obese patients we must be careful when prescribing GC, due to an increased sensibility to GCs and this drugs partially distribute in the excess of bodyweight and that adjustment based on total body weight would lead to an excessive increase in initial plasma GC concentration after a loading dose and would be thus inappropriate.17
To exert their biological function, prohormones of GC (such as cortisone and prednisone) undergo enzymatic reduction by 11β-hydroxysteroid dehydrogenase type 1 in the liver to form active hormones (cortisol and prednisolone). In cases of severe liver disease, prednisolone may be prescribed instead of prednisone. The rest of the GC do not require this process.3 The same enzyme, but type 2, can promote the reverse reaction by dehydrogenation, resulting in the inactivation of active GC. This occurs mainly in the kidney and intestine to generate a negative regulatory mechanism to avoid the mineralocorticoid effect.12,18,19
GC exert their effects by binding to specific receptors that regulate cellular functions. These receptors are present in almost all nucleated cells, allowing for widespread effects throughout the body. GC have been found to regulate up to 10–20% of gene expression in a cell.1,20 They are lipophilic, so they can diffuse freely across the cell membrane. Their mechanisms of action are generically grouped into genomic and non-genomic, but cannot be separated. Practically speaking, the predominance of non-genomic effects will depend on the use of high doses. In the absence of the hormone ligand, GC receptors are mainly cytoplasmic. Free hormone, from plasma and interstitial fluid, enters the cell and binds to the receptor. The receptor-ligand complex is actively transported to the nucleus, where it interacts with glucocorticoid-sensitive elements (GSE) of deoxyribonucleic acid (DNA) and transcription factors, such as nuclear factor k-B (direct and indirect genomic mechanism of action, respectively). There they can regulate the transactivation and transrepression of several target genes. This mechanism of action, called genomic, involves several steps and therefore requires time, usually 4–6h (minimum 30min) before the clinical effect can be observed, and occurs with any therapeutically relevant dose9,20–22 (Fig. 3).
The mechanism of action that does not depend on the interaction of the steroid molecule with the cell nucleus is called the non-genomic effect (Fig. 4). It involves the interaction of GC with receptors on the cell membrane and the generation of physicochemical interactions with biological membranes, thus altering cellular functioning. In this way, through actions of inhibition of ion channels or other pathways, changes in the immune response can occur. To achieve its effect, a shorter sequence of events is required, so it is a faster effect, usually 15min. But it is only achieved with high doses of GC, usually equivalent to 100mg prednisolone. Thus, we can understand how the response to treatment with methylprednisolone, at high doses, is biphasic. Composed of an early, rapid, non-genomic effect, and a late-onset but sustained genomic effect.18,19
Regarding genomic effects, saturation of the cytoplasmic receptor is considered to be the modulator of the therapeutic effect. Unfortunately, precise data on the administered dose and the degree of receptor occupancy are not available, and high interindividual variation is expected because absorption and metabolism, which by itself are inherently variable, are not controlled in these assays. Estimating the biological activity of prednisolone based on known binding constants suggests that oral doses of 7.5–15mg would result in 42–63% receptor saturation 8h after administration. Higher doses, such as 100mg or more, would be required to achieve near-complete receptor saturation.9 Through these mechanisms, GC are able to reduce the activation, proliferation, differentiation, and survival of several types of inflammatory cells such as macrophages and T lymphocytes, while promoting apoptosis, particularly in immature and activated T lymphocytes. These effects are mainly mediated by changes in cytokine synthesis and secretion.1,10,15
In patients with acute adrenal insufficiency, it is worth mentioning that the early administration of glucocorticoids, such as hydrocortisone, favors α1-adrenergic receptor expression. This helps resolve hemodynamic instability and decreases inappropriate arginine-vasopressin secretion, leading to improved free water secretion and a favorable fluid and electrolyte balance. Both of these effects can be observed within seconds to a few minutes after administration.23
PotencyThe potency of different GC effects is commonly expressed as a prednisolone/prednisone equivalent dose, as prednisone was the first synthetic GC used in clinical practice. While the scientific evidence supporting the accuracy of these values is not strong, they remain useful as general therapeutic guidelines in clinical settings. However, it is important to avoid relying on these ratios dogmatically.9 These doses are calculated by determining the ratio between the glucocorticoid used and its potency. For example, the conversion factor of hydrocortisone is 4, so 200mg is equivalent to 50mg of prednisolone (200mg/4=50mg). In cases where the equivalent dose is not known, web-based applications can be used. One easily accessible application can be found at https://accessmedicina.mhmedical.com/calculator.aspx?calc=25.
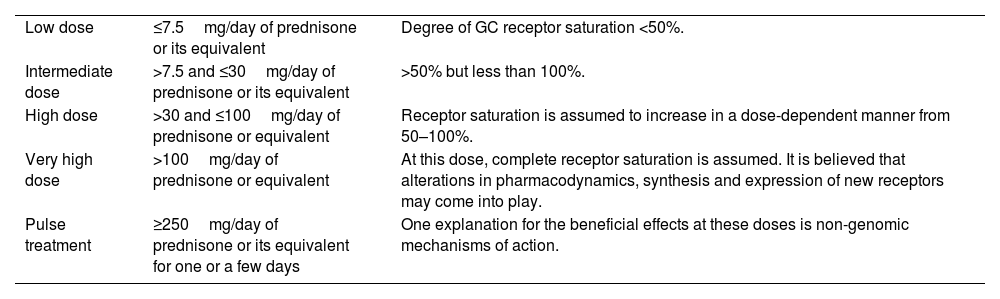
It is important to note that the concept of equivalent doses only applies to doses lower than 100mg of prednisone. Beyond this threshold, non-genomic effects may become more prominent and differ from the classical genomic effects. Therefore, when prescribing higher doses, clinicians should consider the potential differences in effects and adjust the treatment plan accordingly (Table 1). For instance, high-dose GC therapy, such as GC pulses (equivalent to 250–1000mg prednisolone every 24h), typically used in severe exacerbations of immunologically mediated disorders. Generally speaking, methylprednisolone or dexamethasone are preferred over prednisolone because, although all have similar genomic potency, the non-genomic effect of the former is three to five times greater.20 Another example is the very low non-genomic potency of betamethasone, which may be one of the reasons why this drug is rarely used systemically, despite having a similar genomic potency to dexamethasone.1,3,9,10
Comparison of systemic glucocorticoids in their pharmacological characteristics.
| Glucocorticoid | Genomic powera | Non-genomic power | Mineralocorticoida activity | Binding to transcortin | Albumin binding | Biological half-life (h) |
|---|---|---|---|---|---|---|
| Prednisoneb | 4 | 4 | 0.3 | + | − | 18–36 |
| Prednisolone | 4 | 4 | 0.3 | +++ | + | 12–36 |
| Hydrocortisone | 1 | Under | 1 | − | +++ | 8–12 |
| Methylprednisolone | 5 | 10–15 | 0.5 | − | +++ | 18–36 |
| Dexamethasonec | 20–30 | 20 | 0 | − | +++ | 36–54 |
| Betamethasonec | 20–30 | <4 | 0 | − | +++ | 36–54 |
| Fludrocortisonec,a | 10–15 | − | 250 | − | +++ | 18–36 |
There are various ways in which GC can be administered, including oral, intravenous, intramuscular, intra-articular, inhaled, and topical routes. In cases where rapid effects are required, the most effective route is intravenous, due to the high bioavailability achieved. Non-systemic administration of GC (e.g., intraarticular, epidural, etc.), can also have systemic effects for weeks and complications associated with their use.24 The route of administration for GC also depends on their formulation, as they can be available in either a free form or chemically bound to an ester or salt. Most orally administered GC, whether in their free form or as esters or salts, are readily absorbed. However, free forms are not water-soluble, limiting their use to parenteral administration. Ester-bound formulations are liposoluble and can be used in tablets, intralesional, intramuscular, and intra-articular routes. Salt-bound formulations are water-soluble (e.g., sodium succinate and phosphate) and are used for intravenous administration.1,16
The use of GC with different doses is very common. Often, there is a lack of clarity in expressing the intended meaning of semiquantitative terms used for doses, such as low or high. Based on pathophysiological and pharmacokinetic data, standardization has been proposed to minimize problems in interpreting these commonly used terms (Table 2).
Definition for conventional terms for glucocorticoid doses.
| Low dose | ≤7.5mg/day of prednisone or its equivalent | Degree of GC receptor saturation <50%. |
| Intermediate dose | >7.5 and ≤30mg/day of prednisone or its equivalent | >50% but less than 100%. |
| High dose | >30 and ≤100mg/day of prednisone or equivalent | Receptor saturation is assumed to increase in a dose-dependent manner from 50–100%. |
| Very high dose | >100mg/day of prednisone or equivalent | At this dose, complete receptor saturation is assumed. It is believed that alterations in pharmacodynamics, synthesis and expression of new receptors may come into play. |
| Pulse treatment | ≥250mg/day of prednisone or its equivalent for one or a few days | One explanation for the beneficial effects at these doses is non-genomic mechanisms of action. |
Hydrocortisone is most often used for hormone replacement in the setting of adrenal insufficiency because it possesses dual GC and mineralocorticoid activity, which matches that of endogenous cortisol. Prednisolone and methylprednisolone are used to treat a variety of inflammatory and immune disorders. When administered for hormone replacement, they should be combined with a drug possessing mineralocorticoid activity (such as fludrocortisone), to compensate for their low mineralocorticoid effect. For intravenous application, only the succinate presentation (due to its high solubility) is recommended, and it should be administrated in a slow infusion lasting at least 60min. The acetate form is reserved for intra-articular or intramuscular administration.1
Prednisolone is usually the GC of choice for chronic treatments because it has a short half-life, can be taken orally, and is easily dosed in a wide range. Morning administration of this GC should be preferred, as studies shown it to have a lower risk of suppression of the HPA axis.13
Budesonide is a glucocorticoid that has low oral availability. It has been estimated that, due to a high first-pass metabolism in the liver, only 10–15% of the administered drug remains available. This characteristic helps to reduce the risk of adverse effects compared to prednisolone.18 Additionally, budesonide exhibits high anti-inflammatory activity topically. Considering these two characteristics, its presentation with an enteric coating is useful and forms part of the therapeutic approach to induce remission in patients with low-risk Crohn's disease.25,26
Dexamethasone is used especially as an anti-inflammatory in allergic and autoimmune disorders. It is included in some chemotherapeutic regimens and is also frequently employed to assess the functionality of the HPA axis, aiding in the differential diagnosis of adrenal incidentaloma and endogenous Cushing's syndrome. Due to its lack of mineralocorticoid activity and its potential to cause prolonged and severe suppression of the HPA axis, dexamethasone is rarely used for hormone replacement.18
Regarding pregnant women, it should be noted that the ratio of prednisolone between maternal and fetal blood is 10:1. This is because GC with high protein binding (such as prednisolone, methylprednisolone, and hydrocortisone), do not cross the placenta. Additionally, the placental 11β-HSD type 2 enzyme inactivates some GC. On the other hand, fluorinated GC (such as dexamethasone and betamethasone) have a low affinity for transport proteins and are poorly metabolized by 11β-HSD2 in the placenta. Consequently, the concentration ratio of these GC between maternal and fetal blood is around 1:1. With this concept in mind, it is understood why prednisone, prednisolone and methylprednisolone are good choices for trating the pregnant mother. If an effect on the fetus is desired (e.g., fetal heart block due to maternal Sjögren's syndrome or pulmonary maturation, etc.), fluorinated GCs should be indicated.1,19
Finally, in the treatment with topical GCs, it should be kept in mind that systemic absorption is higher in areas with inflamed skin and anatomical regions with thin stratum corneum, such as the eyelids or face. Higher potency GCs are generally used for severe dermatoses in non-facial/non-intertriginous areas, they are especially useful in areas such as palms and soles. Conversely, in those dermatoses that affect areas with thin stratum corneum or when large areas of skin need to be treated, low potency GCs should be preferred for short periods of time.27
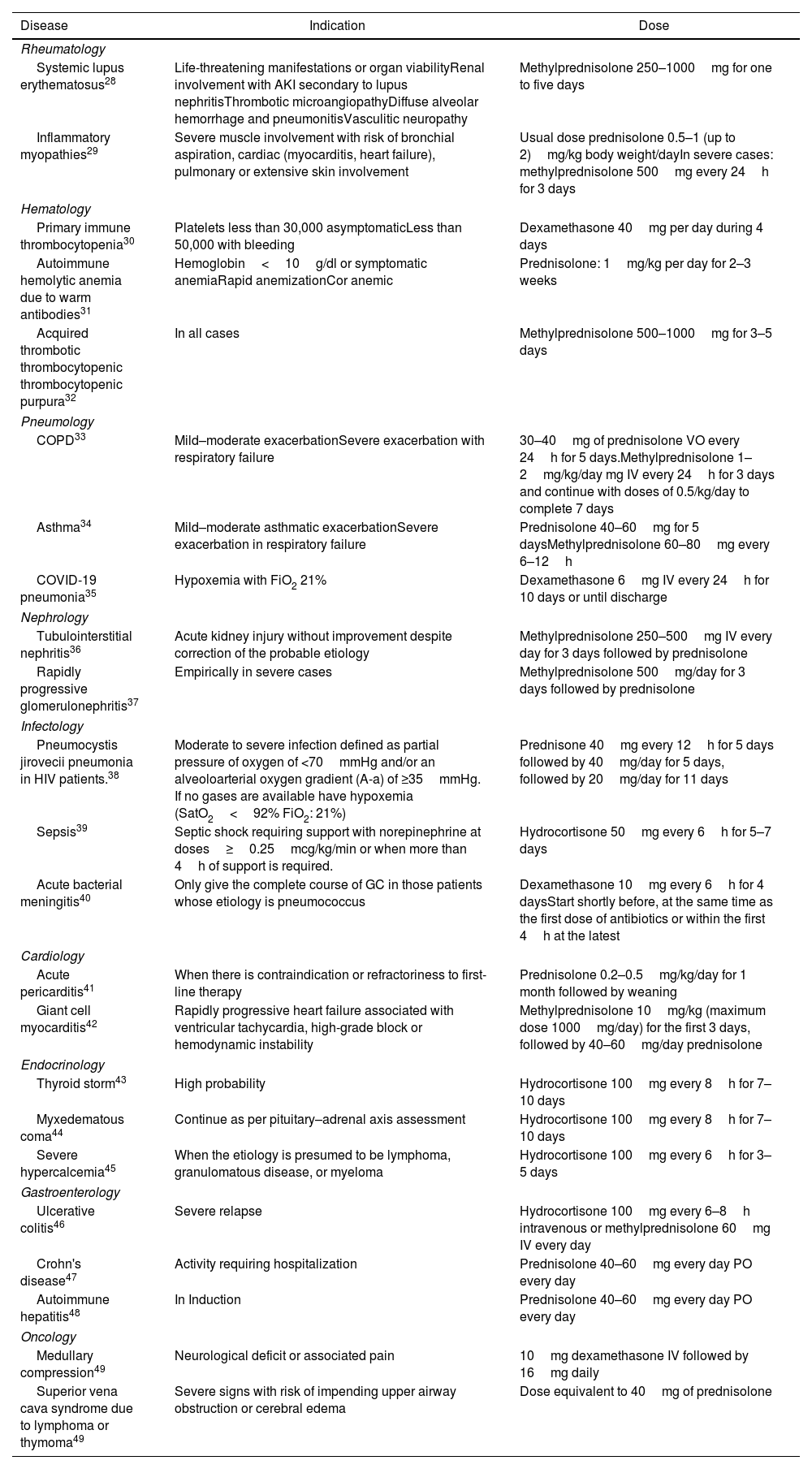
The peculiarities of treatment for diseases that require GC are beyond the scope of this review, in this section we present a summary that summarizes only the general use, indications, and doses in the most frequent uses (Table 3).
Indications for high doses of glucocorticoids in different diseases.
| Disease | Indication | Dose |
|---|---|---|
| Rheumatology | ||
| Systemic lupus erythematosus28 | Life-threatening manifestations or organ viabilityRenal involvement with AKI secondary to lupus nephritisThrombotic microangiopathyDiffuse alveolar hemorrhage and pneumonitisVasculitic neuropathy | Methylprednisolone 250–1000mg for one to five days |
| Inflammatory myopathies29 | Severe muscle involvement with risk of bronchial aspiration, cardiac (myocarditis, heart failure), pulmonary or extensive skin involvement | Usual dose prednisolone 0.5–1 (up to 2)mg/kg body weight/dayIn severe cases: methylprednisolone 500mg every 24h for 3 days |
| Hematology | ||
| Primary immune thrombocytopenia30 | Platelets less than 30,000 asymptomaticLess than 50,000 with bleeding | Dexamethasone 40mg per day during 4 days |
| Autoimmune hemolytic anemia due to warm antibodies31 | Hemoglobin<10g/dl or symptomatic anemiaRapid anemizationCor anemic | Prednisolone: 1mg/kg per day for 2–3 weeks |
| Acquired thrombotic thrombocytopenic thrombocytopenic purpura32 | In all cases | Methylprednisolone 500–1000mg for 3–5 days |
| Pneumology | ||
| COPD33 | Mild–moderate exacerbationSevere exacerbation with respiratory failure | 30–40mg of prednisolone VO every 24h for 5 days.Methylprednisolone 1–2mg/kg/day mg IV every 24h for 3 days and continue with doses of 0.5/kg/day to complete 7 days |
| Asthma34 | Mild–moderate asthmatic exacerbationSevere exacerbation in respiratory failure | Prednisolone 40–60mg for 5 daysMethylprednisolone 60–80mg every 6–12h |
| COVID-19 pneumonia35 | Hypoxemia with FiO2 21% | Dexamethasone 6mg IV every 24h for 10 days or until discharge |
| Nephrology | ||
| Tubulointerstitial nephritis36 | Acute kidney injury without improvement despite correction of the probable etiology | Methylprednisolone 250–500mg IV every day for 3 days followed by prednisolone |
| Rapidly progressive glomerulonephritis37 | Empirically in severe cases | Methylprednisolone 500mg/day for 3 days followed by prednisolone |
| Infectology | ||
| Pneumocystis jirovecii pneumonia in HIV patients.38 | Moderate to severe infection defined as partial pressure of oxygen of <70mmHg and/or an alveoloarterial oxygen gradient (A-a) of ≥35mmHg. If no gases are available have hypoxemia (SatO2<92% FiO2: 21%) | Prednisone 40mg every 12h for 5 days followed by 40mg/day for 5 days, followed by 20mg/day for 11 days |
| Sepsis39 | Septic shock requiring support with norepinephrine at doses≥0.25mcg/kg/min or when more than 4h of support is required. | Hydrocortisone 50mg every 6h for 5–7 days |
| Acute bacterial meningitis40 | Only give the complete course of GC in those patients whose etiology is pneumococcus | Dexamethasone 10mg every 6h for 4 daysStart shortly before, at the same time as the first dose of antibiotics or within the first 4h at the latest |
| Cardiology | ||
| Acute pericarditis41 | When there is contraindication or refractoriness to first-line therapy | Prednisolone 0.2–0.5mg/kg/day for 1 month followed by weaning |
| Giant cell myocarditis42 | Rapidly progressive heart failure associated with ventricular tachycardia, high-grade block or hemodynamic instability | Methylprednisolone 10mg/kg (maximum dose 1000mg/day) for the first 3 days, followed by 40–60mg/day prednisolone |
| Endocrinology | ||
| Thyroid storm43 | High probability | Hydrocortisone 100mg every 8h for 7–10 days |
| Myxedematous coma44 | Continue as per pituitary–adrenal axis assessment | Hydrocortisone 100mg every 8h for 7–10 days |
| Severe hypercalcemia45 | When the etiology is presumed to be lymphoma, granulomatous disease, or myeloma | Hydrocortisone 100mg every 6h for 3–5 days |
| Gastroenterology | ||
| Ulcerative colitis46 | Severe relapse | Hydrocortisone 100mg every 6–8h intravenous or methylprednisolone 60mg IV every day |
| Crohn's disease47 | Activity requiring hospitalization | Prednisolone 40–60mg every day PO every day |
| Autoimmune hepatitis48 | In Induction | Prednisolone 40–60mg every day PO every day |
| Oncology | ||
| Medullary compression49 | Neurological deficit or associated pain | 10mg dexamethasone IV followed by 16mg daily |
| Superior vena cava syndrome due to lymphoma or thymoma49 | Severe signs with risk of impending upper airway obstruction or cerebral edema | Dose equivalent to 40mg of prednisolone |
Because of the diversity of mechanisms of action and target organs, it is not surprising that GC cause a wide range of adverse effects. While several of these are unavoidable, the risk is dose- and duration-dependent (cumulative dose), so a clinician's approach to minimize the patient's exposure to GC will be beneficial. Low-dose treatment is safer than commonly believed. However, there is interindividual variability in sensitivity to adverse effects. Some epidemiological studies suggest that treatment with a daily dose of fewer than 10mg of prednisolone or its equivalent appears to carry no or only a slightly increased risk of adverse events. Whereas if doses of 20–40mg/day are received, the risk of infection is much higher.25,26,50,51 Waljee et al.52 in a retrospective study, refute the concept of the relative safety of short-term GC use within 30 days of initiation of therapy. The study showed an increased risk of sepsis, venous thromboembolic events, and fractures. Although these data are from an observational study and the risk of bias by indication and severity is inherent, these risks are alarming and require further exploration. In the study conducted by Huscher et al.,53 two distinct dose-related patterns of adverse events were identified. The first pattern, referred to as the “linear pattern,” demonstrated a gradual increase in adverse events with escalating doses. This pattern was observed for cushingoid phenotype, ecchymosis, mycosis, parchment-like skin, and sleep disturbance. The second pattern, known as the “threshold pattern,” revealed a significant rise in the frequency of events beyond a specific threshold value. This threshold was observed at dosages exceeding 7.5mg/day and was associated with glaucoma, depression, and increased blood pressure. Additionally, dosages of 5mg/day or higher were linked to epistaxis and weight gain. Notably, a particularly low threshold was observed for eye cataract occurrence (<5mg/day).
A recent systematic review with meta-analysis, published by Edel et al.54 demonstrates how physicians are often concerned about the safety profile of GC pulses. The incidence of adverse events associated with glucocorticoid pulse therapy is estimated at a rate of 35 events per 100 patient-years. The most commonly reported adverse events include hypertension, primarily diastolic blood pressure elevation, hyperglycemia, flushing, chest pain, heart rhythm disorders such as palpitations and tachycardia, and headache.55 Therefore, their utilization should be approached with caution and restricted to cases where they are deemed clinically necessary.
It is no longer acceptable to consider glucocorticoid toxicity as an unavoidable consequence in order to control autoimmune or allergic diseases and achieve remission. In order to mitigate the adverse effects associated with GC therapy, several strategies are implemented. These include the utilization of the minimum effective dose of GC for the shortest duration necessary to achieve treatment objectives, the careful management of preexisting comorbidities that may heighten risk when glucocorticoids are required, and the diligent monitoring of patients undergoing treatment for potential adverse effects, with the consideration of additional interventions when deemed beneficial.56–58
In this section, we will give some simple guidelines for the prevention of adverse events that usually occur in the long term, summarized in Table 4. In the following paragraphs, we will go into the prevention and treatment of adverse events that usually occur with high doses in short cycles.
Adverse effects of glucocorticoids on various systems and strategies to prevent them.
| Committed organ | Side effect | Recommendation |
|---|---|---|
| Bone | Secondary osteoporosisIn patients with doses equivalent to or greater than 7.5mg of prednisolone for periods greater than 3 months | Calcium supplement 1000mg/dayVitamin D3 levels >30ng/ml (supplement with 600–800IU/day)Correcting factors that increase the risk of falls |
| Muscle | MyopathyAtrophy | Physical activity |
| Endocrinological | Adrenal insufficiencyDyslipidemia | Gradual disassembly and use of less than 4 weeksChecking biannual lipid profiles |
| Ocular | GlaucomaProptosisHerpes virus infection | Baseline ophthalmology examinationCheck intraocular pressure after 3 months of systemic steroidsAvoid ophthalmic route |
| Digestive | Peptic ulcerGastrointestinal bleedingFatty liver | Avoid concomitant use with non-steroidal anti-inflammatory drugs or antiplatelet drugs, or in presence of other risk factors administer proton pump inhibitors |
| Neuropsychiatric | AnxietyDepressionIrritabilityInsomnia | Identify individuals at riskAvoid concomitant use with tricyclic antidepressantsDose glucocorticoids in the morning onlyAdvise family members to contact clinicians withconcern of any change in behavior |
| Hematological | Venous thromboembolism | Avoid prolonged restUse of thromboprophylaxis in appropriate settings |
Glucocorticoid-induced diabetes mellitus is a widely recognized adverse effect. The proposed mechanisms include increase hepatic glucose production and induce insulin resistance by inhibiting uptake and metabolism in peripheral tissues. Additionally, they have a direct effect on the endocrine cells of the pancreas, resulting in decreased insulin secretion and increased glucagon.59 It is prevalence is likely underestimated due to variations in study populations and their varying susceptibility to hyperglycemia. High doses of GC usually result in a rapid increase in blood glucose values in 50% of hospitalized patients.60 One-third of the cases occur in patients with a previous diagnosis of diabetes and sometimes even manifest diabetes in a patient with no known diagnosis. Postprandial hyperglycemia and only slightly elevated fasting glucose concentrations are characteristic of GC-induced diabetes mellitus. The impact on glycemic control varies depending on the dosage and duration of exposure. For patients receiving prednisolone at 8:00a.m., peak hyperglycemia is usually 8–16h after administration. All patients should have blood glucose readings between 6–11am and 5–10pm for 48h. If blood glucose is above 180mg/dl, a diagnosis of corticosteroid-induced diabetes is made and appropriate management should be performed. If the value is between 140 and 180mg/dl, glucose monitoring should continue. And if it is less than 140mg/dl, it can be followed with once-daily measurements. If the hyperglycemia is solely inducing by GC, the use of NPH insulin is useful. The latter should be applied at the time the GC is given, due to the similarity in the pharmacokinetics of this compared to the hyperglycemia curve generated by administering steroid therapy.2,61 The efficacy of deflazacort vs. prednisolone seems to be similar, however, small studies have shown that it has a lower interference on glucose metabolism, weight gain, and bone mass loss, so it could be preferred in diabetic patients or patients with osteoporosis, however, the cost is much higher for deflazacort, therefore further studies are required to evaluate the cost-effectiveness in these population groups.62,63
Strongyloides stercoralis hyperinfection syndromeIn hosts with chronic infection by this intestinal nematode endemic in some countries, the use of GC can favor a condition known as “hyperinfection syndrome”, which refers to a marked increase in the autoinfection cycle by filarial larvae. This severe form of the disease is associated with an immunosuppressive state, debilitating diseases, human T-cell lymphotropic virus type 1 infection, and Cushing's disease. In developed countries, the main factor associated with hyperinfection is GC therapy, which can generate marked parasitemia and intestinal microperforations that favor bacterial translocation, increasing the risk of bacteremia by Gram-negative bacilli, resulting in a mortality rate close to 100% in the absence of treatment.64 Hyperinfection syndrome can occur independently of the dose, duration, and route of steroid administration, even with short cycles (6–17 days) of steroids and also in immunocompetent patients, without other conditions of immune suppression. The most accepted recommendation is the use of deworming with Ivermectin 200μg/kg (1drop/kg) once prior to initiation GC therapy forpatients receiving doses equivalent to 20mg prednisolone for more than 2 weeks or GC pulses.65
Suppression of the hypothalamic–pituitary–adrenal axis and adrenal insufficiencyThe physiological secretion of cortisol in humans under basal condition per day has been calculated to range between 5.7 and 10mg/m2/day. Therefore, 15–25mg of oral cortisol, equivalent to about 4–6mg of prednisolone, would be sufficient to cover this need in people with adrenal insufficiency. Fig. 1 shows that systemically absorbed GC, including exogenous ones, exert negative feedback at the pituitary and hypothalamic levels, leading to a decrease in cortisol synthesis at the adrenal level and long-term hypoplasia or atrophy of the adrenal cortex, specifically the zona fasciculate and reticular but the aldosterone-synthesizing capacity remains unaffected. Abrupt discontinuation or rapid tapering of GC in these patients may result in symptoms due to tertiary adrenal insufficiency.66 Not all patients prescribed these drugs require gradual discontinuation; the risk depends on the potency, dose, and route of administration and duration of the GC used. Moreover, administering GC at night and in multiple doses, as opposed to a single daily dose in the morning, along with the utilization of long-acting GC, would result in greater suppression of the HPA axis.67 Patients with a cushingoid appearance or who have used daily prednisolone equivalent doses of 7.5mg or more for at least 3 weeks are considered at risk for axis suppression and should undergo gradual and slow dose reduction. Therefore, we can conclude, that a patient who has received any dose of GC for less than 2–3 weeks is not at risk of adrenal insufficiency upon discontinuation of treatment, and abrupt discontinuation of the drug could be performed.68 It is important to note that the dose of GC should be tapered once the maximum desired therapeutic benefit has been achieved and it is determined that the disease will not recur or a GC-sparing drug has been introduced. Another indication is when therapeutic failure has occurred after an adequate trial or when side effects, such as osteoporosis or hypertension, become severe or uncontrollable with medication. Some complications require immediate cessation or very rapid tapering, these are acute GC-induced psychosis unresponsive to antipsychotics, herpesvirus-induced corneal ulceration, which can rapidly lead to corneal perforation and permanent blindness, and severe infections in general that put the patient's life at risk.
Under stressful conditions, the basal secretion of GC increases. It is for this reason, that in patients taking long-term low-dose GC and facing conditions such as fever or infection, it is recommended based on expert opinion, to increase the dose up to 15–20mg of prednisolone or its equivalent usually for 5–7 days. Those who are to be taken for emergency major surgery may be prescribed a dose of hydrocortisone. This may be 25–50mg of hydrocortisone every 8h for 2–3 days. In patients who come with intermediate-high doses of GC, and develop a complication that warrants suspension or rapid reduction, the dose is usually lowered to 15–20mg of prednisolone equivalents, so that the patient can cope with the infection without developing adrenal insufficiency.69 If the disease that indicated GC administration is still very active in the presence of severe infection, one strategy is the administration of intravenous immunoglobulin.70
In patients requiring long-term glucocorticoid treatment, it is essential to strive for the lowest possible dose, preferably below 7.5mg/day. Prolonged use of glucocorticoid doses equal to or exceeding this threshold has been consistently linked, in a dose-dependent manner, to irreversible damage and an increased risk of mortality over the course of several years in autoimmune diseases.71,72
ConclusionMore than 75 years have passed since GCs were first used; currently they are used in medical practice across wide range of scenarios, clinicians must be aware of the mechanisms of action, indications and adverse effects in order to make a more rational use of them. It is essential to prescribe an appropiate dosage to avoid subtherapeutic doses in severe scenarios or very high doses that may not provide additional benefit and increase the risk of complications. Potential adverse effects should be identified before, during and after the administration of these drugs, in order to implement prevention, follow-up and treatment strategies. Further clinical studies are needed to draw conclusions in order to establish more effective and safer administration guidelines.
FundingNo funding was received for this study.
Conflict of interestsThe authors have no conflict of interests to disclose.