The zika virus, another re-emerging Flavivirus transmitted to humans by mosquitoes, is responsible for the most recent fever outbreak in the Americas and the Pacific, starting in 2015. The immunologically naïve population in the Americas favors the spread of epidemics. The zika fever is characterized by febrile illness, malaise, conjunctivitis and a maculopapular rash. Similar to other arboviroses recently spread in the Americas, there is no specific or effective antiviral therapy and vaccines are still in trials. The only effective preventive measures consist of individual protection against mosquito bites and vector control. This febrile illness increases the epidemiological and public health challenge existing in America, where the population is already fighting against dengue and chikungunya fever. Disease prevention is important due to the economic burden it entails. The fact of sexual and transfusion virus transmission is a great challenge to overcome. Doctors need to distinguish between dengue, chikungunya and other diseases to give a successful treatment and prevent the disease spreading.
Among many public health alerts, the global spread of arboviruses is of concern and alarm. The Zika virus is transmitted to people through the bite of an infected mosquito from the Aedes genus, mainly Aedes aegypti in tropical and subtropical regions and Aedes albopictus in temperate climates. These are the same mosquitoes that transmit dengue, Chikungunya and yellow fever. The disease is named Zika virus disease (ZVD) instead of Zika fever because of the frequent subfebril and afebrile manifestations. Last, but not least important, the Zika virus is the third recent global infectious disease outbreak, following closely behind H1N1 flu and the Ebola virus, which has had detrimental implications for pregnant women and their unborn children.
EpidemiologyThe first isolation was made in April 1947 from the serum of a pyrexial rhesus monkey in the canopy of Zika forest, Uganda,1 followed by an isolation from Aedes africanus mosquitoes in January 1948, in the same forest. The first human cases were detected in Uganda and the United Republic of Tanzania in 1952, where neutralizing antibodies to the Zika virus were detected in sera.2,3 A couple of years later, in 1954, the virus was isolated from a girl in Eastern Nigeria.4
From the 1960s to the 1980s, the zika virus was detected in mosquitoes and sentinel rhesus monkeys in countries of equatorial Africa. Sporadic human cases were identified, mostly by serological methods, but such cases were rare and the disease was regarded as benign.
In 1969, the Zika virus expanded its geographical distribution in equatorial Asia, where it is the first large outbreak in humans on the Pacific island of Yap, in the Federated States of Micronesia. An estimated 73% of Yap residents over three years of age were infected with the Zika virus. No deaths, hospitalizations, or neurological complications were reported.
From 2012 to 2014, the Zika virus caused outbreaks in French Polynesia, Easter Island, Cook Islands and New Caledonia. Meanwhile, the virus continued circulating in Cambodia, Malaysia, Thailand, Nigeria, Senegal and Uganda.
By March 2015, an illness characterized by a skin rash was detected in northern Brazil. Nevertheless, it was not until May when the Zika virus infection was confirmed. By July, Brazil reported laboratory-confirmed Zika cases in twelve states. In October 2015, Colombia reported PCR-confirmed patients with locally acquired Zika infections and posteriorly 156 confirmed cases in thirteen municipalities. The Zika virus was limited to Brazil and Colombia until November 2015, when it spread to Suriname, Guatemala, Mexico, El Salvador, Paraguay and Venezuela. In December, Panama reported its first confirmed cases, as well as Haiti, Puerto Rico, Martinique, Honduras and French Guiana.5
In January 2016, Bolivia and Saint Martin detected their first indigenous cases, as well as Barbados, U.S. Virgin Islands, Dominican Republic, Nicaragua and Jamaica.6 As of April 28, 2016, the new countries with confirmed local transmissions are: Curacao, Costa Rica, Republic of Trinidad and Tobago, Aruba, Bonaire, Sint Maarten, Saint Vincent and the Grenadines, Dominica, Cuba, Saint Lucia and Belize. This makes a total of 35 countries/territories with ongoing autochthonous Zika virus transmission and 7982 accumulated confirmed cases.7
In Mexico, the first imported case was identified in November 17, 2015, when a traveler came back from Colombia to Querétaro. Later in that month, in the 47th Epidemiological Week, the first two cases were reported in Monterrey, Nuevo León, and in Huixtla, Chiapas. None of the patients had traveled recently.8 New cases were detected until the 52nd Epidemiological Week in Jalisco, Chiapas and Nuevo León.9 The first Epidemiological Week of 2016 lacked confirmed cases. However, Chiapas has been reporting new cases since the 2nd Epidemiological Week.10 Up to April 29, 2016, there have been 185 confirmed autochthonous cases in Mexico reported from the states of Chiapas, Guerrero, Jalisco, Michoacán, Nayarit, Nuevo León, Oaxaca, Sinaloa, Tabasco, Veracruz and Yucatán.11
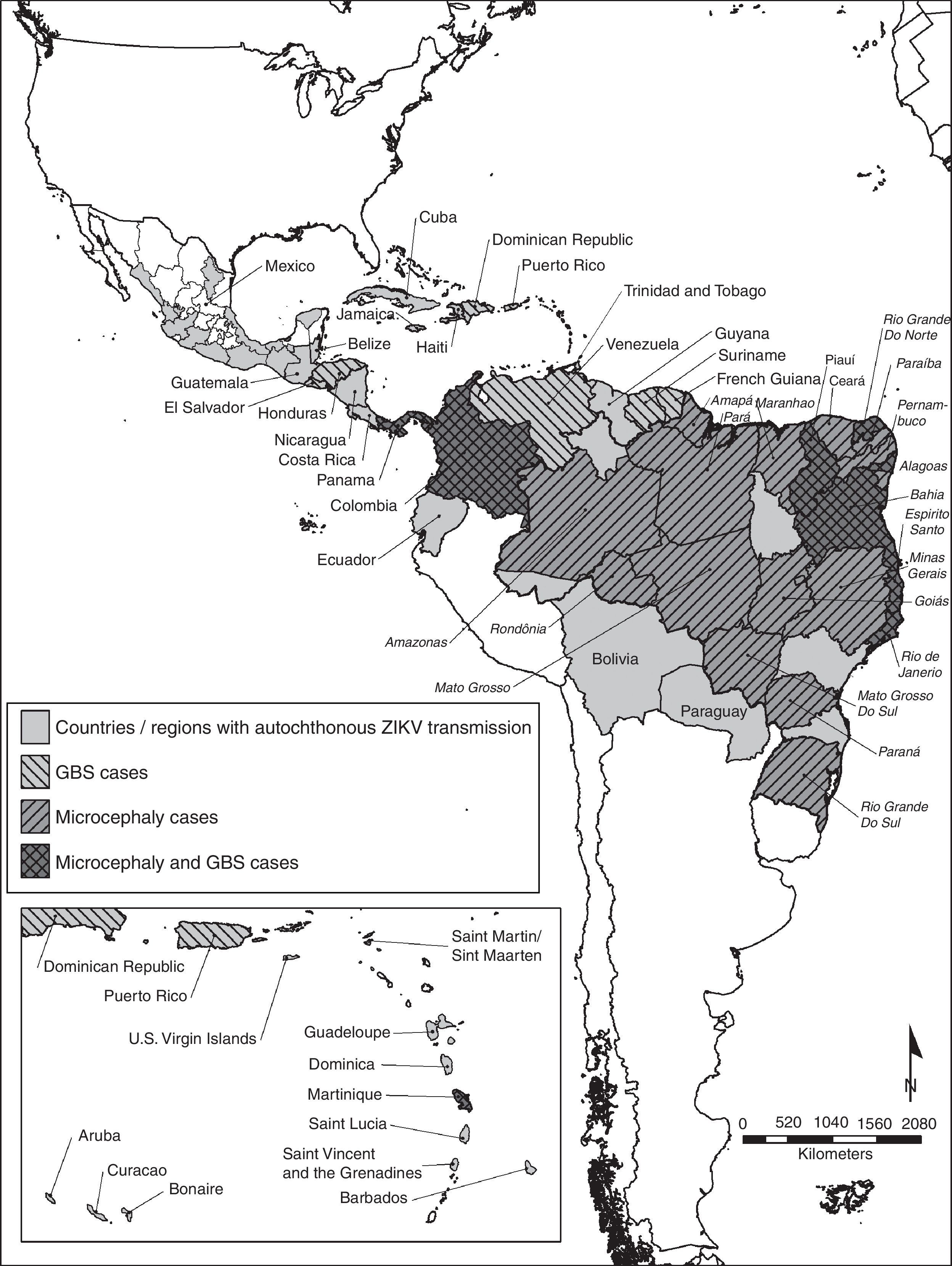
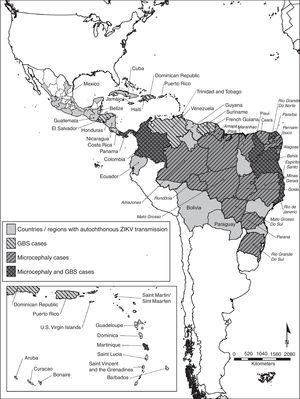
Even though there is no autochthonous ongoing transmission in the United States, the presence of Ae. albopictus in a great proportion of the continental US poses a risk for autochthonous Zika virus transmission.12 The geographic distribution of the autochthonous transmission of the Zika virus can be observed in Fig. 1.
Countries and regions in the Americas with autochthonous Zika virus transmission and complications. The map illustrates the countries and regions where autochthonous Zika virus transmission is taking place. It also shows the countries where Guillain–Barré syndrome cases were reported, as well as confirmed microcephaly cases. Mexico and Brazil are divided by states. The Brazilian state names are in italics. Latin and Non Caribbean are not labeled due to the map resolution. Information updated April 28, 2016. ZIKV: Zika virus; GBS: Guillain–Barré syndrome.
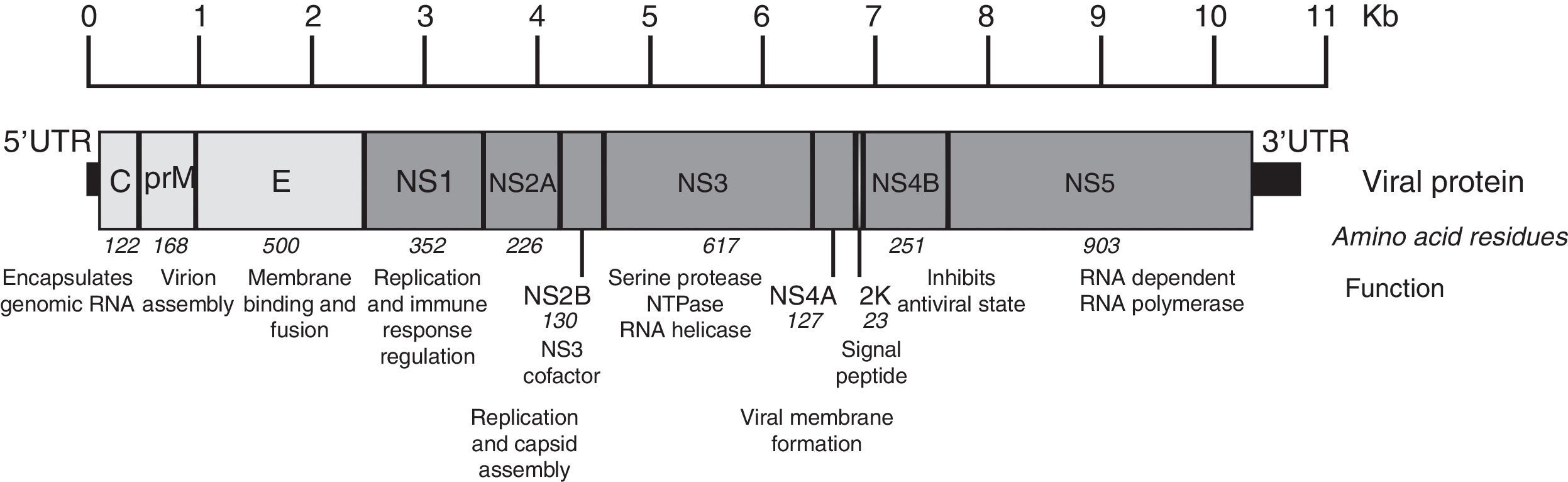
The Zika virus is a member of the Flavivirus genus of the Flaviviridae family. This virus is related to other pathogenic vector borne Flavivirus, where we can find the Dengue virus, the West Nile Virus and the Saint Louis encephalitis virus. It is a single-stranded, positive-sense, RNA virus with a genome approximately 11kb in length.13 The single open reading frame (ORF) encoding a polyprotein is framed by 5′ and 3′ untranslated regions. The encoding polyprotein is translated and processed by viral and cellular proteases, co- and post-translationally, into three structural (capsid, precursor membrane or membrane, and envelope) and seven nonstructural (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5) proteins (Fig. 2). The NS1, NS3 and NS5 proteins are large and highly conserved, meanwhile the NS2A, NS2B, NS4A and NS4B proteins are small and hydrophobic.14
Zika virus genome organization. The figure shows the structural and nonstructural proteins the way they are organized throughout the genome as well as the untranslated regions in 5′ and 3′. The name, size in amino acids and function is showed for each protein. The figure is drawn to scale based on the reference ZIKV genome with GenBank access number: NC_012532.1.13 The information regarding viral protein function was obtained from the UniProt database using dengue virus due to the lack of published data. The accession number used was P17763.77 NS: nonstructural protein; C: capsid; E: envelope; prM: precursor membrane; UTR: untranslated region; Kb: kilobases.
After a mosquito bite, the Zika virus can infect human dermal fibroblasts, epidermal keratinocytes and immature dendritic cells. AXL, a phosphatydylserine receptor, has been described as a ZIKV entry receptor and is also related with cellular autophagy, enhancing ZIKV replication in permissive cells. DC-SIGN, Tyro3 and TIM-I also seem to mediate ZIKV entry.15 Information concerning ZIKV replication is scarce; therefore we present the common features of the Flaviviridae family life cycle. After the viral particle interacts with the host receptors, it is internalized by clathrin-mediated endocytosis. The low pH of the endosome induces fusion of the virion envelope with cellular membranes. Following uncoating of the nucleocapsid, the RNA genome is released into the cytoplasm. The genome serves three discrete roles within its life cycle: as the messenger RNA (mRNA) for translation of all viral proteins, a template during RNA replication, and the genetic material packaged within new virus particles. RNA replication occurs entirely in the cytoplasm in close association with intracellular membranes. Progeny virions assemble by budding into an intracellular membrane compartment, most likely the endoplasmic reticulum (ER), then transit through the host secretory pathway and are released at the cell surface.16
Phylogenetic analysis among the genus Flavivirus places ZIKV at clade X, with the Spondweni virus, in the mosquito-borne cluster.17 Recent phylogenetic analysis divides ZIKV into three different lineages: West African, East African and Asian. The three distinct ZIKV lineages share a common ancestor, possibly with Ugandan lineages around 1920.18 The Asian lineage is responsible for the current outbreak in America.19
TransmissionVector transmissionThe Zika virus is mostly transmitted to people through the bite of an infected mosquito from the Aedes genus, including Aedes aegypti in tropical and subtropical regions and Aedes albopictus in temperate regions. Given numerous virus isolations from mosquitoes, they are clearly the natural reservoirs. Vertebrate hosts are most likely amplifying or dead-end hosts, since no vertebrate in nature has ever been conclusively determined to serve as a true reservoir for any arbovirus.20
Some of the prevalent vectors identified in Africa include Ae. luteocephalus, Ae. africanus, and Ae. furcifer. Nevertheless, the dominant species varies considerably depending on sampling location. Interestingly, the involvement of Ae. aegypti has been small in Africa.21 On the other hand, Ae. aegypti has been the single most important vector in Southeast Asia and the Pacific. In the outbreak on Yap Island in the Pacific, Ae. hensilli was implicated as a possible vector.22
Ae. albopictus is also susceptible to infection and able to transmit ZIKV.23 The highest concern is that Ae. albopictus has been found in temperate climates where Ae. aegypti is absent, allowing virus transmission in those regions. This includes 37 of 48 contiguous states of the United States of America, Albania, Bulgaria, Croatia, Southern France, Greece, Italy, Malta, Montenegro, Slovenia, Eastern Spain, and Southern Switzerland.24,25
Non-vector transmissionEven though the main transmission route of the Zika virus is by the bite of an infected mosquito, cases of non-vector transmission have been reported.
During the French Polynesian outbreak, 3% of 1505 blood donors, asymptomatic at the time of blood donation, were found positive for ZIKV by PCR.26,27 In this recent outbreak, two possible cases of transfusion–transmission have been described in Campinas, Brazil and are being investigated.28 For these reasons, the Food and Drug Administration (FDA) has made recommendations to prevent transfusion–transmission. Some of these recommendations are: defer donors at risk for ZIKV infections for 4 weeks, use of an FDA-approved pathogen reduction device, and test local blood donations with an FDA-licensed blood donor screening test for ZIKV when available.29 However, a recent publication affirmed that screening potential blood donors based on symptoms or serological testing of donated blood would do little to protect the blood supply, reducing the risk of an infected donation by at most 30%. The authors advise that high incidence areas should consider PCR testing to identify safe components for use in pregnant women.30
In addition, the Zika virus can be sexually transmitted from a man to his sex partner(s). During February 2016, the CDC received reports of 14 instances of suspected sexual transmission of the Zika virus. Among these, two laboratory-confirmed cases and four probable cases of the Zika virus disease have been identified among women whose only known risk factor was sexual contact with a symptomatic male partner with recent travel to an area with ongoing Zika virus transmission.31 To date, all reported cases of sexual transmission of the Zika virus have been from symptomatic male partners. Sexual transmission of the Zika virus from infected women to their sex partners and from persons who are asymptomatically infected has not been reported.31 There have been two reports of replication-competent Zika virus isolated from semen at least 2 weeks after onset of illness, when blood plasma specimens were negative by RT-PCR.32,33 Viral ARN has been detected in semen by RT-PCR as long as 62 days after illness onset, but the duration of persistence of infectious Zika virus in semen remains unknown.34 There is now a report that indicates that the Zika virus can be transmitted through anal sex, as well as vaginal sex.35 Therefore, the Centers for Disease Control and Prevention issued interim guidance for the prevention of sexual transmission of the Zika virus. Couples in which a woman is pregnant should use condoms or abstain from sex for the duration of the pregnancy. Likewise, couples in which a man had a confirmed Zika virus infection or clinical illness should consider using condoms or abstaining from sex for at least 6 months after onset of illness. If the couple lives in an area with active Zika virus transmission, the use of condoms or abstaining from sex while active transmission persists are recommended.36 Evidence implies trans-placental transmission and perinatal transmission during delivery, with Zika virus RNA being found in amniotic fluid and in paired blood samples taken from newborn infants and mothers.37,38 There is no evidence to support transmission by breastfeeding or via contact with saliva, urine, or respiratory droplets.
Clinical featuresThe first clinical characterizations described Zika virus infections as mild and self-limiting.
After an experimentally induced Zika virus infection in a human volunteer, it resembled the condition observed in the girl in Eastern Nigeria reported by MacNamara closely.4 The infection was a short-term fever, without evidence of involvement of any particular tissue. In both cases the only manifestations were fever and headache.39 The clinical picture of the infection described by a worker that was infected collecting mosquitoes in the Zika forest was that of a mild febrile illness of short duration accompanied by a generalized maculopapular rash.40 In Java, Indonesia, all patients had high fevers upon examination. Six of seven patients had stomach ache, five had malaise, five experienced dizziness and four were anorexic. Less frequently reported symptoms and signs were diarrhea, constipation, hypotension and chills. Interestingly, arthralgia, myalgia, vomiting, conjunctivitis, hematuria, lymphadenopathy and leg pain were present in one of the patients. Furthermore, none of the patients had a rash.41 At a study made in Thailand with cases from 2012 to 2014, the clinical presentation was mild and nonspecific. All subjects presented fever and a maculopapular rash. Other symptoms included sore throat, arthralgia, myalgia, rhinorrhea, and headache. Only two patients complained of conjunctivitis, which was less than the rate previously reported in ZIKV cases outside Africa.42
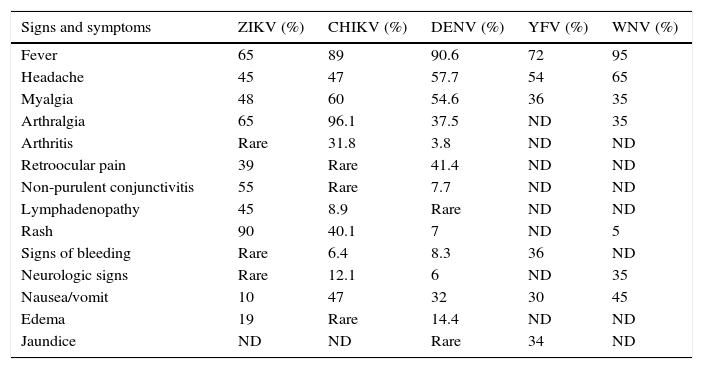
The incubation period of the Zika virus disease is not clear, but is estimated to be 4–7 days.30 The symptoms are similar to other arbovirus infections such as dengue, and include fever, maculopapular rash, pruritus, conjunctival hyperemia, myalgia, arthralgia, malaise, headache, retro-orbital pain and digestive disorders. These symptoms are usually mild and last for 2–7 days.22,43 In addition, common presentations accompanying the febrile illness are frequently confused with dengue virus infection, which may result in underreporting of Zika virus infection. In Table 1, we present the frequent signs and symptoms of the most common arboviroses. This can be of great help to clinicians regarding differential diagnosis.
Differential diagnostic of most common arbovirus infections in acute presentations.
| Signs and symptoms | ZIKV (%) | CHIKV (%) | DENV (%) | YFV (%) | WNV (%) |
|---|---|---|---|---|---|
| Fever | 65 | 89 | 90.6 | 72 | 95 |
| Headache | 45 | 47 | 57.7 | 54 | 65 |
| Myalgia | 48 | 60 | 54.6 | 36 | 35 |
| Arthralgia | 65 | 96.1 | 37.5 | ND | 35 |
| Arthritis | Rare | 31.8 | 3.8 | ND | ND |
| Retroocular pain | 39 | Rare | 41.4 | ND | ND |
| Non-purulent conjunctivitis | 55 | Rare | 7.7 | ND | ND |
| Lymphadenopathy | 45 | 8.9 | Rare | ND | ND |
| Rash | 90 | 40.1 | 7 | ND | 5 |
| Signs of bleeding | Rare | 6.4 | 8.3 | 36 | ND |
| Neurologic signs | Rare | 12.1 | 6 | ND | 35 |
| Nausea/vomit | 10 | 47 | 32 | 30 | 45 |
| Edema | 19 | Rare | 14.4 | ND | ND |
| Jaundice | ND | ND | Rare | 34 | ND |
ZIKV: Zika virus; CHIKV: chikungunya virus; DENV: dengue virus; YFV: yellow fever virus; WNV: West Nile virus; ND: no data.
When Zika virus infections were first noted, there were no reports of complications, but in recent ZIKV outbreaks the incidence of neurological disorders has increased. Evidence that neurological disorders, including microcephaly and Guillain–Barré syndrome, are linked to Zika virus infection remains circumstantial, but a growing body of clinical and epidemiological data points toward a causal role for the Zika virus.
MicrocephalyThere are 6 countries, territories and areas reporting microcephaly cases potentially associated with Zika virus infection. These are Brazil, Cabo Verde, Colombia, French Polynesia, Martinique, and Panama (Fig. 1).44
According to the Ministry of Health of Brazil, from October 22, 2015 through April 23, 2016, there have been reports of 7228 suspected cases of microcephaly or other nervous system malformations among newborns across the country. This contrasts with the period from 2001 to 2014, when an average of 163 microcephaly cases was recorded nationwide per year.45 Up to April 23, 2016, Brazil Health authorities have reviewed 3518 cases, 49% of the total. They identified 1198 confirmed cases of microcephaly and/or other central nervous system (CNS) malformations with evidence suggestive of congenital infection, and discarded 2320.46 The 1198 confirmed microcephaly cases occurred in 435 municipalities located in 22 Brazilian Federal Units: Alagoas, Bahia, Ceará, Maranhão, Paraíba, Pernambuco, Piauí, Rio Grande Do Norte, Sergipe, Espírito Santo, Minas Gerais, Rio de Janeiro, Amapá, Amazonas, Pará, Rondônia, Distrito Federal, Goiás, Mato Grosso, Mato Grosso Do Sul, Paraná, and Rio Grande Do Sul.46 There have been 251 deaths (including miscarriages or stillbirths) reported among microcephaly and/or CNS malformation cases. Fifty-four of these deaths were confirmed as having microcephaly and/or CNS malformation, 167 remain under investigation and 30 were discarded.46 There is evidence that in addition to microcephaly, there may be a link between Zika virus infection and hydrops fetalis and fetal demise.47
A recent article reports an increase in congenital cerebral malformations and dysfunction in fetuses and newborns in French Polynesia, following an epidemic of the Zika virus, from October, 2013 to March, 2014. A retrospective review identified 19 cases, including eight with major brain lesions and severe microcephaly, six with severe cerebral lesions without microcephaly and five with brainstem dysfunction without visible malformations. Of the five-microcephaly cases that were tested virologically, viral ARN was detected by RT-PCR and infectious ZIKV isolates were obtained in four. When interviewed, the mothers of four cases reported clinical infection in the first trimester of pregnancy. The remaining mother could not be reached.48
A case series of pregnant U.S. women that traveled to Zika affected areas reported that infection during pregnancy was associated with a range of outcomes, including early pregnancy losses, congenital microcephaly, and apparently healthy infants. In addition, viral RNA was detected in fetal remains of early pregnancy loss, amniocentesis fluid, and placenta.49
On March 30th, Colombia reported 50 live births with microcephaly between January 4th, 2016 and March 20th, 2016. Of the 50 cases registered, 16 were discarded for microcephaly with suspected association with the Zika virus. Of the remaining 34 cases, two were ruled out for not meeting the national criteria for association with microcephaly by Zika virus. The remaining cases (32) are under investigation. So far, eight of these 32 cases of microcephaly presented positive Zika virus results by RT-PCR.44
A recent study from Pernambuco, Brazil, established that 30 of 31 studied microcephaly cases had Zika-specific IgM in their CSF. Since IgM does not cross either the placenta barrier or the blood–brain barrier, the presence of IgM in the CSF indicates that the neonate had the infection in the CNS. This is strong evidence that microcephaly was a consequence of Zika virus infection.50
A group of researchers evaluated the available data regarding ZIKV and microcephaly using criteria that have been proposed for the assessment of potential teratogens. They concluded that there is a causal relationship between prenatal Zika virus infection and microcephaly and other serious brain anomalies.51
The link between ZIKV infection and microcephaly becomes stronger with every new case report published. However, case reports, unlike cohort studies, do not establish a causative link between the virus and microcephaly. More research is needed to clearly establish the relationship between ZIKV infection and microcephaly.
Guillain–Barré syndromeAccording to the WHO, during 2015 and 2016, thirteen countries and territories have reported an increased incidence of Guillain–Barré syndrome (GBS) and/or laboratory confirmation of a Zika virus infection among GBS cases.52
Countries where there is increased incidence of GBS cases, with at least one GBS case with confirmed Zika virus infection are: Brazil, Colombia, Dominican Republic, El Salvador, French Polynesia, Honduras, Suriname, and Venezuela. In contrast, the countries reporting GBS with laboratory confirmed Zika virus infections without increase of GBS incidence are: French Guiana, Haiti, Martinique, Panama and Puerto Rico.52 Countries or territories where GBS cases were reported can be seen in Fig. 1.
There is little information in respect to the clinical characteristics of the Guillain–Barré syndrome cases caused by this virus. The most complete description is from a case-control study made in French Polynesia, where they diagnosed 42 cases of Guillain–Barré syndrome. Forty-one (98%) patients had anti-Zika virus IgM or IgG, and all (100%) had neutralizing antibodies against the Zika virus.53
Most patients had electrophysiological findings compatible with the acute motor axonal neuropathy (AMAN) type of the syndrome, and had rapid evolution of the disease.53 The clinical outcome of these patients with the Zika virus and Guillain–Barré syndrome was generally favorable, despite a rapid onset and short plateau phase, as may be seen in other patient groups suffering from the AMAN type of Guillain–Barré syndrome. Even though it is very likely that these patients had been recently infected with the Zika virus, it is possible that the disease was due to dengue or might possibly have been unrelated to Flavivirus infection.53
A recent case report showed that a housekeeper from Rio de Janeiro that presented clinical features consistent with paraparetic Guillain–Barré syndrome, had a positive PCR test for ZIKV in serum, cerebrospinal fluid, saliva and urine. It is noteworthy that the patient's serum and cerebrospinal fluid were negative for dengue and chikungunya by real-time PCR.54 This study helps to confirm the association between ZIKV and GBS.
Substantial new research has strengthened the association between the Zika infection and the occurrence of neurological disorders.55 However, more investigation is needed to better understand this relationship. Confounding factors include the contemporary circulation of dengue and chikungunya in the Americas, which are transmitted by the same species of mosquito.
Clinical and laboratory diagnosis of ZikaEven though the Zika virus was discovered decades ago, there are no licensed or broadly distributed diagnostic tests. According to the WHO, a suspected case of Zika virus disease is defined as a patient with a rash and two or more of the following signs or symptoms: fever, usually <38.5°C, conjunctivitis (non-purulent/hyperemic), arthralgia, myalgia, and/or peri-articular edema. A arobable case of Zika virus disease is a patient who meets the criteria of a suspected case and has Zika IgM antibodies, with no evidence of infection with other flaviviruses. A confirmed case of Zika virus disease is a patient who meets the criteria for a suspected case and has laboratory confirmation of recent Zika virus infection. This confirmation can be the following: viral RNA (serum, urine, saliva, tissue or whole blood), positive Zika IgM antibodies and plaque reduction neutralization (PRNT90) for Zika virus titers ≥20 and four (or more) times greater than the titers for other flaviviruses, with the exclusion of other Flaviviruses. In autopsy specimens, detection of the viral genome should be by molecular techniques, or antigen detection by immunohistochemistry.56
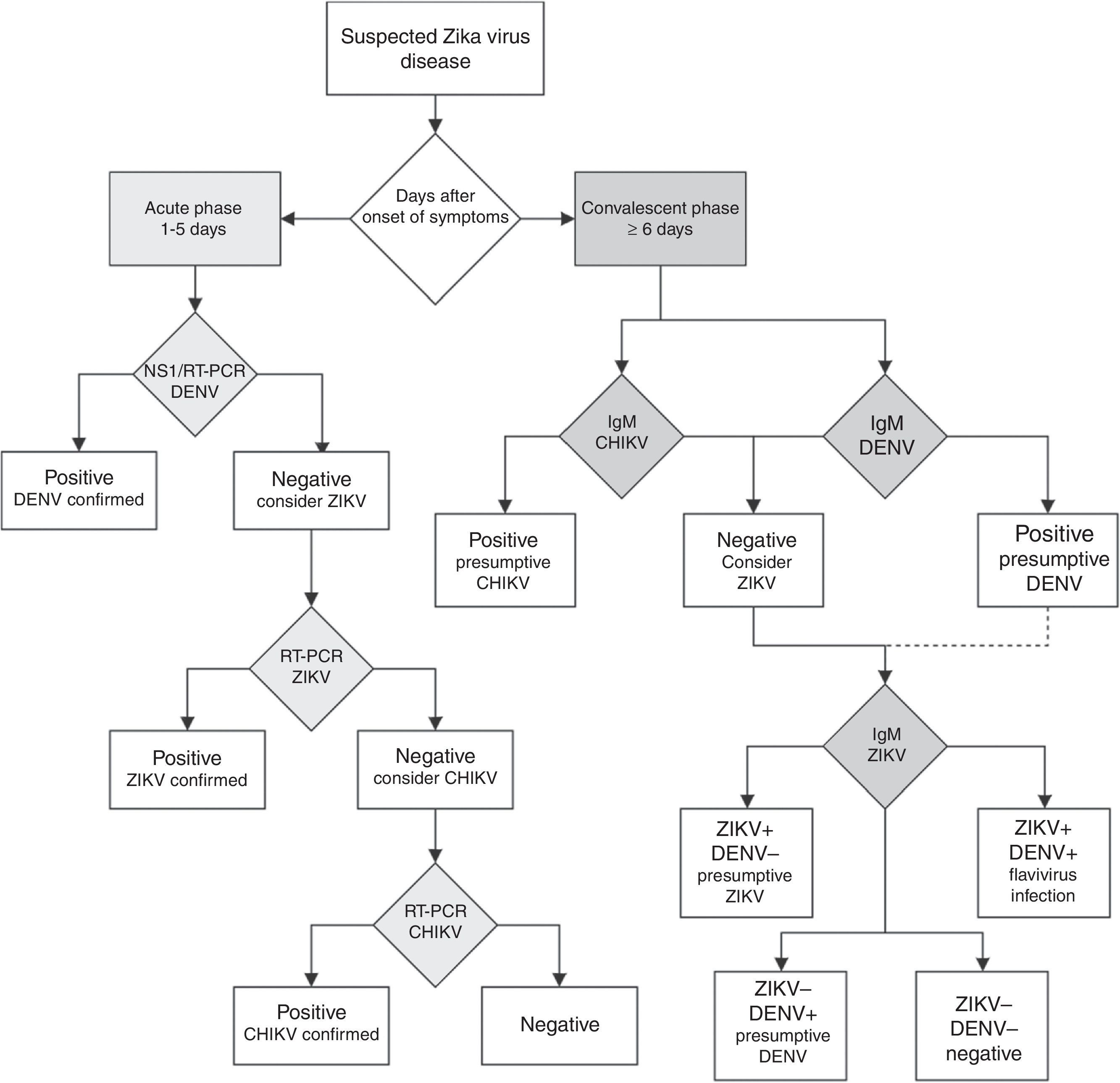
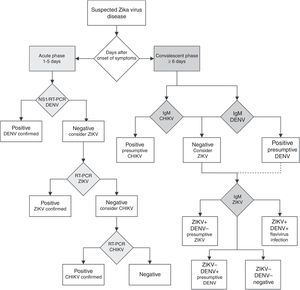
Unfortunately, if the Zika virus disease is suspected in a population where other flaviviruses are endemic, serological diagnosis of ZIKV is difficult to interpret because the high degree of cross-reactions in the IgM and IgG assays could lead to false-positive results.57 In countries where laboratory capacities are limited, the arbovirus diagnosis is often performed by serologic testing by IgM ELISA or rapid tests. If rapid tests are used for dengue, it is recommended to use a combined NS1 antigen and IgM antibody test to increase the sensitivity and specificity of dengue fever diagnosis.58 If several patients are negative to a DENV NS1 test within the first week of a “dengue-like disease,” the Zika virus disease or other arboviruses should be suspected. In countries with advanced laboratory capacities, a RT-PCR assay should be the first-line test. Patients within the acute phase of infection with a dengue or chikungunya-like syndrome, or fever and rash, with negative DENV and CHIKV RT-PCR assays should be tested with a specific ZIKV RT-PCR assay.57 The algorithm for Zika virus detection in Fig. 3 can be a useful guide for clinicians.
Diagnostic algorithm for detecting Zika virus and related arbovirus. This algorithm is adapted from the one proposed by the Pan American Health Organization.65 Due to cross-reactivity in secondary Flavivirus infections, ELISA for IgM against Dengue virus is suggested. NS1: non-structural protein 1; RT-PCR: real-time polymerase chain reaction; DENV: dengue virus; ZIKV: Zika virus; CHIKV: chikungunya virus; IgM: immunoglobulin M.
There are two strategies for the molecular detection of ZIKV. One is the detection of Flaviviruses using consensus primers and posterior detection of specific ZIKV ARN.59 The other strategy is to use specific ZIKV primers and probes. Several protocols have been developed to target the E-encoding gene,60 the membrane-envelope junction (M/E-encoding gene), the partial envelope (pE)-encoding gene, and the NS5-encoding gene.61,62 Furthermore, ZIKV RT-PCR do not cover the genetic diversity and geographic distribution of all ZIKV strains.61 The reason is that the primers and probes have been designed using only the few full ZIKV genome sequences available. On March 17, 2016, the FDA issued an Emergency Use Authorization (EUA) to authorize the emergency use of the CDC's Trioplex Real-time RT-PCR Assay for the qualitative detection and differentiation of RNA from the Zika virus, dengue virus, and chikungunya virus in human sera or cerebrospinal fluid, and for the qualitative detection of Zika virus RNA in urine and amniotic fluid.63 On April 28, 2016, Focus Diagnostics, Inc.’s Zika Virus RNA Qualitative Real-Time RT-PCR test was authorized under the EUA for the qualitative detection of RNA from the Zika virus in human serum specimens. This is the first commercial test to detect the Zika virus that has been authorized by the FDA for emergency use.63
Zika virus serology is usually performed by ELISA, with confirmation by a plaque reduction neutralization test (PRNT, which is the “gold standard” for anti-Flavivirus antibody differentiation) according to standard protocols. However, PRNT is done only in highly specialized laboratories, is expensive, and may require regulated laboratories because of the manipulation of live viruses.64 To date, there is no validated commercial serology kit for ZIKV, but on February 26, 2016, the FDA issued an Emergency Use Authorization for the emergency use of CDC Zika Immunoglobulin M Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA) for the presumptive detection of Zika virus-specific IgM in human sera or cerebrospinal fluid.63 Samples for serologic or molecular testing should have special storage conditions, ranging from refrigeration (2–8°C) to freezing (−10 to −20°C or −70°C) depending of the time and site of testing.65
TreatmentSimilar to other arboviral diseases, there is no specific antiviral drug treatment for ZIKV infection. Symptomatic treatment is recommended after excluding more serious conditions like malaria, dengue, and bacterial infections. In acute infection, treatment is symptomatic and supportive, consisting of rest and the use of acetaminophen to relieve fever (<4g/day). The use of ibuprofen, naproxen, or another non-steroidal anti-inflammatory agents (NSAID) to relieve the arthritic component of the disease can be used when dengue infection is discarded. Patients should be advised to drink plenty of fluids to replenish fluid lost from sweating, vomiting, and other insensible losses.66
There are specific guidelines for health care providers caring for infants and children with possible Zika virus infection at the official CDC website. These guidelines recommend clinical evaluation and laboratory testing for infants with possible congenital Zika virus infection, with or without microcephaly or intracranial calcifications.67
The CDC has also put out guidelines for health care providers caring for women of reproductive age with possible Zika virus exposure. Women who have had Zika virus disease or exposure without clinical illness, should wait at least 8 weeks after symptom onset to attempt conception. Men with Zika virus disease should wait at least 6 months after symptom onset, or 8 weeks after exposure to the virus without clinical illness, to attempt conception. These guidelines also provide updated recommendations for the testing of pregnant women with possible Zika virus exposure.68 It is important to note that the current guidance is based on a limited body of evidence.
PreventionPending vaccine development, the only effective preventive measures consist of individual protection against mosquito bites and vector control. Control of both adult and larval mosquito populations uses the same model as for dengue and has been relatively effective in many countries and settings. Mosquito control is the best available method for preventing ZIKV infection. Breeding sites must be removed, destroyed, frequently emptied, and cleaned or treated with insecticides.43
For protection, clothing which minimizes skin exposure to the day-biting vectors is advised. Repellents can be applied to exposed skin or to clothing, in strict accordance with product label instructions. Repellents should contain DEET (N, N-diethyl-3-methylbenzamide), IR3535 (3-[N-acetyl-N-butyl]-aminopropionic acid ethyl ester) or icaridin (1-piperidinecarboxylic acid, 2-(2-hydroxyethyl)-1-methylpropylester). Mosquito coils or other insecticide vaporizers may also reduce indoor biting.69
As previously mentioned, the correct and consistent use of condoms during any sexual intercourse is encouraged to prevent the sexual transmission of the Zika virus. Another, although difficult, option is sex abstention while active virus transmission persists.36
VaccinesUp to March, 2016 there is no available vaccine against the Zika virus. There are several proposals for a vaccine, using the same methodology as other anti-flaviviruses vaccines70; nevertheless, the process is still long. There are ethical issues involving pregnant women and the “safe use” of vaccines, which are still a barrier.71
Future directionsThere are still many unanswered questions regarding the Zika virus disease. Clinical and epidemiological studies must be performed to describe viral dynamics and the expansion of the outbreak. More studies are needed to confirm the suspected association between ZIKV infection with microcephaly and Guillain–Barré syndrome. Research must be done to identify an antiviral, prophylactic, or immunotherapy vaccine as well as diagnostic ELISA testing.
Conflicts of interestThe authors declare that there were no conflicts of interest in writing this manuscript.