The Embryology Interest Group of the Association for the Study of Reproductive Biology has established a system of embryo scoring based on the bibliography published to date and the experience of the members of the group. This article sets out the morphological characteristics evaluated at each stage, as well as updating previous editions, from oocyte to blastocyst, and their influence on the potential for embryo development and implantation. Four categories are proposed with the purpose of standardising criteria and making it easier to run multicentre studies.
Desde el Grupo de Interés de Embriología (GIE) de la Asociación para el Estudio de la Biología de la Reproducción (ASEBIR) se ha establecido un sistema de clasificación embrionaria basado tanto en la bibliografía publicada hasta la fecha como en la propia experiencia de los miembros del grupo. En el presente artículo se detallan las características morfológicas evaluadas en cada estadio, desde oocito hasta blastocisto y su influencia en el potencial de desarrollo e implantación embrionario. Se proponen 4 categorías con el propósito de unificar criterios y facilitar la realización de estudios multicéntricos.
Since the birth of the first baby resulting from in vitro fertilisation there have been great changes in clinical practice and technology but, in spite of years of research, embryo selection continues to be based mainly on morphology criteria.
Regrettably, the lack of consensus in embryo morphology assessment and selection leads to a series of problems such as the impossibility of generating multicentre studies with common assessment of embryo quality, correct interpretation of clinical reports from other laboratories, and the impossibility of comparing the results among different centres.
In an attempt to respond to this situation, the Embryology Interest Group (GIE in its Spanish initials) of the Spanish Association for the Study of Reproductive Biology (ASEBIR in its Spanish initials) classified embryos in categories based on the sequential evaluation of the embryo in the different stages of development titled “Criterios ASEBIR de valoración morfológica de oocitos, embriones tempranos y blastocistos humanos” (ASEBIR criteria for morphological assessment and selection of early embryos and human blastocysts) (Ardoy et al., 2007, 2008; Hurtado de Mendoza et al., 2015).
The 2015 update included proposals agreed by the scientific societies (Balaban et al., 2011; Magli et al., 2012) as well as several works dealing with scoring: a survey on the use of the ASEBIR criteria (Hurtado de Mendoza, 2011), the results obtained in external quality controls (Castilla et al., 2010) and, finally, the first work on validation of the ASEBIR criteria, a multicentre study that proved its predictive validity in Day 3 (D+3) stage (Pons et al., 2014).
The current scoring system includes all the parameters that are clearly related to embryo implantation chances. Some parameters that were not included in the scoring system have been proposed as additional criteria to help in selecting embryos of the same category. Finally, certain morphological parameters need greater study in order to form a relevant part of the decision chart. It must be made clear that the morphology assessment, selection criteria and their categorisation have been described exclusively for fresh oocytes and embryos.
ASEBIR's GIE is aware that new technologies have been introduced in recent years, especially the evaluation of embryo morphokinetics using time-lapse technology (Wong et al., 2010; Meseguer et al., 2011; Basile et al., 2015). This has made it possible to become aware of events that cannot be assessed using static sequential observation, for example direct cleavage or blastomere fusion. The morphokinetic parameters will produce greater knowledge that will make it possible for us to modify some of the established criteria. Because the ASEBIR scoring system is dynamic, its future updates will continually incorporate the parameters that show a clinical improvement in the results.
The aim of this work is to review the morphological parameters at each stage of the embryo development included in the proposed system of scoring by categories and their relationship with embryo implantation potential.
Day 0: oocyte assessment and selectionIt is estimated that 60–70% of the oocytes retrieved in ovarian stimulation cycles present morphological alterations, both cytoplasmic and extracytoplasmic, that could affect the future development of the embryo.
With regard to alterations in the cytoplasm, the clustering of organelles and their granularity are among the most frequent. When it is central, it is associated with low implantation potential and high miscarriage rate (Rita de Cássia et al., 2010; Rienzi et al., 2012). The aggregates of smooth endoplasmic reticulum are considered to be a severe anomaly associated with abnormal embryo development, low rate of blastocyst formation, high percentage of biochemical pregnancies and obstetric complications in the pregnancies derived from these embryos (Meriano et al., 2001; Sá et al., 2011; Rienzi et al., 2011). Despite births of healthy children have been reported (Shaw-Jackson et al., 2014; Balaban et al., 2011) it is recommended not to inseminate oocytes with these characteristics, although the final decision must be taken case by case. Vacuoles are another frequent dysmorphism which, when they persist, can interfere with the cleavage planes of the embryo, leading to low blastocyst formation rates (Ebner et al., 2005). Lastly, there is no agreement in the bibliography about the influence of cytoplasmic inclusions on fertilisation, cleavage and embryo quality, or blastocysts formation rate (Otsuki et al., 2007; Rienzi et al., 2011, 2012).
As for extracytoplasmic alterations, the cellular debris in the perivitelline space may be due to an excessive administration of gonadotropins in the ovarian stimulation cycles (Balaban and Urman, 2006). However, there is not sufficient evidence to support a prognosis associated with this observation. The presence of a large perivitelline space is associated with over-maturation of the oocyte (Miao et al., 2009; Hassa et al., 2014), being related to a low fertilisation rate, though it does not seem to affect embryo quality (Ten et al., 2007; Setti et al., 2011; Rienzi et al., 2012).
The size of the oocyte reflects genetic anomalies. It is advisable not to inseminate giant oocytes (>200μm) as they often contain an additional chromosomes set (Rosenbusch et al., 2002).
Nor should oocytes with a large polar body (PB) (>30μm) be inseminated due to their risk of being aneuploid (Balaban et al., 2011).
The appearance of cumulus corona radiata has not been shown to have any direct relationship with later embryo development.
Although the oocyte is not included as of now as a relevant parameter in embryo scoring, it is recommended to take into consideration these intra- and extracytoplasmic morphological characteristics, which could compromise future embryo development.
Day 1: zygote and early cleavageIn the zygote stage, between 16 and 18h post-insemination (hpi), the pronuclei (PN) and PB can be observed. This is an essential evaluation for assessing fertilisation properly (2PN2PB) as well as for identifying alterations in it (Balaban et al., 2011).
It is recommended to discard the fertilised oocytes coming from ICSI cycles that present 1PN2PB (Ardoy et al., 2008; Azevedo et al., 2014), as it is a pattern related with aneuploidies (Mateo et al., 2013) or parthenogenetic activation (Staessen and Van Steirteghem, 1997). In case this zygote comes from conventional IVF, each laboratory should decide for itself whether or not to continue with its culture (Hurtado de Mendoza et al., 2015). Zygotes with more than 2PN should also be discarded as approximately 50% will be triploid, as well as those that present 2PN and one or more micronuclei, with higher aneuploidy rates (Palermo et al., 1995; Boada and Ponsá, 2008).
Additionally, the symmetry, position and location of the PN in the cytoplasm could be assessed; as well as the number, symmetry and location of the nucleolar precursor bodies (NPBs) (Tesarik and Greco, 1999; Scott, 2003; Nicoli et al., 2013). Despite the lack of consensus about the value of the different pronuclear patterns, there is agreement about the lesser potential of the zygotes that present a single NPB in one PN, separated PN or PN of different sizes (Balaban et al., 2011).
The number of PBs is also an important parameter in evaluating ploidy. The embryos with a single PB must be discarded. However, there is no evidence that the appearance or location of PB with respect to the PN (Gianaroli et al., 2003; Nicoli et al., 2013) should be included in the morphological assessment and selection.
The presence of a cytoplasmic halo is considered as a positive characteristic, provided that it is not excessive (Zollner et al., 2002; Balaban and Urman, 2006).
In the interval between 25 and 27 hpi the early cleavage is assessed. In this observation it is evaluated whether the first mitotic cleavage has occurred, as well as size similarity of the blastomeres, multinucleation and fragmentation. The relationship between early cleavage (2 blastomeres) and clinical outcomes in implantation and pregnancy rates is controversial (Emiliani et al., 2006; de los Santos et al., 2014), as with embryo quality (Rienzi et al., 2005; Çiray et al., 2006), development to blastocyst stage (Guerif et al., 2007) or chromosomal anomalies (Arroyo et al., 2015).
If the embryo has 3 blastomeres at the early cleavage assessment (25–27 hpi), it could come from fast or direct cleavage (1–3 cells). In these cases, its implantation potential will be decreased (Rubio et al., 2012). As it is impossible to ascertain this situation without time-lapse equipment, this information could only be used to select among embryos of the same quality.
Time-lapse technology has been used to relate morphokinetic parameters of the zygote with clinical outcomes. Thus, the time of the 2nd PB extrusion, the PN fading and the duration of these have been associated with implantation rates (Aguilar et al., 2014). According to some authors, the duration of the presence of the PN is crucial; the zygotes that develop to competent blastocysts, have their 2PN visible between 7.7 and 22.9 hpi (Chamayou et al., 2013) whereas the early breakdown of the PN (before 20h 45min), compromises the possibility of generating a live birth (Azzarello et al., 2012).
It is advisable to use early cleavage as a secondary parameter to decide among embryos of similar quality (Balaban et al., 2011).
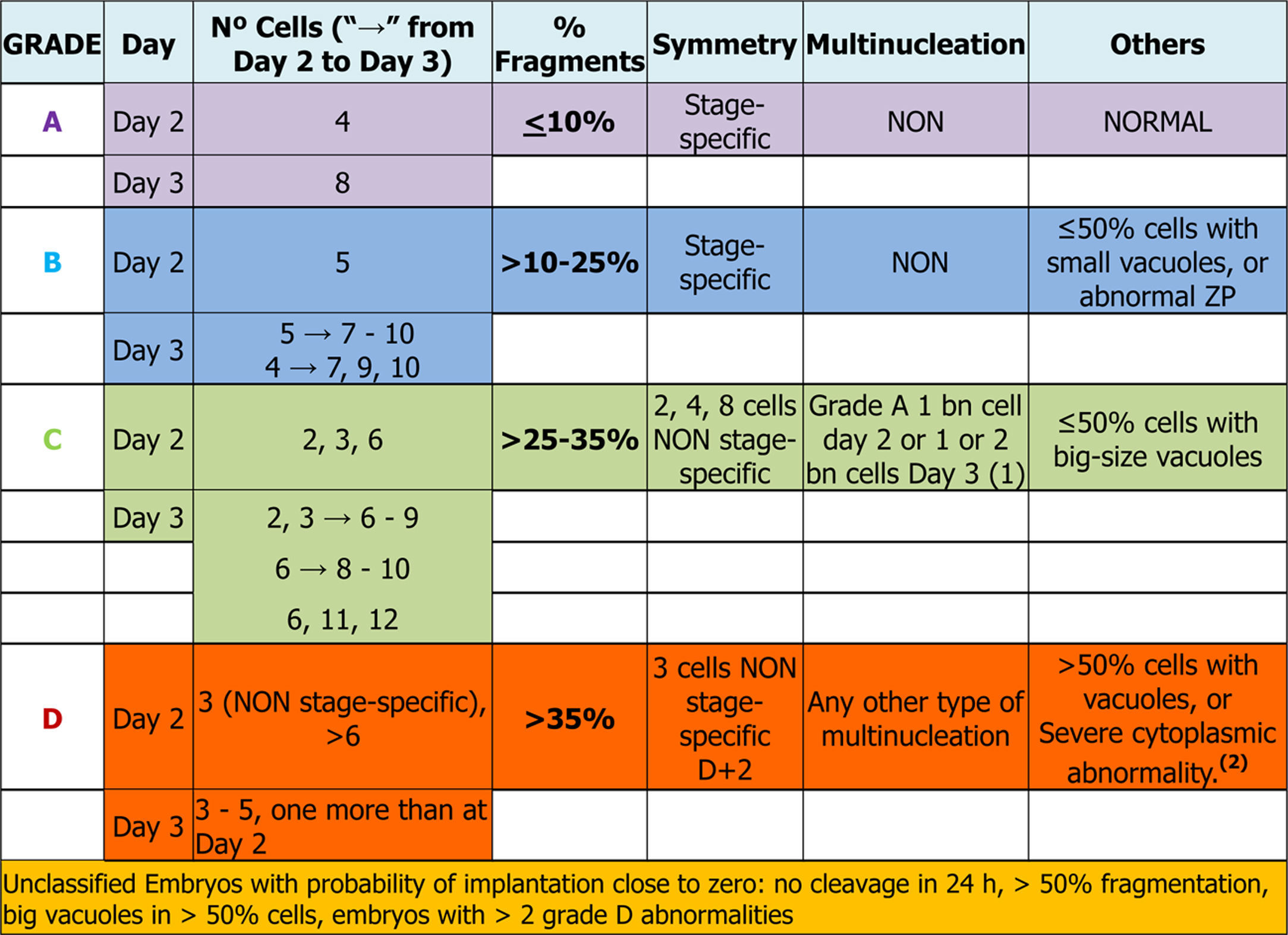
Days 2 and 3: early embryosEmbryo scoring is established in early embryos. This can only be applied to those that come from 2PN2PB zygotes, and four categories are defined related to the implantation potential of the embryo, A being the category with highest implantation potential, B and C intermediate and D the lowest.
Number of blastomeres and cleavage rateThe number of blastomeres is one of the most important indicators of embryo development potential.
On day 2 of culture (D+2) the observation must be made between 43 and 45 hpi and on day 3 (D+3), between 67 and 69 hpi (Balaban et al., 2011). It is widely agreed that the embryos with high implantation potential and live birth rates are those that have 8 cells on D+3 and come from embryos that have 4 cells on D+2 (Racowsky et al., 2011).
On the other hand, many works have observed that both the embryos that divide more slowly (<4 cells on D+2 or ≤6 cells on D+3) and those that divide faster (>4 cells on D+2 or >9 cells on D+3), present a lower implantation potential and a higher aneuploidy rate compared to embryos with an optimal cleavage rate (Magli et al., 2007; Racowsky et al., 2011). According to some published data (Holte et al., 2007; Luna et al., 2008) and the results of the multicentre study performed by the GIE (data not published), the embryos that divide faster have higher implantation rates than those that divide more slowly.
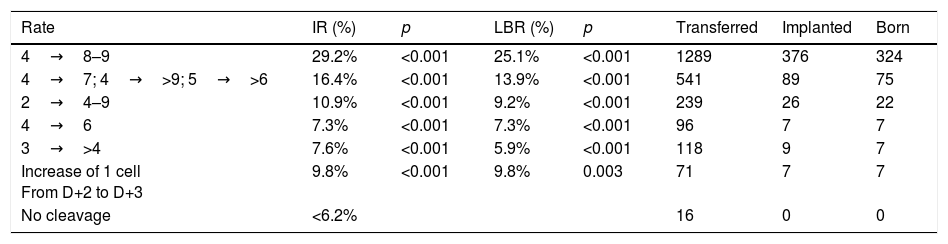
On D+3, the morphological scoring of the embryo will depend on the cleavage rate compared to D+2. Table 1 shows the results obtained in the multicenter study for validation of the ASEBIR criteria (Pons et al., 2014). It compares the embryos cleavage rate with their implantation and live birth rates. The later extension of the study, in which 550 embryos have been added, confirms the tendency already observed initially that embryos with 4 blastomeres on D+2 and 7 blastomeres on D+3 have lower implantation potential than those that have 8 or 9 blastomeres.
Data multicentre cleavage rate between D+2 and D+3 and implantation rate. Year 2017. Cleavage rate D+2–D+3: increase in number of blastomeres between both days of development. IR: implantation rate. p: p-value; significance p<0.05. LBR: recent live birth rate. Transferred: number of embryos transferred of each type. Implanted: number of embryos implanted of each type. Born: number of children born of each type.
| Rate | IR (%) | p | LBR (%) | p | Transferred | Implanted | Born |
|---|---|---|---|---|---|---|---|
| 4→8–9 | 29.2% | <0.001 | 25.1% | <0.001 | 1289 | 376 | 324 |
| 4→7; 4→>9; 5→>6 | 16.4% | <0.001 | 13.9% | <0.001 | 541 | 89 | 75 |
| 2→4–9 | 10.9% | <0.001 | 9.2% | <0.001 | 239 | 26 | 22 |
| 4→6 | 7.3% | <0.001 | 7.3% | <0.001 | 96 | 7 | 7 |
| 3→>4 | 7.6% | <0.001 | 5.9% | <0.001 | 118 | 9 | 7 |
| Increase of 1 cell From D+2 to D+3 | 9.8% | <0.001 | 9.8% | 0.003 | 71 | 7 | 7 |
| No cleavage | <6.2% | 16 | 0 | 0 |
As a consequence, this new revision of the scoring criteria of early embryos modifies the scoring of embryos that develop from 4 blastomeres on D+2 to 7 blastomeres on D+3 according to current evidence on their implantation potential. These embryos, which were classified in category A in the previous update (Hurtado de Mendoza et al., 2015), will now be classified in category B.
This modification is in line with the Istanbul Consensus (Balaban et al., 2011) and with the data provided by time-lapse technology which, although the predictive value for the highest implantation potential is still questioned, is very useful. It has been seen that embryos that reach 8 cells earlier have greater implantation potential (Dal Canto et al., 2012), as do embryos where the second cell cycle is more synchronic (Kirkegaard et al., 2013; Chamayou et al., 2013).
As always, it must be mentioned that due to the nature of the study, with a relatively low sample size in some cleavage rates groups, these results may change in the future as a result of new evidence.
Continuing with the data provided by time-lapse technology on the cleavage rate, kinetic markers have been identified as having prognostic value for blastocyst formation rate (Wong et al., 2010; Motato et al., 2016). Moreover, the identification of atypical phenotypes such as abnormal syngamy, abnormal first cytokinesis, anomalous development or chaotic development, are related to the prognosis for embryo development (Wirka et al., 2014). Direct cleavage from 1–3 cells has also been linked to poor embryo quality and low implantation rate (Cruz et al., 2012; Rubio et al., 2012; Kirkegaard et al., 2013).
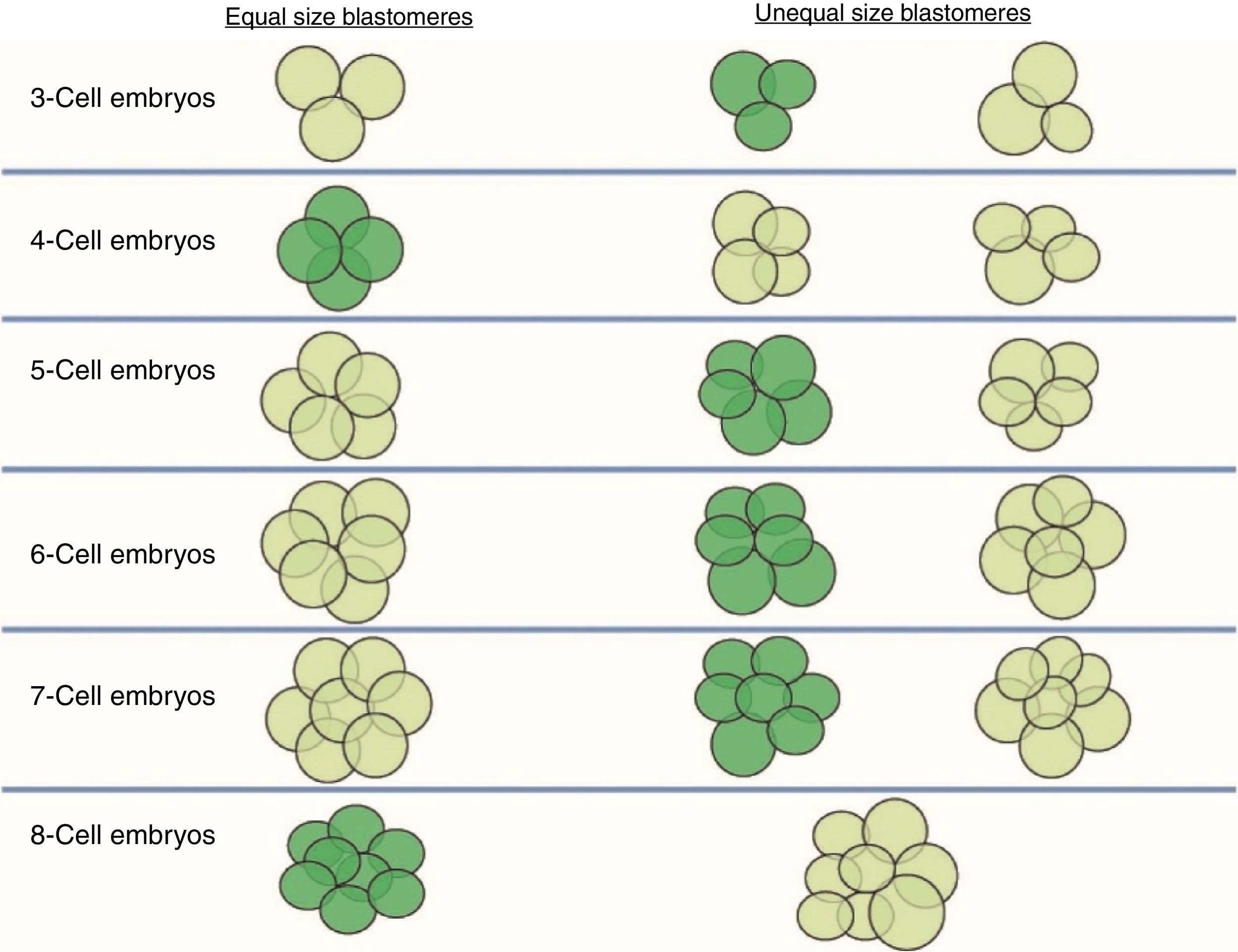
Size of the stage-specific blastomeresThe ASEBIR's GIE recommends using the term “stage-specific” (SS) to unify and define the cellular symmetry of the embryos (Prados et al., 2012). An embryo is considered stage-specific when the size of the blastomeres is consistent with their cleavage cycle, while in non-stage-specific embryos the sizes of the blastomeres are incompatible with their cleavage cycle (Fig. 1).
Stage-specific cleavage patterns. Diagram illustrating the concept of “stage-specific” cleavage patterns (coloured dark green) versus “non-stage-specific” cleavage patterns (coloured light green) starting from 3 cells. As can be seen, the proper relationship of cell sizes coincides with equality of blastomere size only in the cases of 4 and 8 cells (image provided by the European Society of Human Reproduction and Embryology). This image appears in the chapter: “The cleavage stage embryo” (Prados et al., 2012) of the “Atlas of human embryology: from oocytes to preimplantation embryos” (Magli et al., 2012).
Thus, we should only observe blastomeres of similar sizes in the stage-specific embryos of 2, 4, 8 and 16 cells, in which all the blastomeres have completed the same cleavage cycle. On the other hand, in stage-specific embryos in intermediate stages (3, 5, 6, 7, 9, 10 cells) we should expect cells of different sizes because the blastomeres are not dividing synchronically and they are in different cell cycles.
The concept of unequal blastomere size is connected exclusively with uneven cleavage and is defined as asymmetrical division in a 4-cell embryo, where the difference between the diameter of the largest and smallest blastomeres exceeds 20% of the diameter of the largest (Hardarson et al., 2001).
According to some authors, uneven cleavage is associated with a decreased implantation potential (Steer et al., 1992; Veeck, 1999; Van Blerkom et al., 2000; De Placido et al., 2002; Hnida et al., 2004), with an uneven distribution of the genetic material (Ebner et al., 2003), and with increased multinucleation (Hardarson et al., 2001).
A retrospective study (Sela et al., 2012) reported that SS embryo ratio is higher in the group of successful embryo implantation compared to the group of non-successful implantation (80% vs 70%).
Cell fragmentation: degree and typeFragmentation is defined as the presence of anucleated structures of blastomeric origin, formed of portions of cytoplasm delimited by a cell membrane (Johansson et al., 2003).
The assessment and selection of this parameter is applicable both to D+2 and to D+3 and four quality groups are established according to the degree of fragmentation: ≤10%, >10% to 25%, >25% to 35% and >35%.
As well as the degree of fragments, it is important to consider their size and distribution. When they are large and predominate in the embryo, they can be confused with small blastomeres (Johansson et al., 2003) and compromise both the blastocyst formation and the implantation rate (Alikani et al., 2000; Racowsky et al., 2003). Likewise, the type of fragmentation, its percentage and its distribution can compromise the chromosome complement of the embryo (Munné, 2006; Magli et al., 2007). According to their distribution, chromosomal anomalies increase in embryos that present scattered fragmentation, compared with those in which it is concentrated (Magli et al., 2007).
More recently, transmission electron microscopy (TEM) has shown that the mitochondria are the most abundant cell organelle in the fragments (Halvaei et al., 2016), which could be an argument against their extraction in moderately fragmented embryos.
Currently, with the use of time-lapse technology, phenomena such as fragments reabsorption have been observed (Hardarson et al., 2002), which could directly affect the embryo scoring. Other authors have also found a correlation between fragmentation degree and the appearance of the meiotic spindle and first mitotic cycles (Stensen et al., 2015).
ASEBIR's GIE recommends that embryos with over 50% of fragmentation should not be transferred or frozen, since their implantation rate is practically zero.
Visualisation of nuclei and multinucleationMultinucleation is defined as the presence of more than one nucleus in at least one blastomere of an embryo. It can be assessed both on D+2 and on D+3 (Van Royen et al., 2003; Elder and Cohen, 2007). Two types of multinucleation can be observed: binucleation (BN) when there are 2 nuclei per blastomere and multi/micronucleation (MN) when there are more than 2 nuclei per blastomere.
The presence of multinucleation is frequent; however, its incidence varies widely in the different studies, there being significant variations between observers (Paternot et al., 2009).
Numerous studies show that the assessment of nucleation is a parameter with high predictive value for the viability of an embryo (Pelinck et al., 1998; Meriano et al., 2004). Recent studies carried out with time-lapse technology show that this event is particularly frequent in the 2-cell stage (Meseguer et al., 2011; Hashimoto et al., 2016; Balakier et al., 2016).
Thus, the visualisation of a single nucleus per blastomere is considered a good prognosis factor (Moriwaki et al., 2004), whereas the presence of MN has been linked to an increase in chromosomal abnormalities (Hardarson et al., 2001; Munné, 2006; Parriego i Beltran et al., 2016) and a higher miscarriage rate (Scott et al., 2007; Fauque et al., 2013). Also, the type of multinucleation can change the prognosis: according to Meriano et al. (2004), the implantation rate of embryos with micronucleated cells is lower than that of embryos with BN cells.
Despite the poor prognosis, live births from MN embryos have been reported (Fauque et al., 2013; Yilmaz et al., 2014; Hashimoto et al., 2016; Parriego i Beltran et al., 2016) and more recently it has been shown that they can generate euploid embryos (Yilmaz et al., 2014; Balakier et al., 2016; Hashimoto et al., 2016).
In the multicentre study carried out by ASEBIR's GIE (data not published) it was observed that when MN embryos have the characteristics of an optimal quality embryo (categories A and B), the implantation rate was not affected. Despite these data, because the sample size was too small to refute the previously published studies, it was decided to make an exception for those embryos that show all the parameters of an A score but have one BN blastomere on D+2 or 1/2 BN blastomeres on D+3, assigning them to category C. The MN embryos that did not meet these requirements were classified in category D, because of the proven relationship of multinucleated embryos with chromosomal abnormalities and high miscarriage rates (Hardarson et al., 2001; Munné, 2006; Agerholm et al., 2008).
For this reason, in accordance with the Istanbul Consensus (Balaban et al., 2011) ASEBIR recommends transferring MN embryos only when no other embryos are available. Also, it is convenient to culture these embryos till D+5 in order to make a better selection.
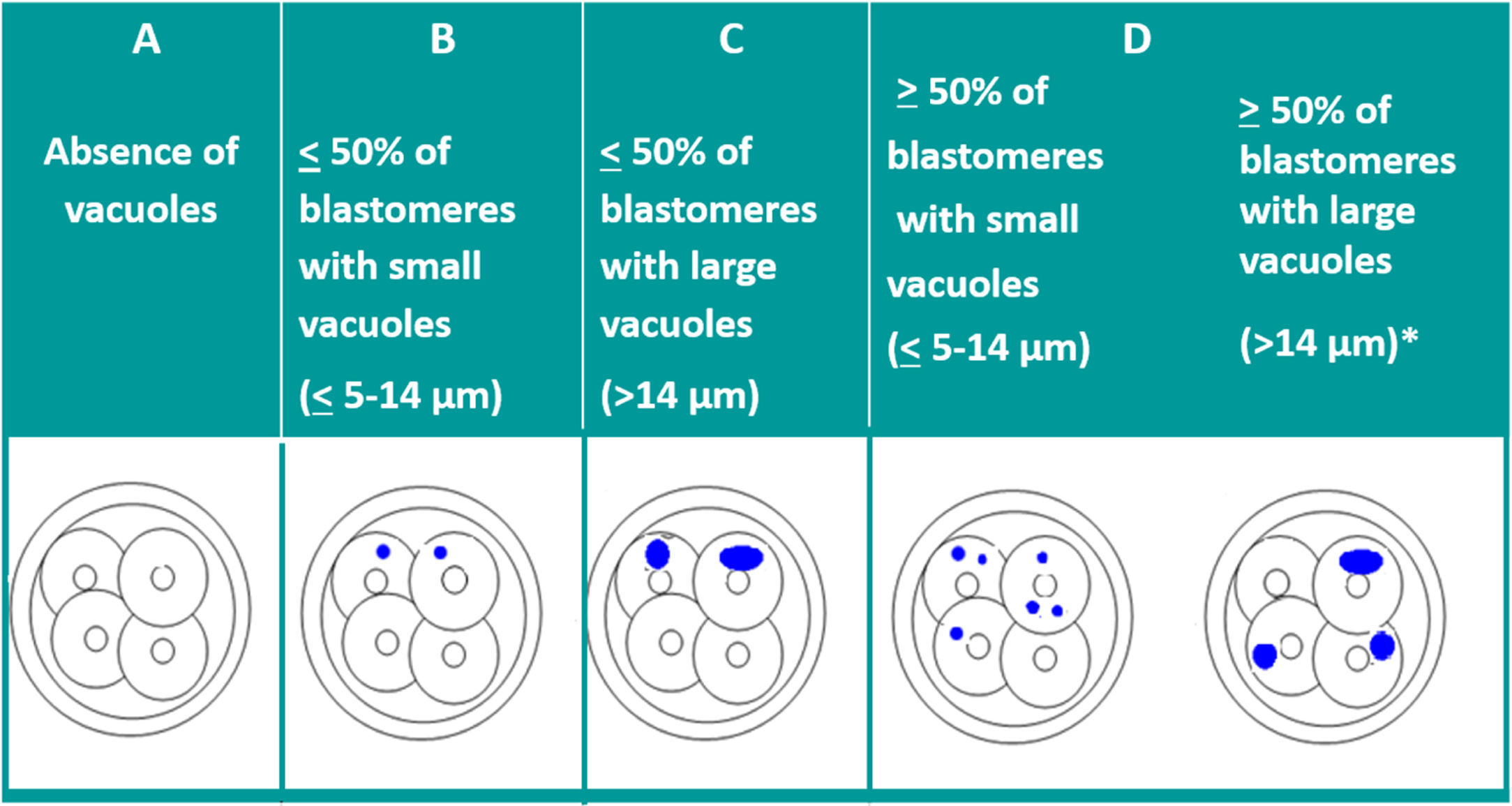
VacuolisationThe impact of the vacuoles on embryo development seems to depend on their size and number. The presence of small vacuoles (<5μm in diameter) does not seem to impair the development of the embryo (Veeck, 1999). On the other hand, the presence of extensive vacuolisation may be harmful especially for its spatial development (Prados et al., 2012).
With the aim of quantifying the impact of the presence of vacuoles, and after carrying out a pilot study, ASEBIR's GIE classifies embryos by size and number of vacuoles as can be seen in Table 2.
Zona pellucidaA healthy zona pellucida (ZP) has a round outline, an approximate thickness of 19.5±2.2μm (Pelletier et al., 2004), with no abnormalities, absence of internal partitioning or septum, and translucent (Goyanes et al., 1990; Veeck, 1999; Gabrielsen et al., 2001).
Abnormalities in the ZP are related to a low implantation rate, probably due to difficulties in hatching (Veeck, 1999; Gabrielsen et al., 2001; Calderón et al., 2002). The GIE emphasises that, while it is important to distinguish between anomalies and dysmorphisms (slight variations from normality), they do not seem to affect the implantation and live birth rates (data not published. Source: GIE multicentre study).
In conclusion, with respect to embryo scoring, slight dysmorphisms in the ZP do not penalise the category of the embryo. Only abnormalities such as excessive thickness, presence of septum, oval shape or dark colour assign the embryo to category B, in the absence of other morphological alterations.
Other anomaliesPitting is characterised by the presence of small pits of approximately 1.5μm in the cortical area of the cytoplasm (Biggers and Racowsky, 2002), its orange-peel appearance must not be confused with extensive vacuolisation (Wiemer et al., 1996; Desai et al., 2000). There appears to be no correlation between pitting and embryo quality (Rienzi et al., 2003) or the possibility of pregnancy (Desai et al., 2000), though it has been linked to a higher incidence of early miscarriages (Ebner et al., 2005). Some studies suggest that culture conditions may be responsible for cytoplasmic pitting (Biggers and Racowsky, 2002; Ebner et al., 2005).
The cortical halo is a clear cortical area, a reflection of the retraction of the cytoplasm. It is linked to the loss of regulatory proteins and mitochondria eliminated through fragments, so their presence could compromise future embryo development (Veeck, 1999; Van Blerkom, 2007).
The irregular shape of the blastomere could be due to physiological alterations or to the process of embryo cleavage (Goyanes et al., 1990). Embryos with regular blastomeres achieve greater rates of blastocyst formation, though no differences have been found in live birth rates (Guerif et al., 2010).
There are not sufficient data to include compaction or early adhesion in the scoring diagram (Wiemer et al., 1996; Desai et al., 2000). The start of the adhesion on D+3, provided the embryo has 7 or 8 blastomeres, is considered as good prognosis. On the other hand, if adhesion starts on D+2 or compaction is very advanced on D+3, they are considered as poor prognosis factors.
After gathering together everything in the above section, the embryo-grading chart was established as seen in Table 3, depending on the expected implantation potential. Grade A being the highest implantation capacity and grade D the lowest (Table 3).
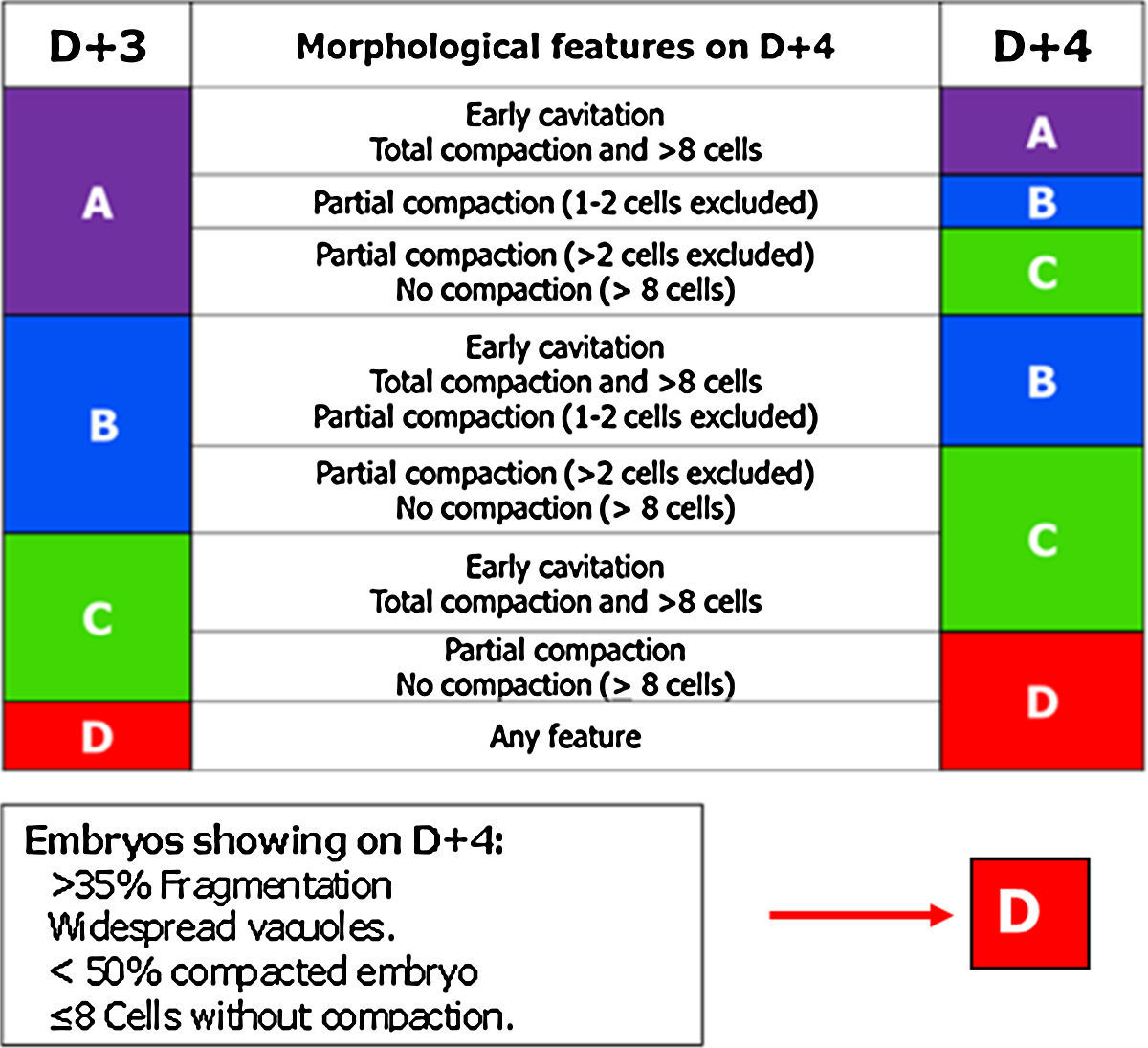
Day 4: morulaOf all the cell stages, the morula, on day 4 (D+4) is the least studied. Embryo transfer is not being performed routinely on D+4 probably due to the lack of morphological criteria with predictive value for pregnancy in this stage. But compaction is a key point to take into account in embryo development (Gardner and Balaban, 2016) and for that reason the GIE decided to include the morula in the embryo scoring system.
The most relevant parameters evaluated on D+4 are the beginning of the 4th round of mitotic divisions, adhesion, cell compaction and the morphological anomalies of poor prognosis (fragmentation, vacuolisation and intracellular cavitation).
The observation interval for D+4 has been set, in accordance with the Istanbul Consensus, between 90 and 94 hpi (Balaban et al., 2011).
In this stage, the optimal quality embryo must have started the 4th round of mitotic divisions (>8 blastomeres) (Feil et al., 2008; Balaban et al., 2011).
Cell adhesion is the first step in the process of embryo compaction. Blastomeres have begun to compact tightly showing wide areas of intercellular contact, but individual blastomeres can still be identified.
At the complete compactation stage, the embryo appears as a compact mass of cells where the blastomeres are completely compacted but the nuclei could still be identified.
Tight junctions between blastomeres prevent the visualisation of each individual blastomere and bring the start of embryo polarisation (Gardner, 1989; Alikani, 2005; Cockburn and Rossant, 2010). The embryo genome activation is a prerequisite for correct compaction (Balaban et al., 2000; Behr et al., 2000; Alikani, 2005). According to Tao et al. (2002), the optimal compaction process must include all the blastomeres, whereas the exclusion of more than 50% of the embryo is correlated with poor prognosis.
Recently, an observational and qualitative study described that the cells excluded from the compacted morulae have a higher incidence of aneuploidy than the cells of the corresponding trophectoderm, and it has been postulated that there may be a self-correction mechanism of the aneuploidy in mosaic embryos. Also, this process seems to be less efficient in women of advanced age (Lagalla et al., 2016).
In D+4 stage it is common to find morulae that present vacuoles and/or cytoplasmic fragments. Both characteristics are indicative of the start of apoptosis. The fragments and cells that enter apoptosis do not compact and are excluded from the compaction.
Finally, based on the characteristics mentioned above, ASEBIR proposed a scoring for D+4 (Table 4).
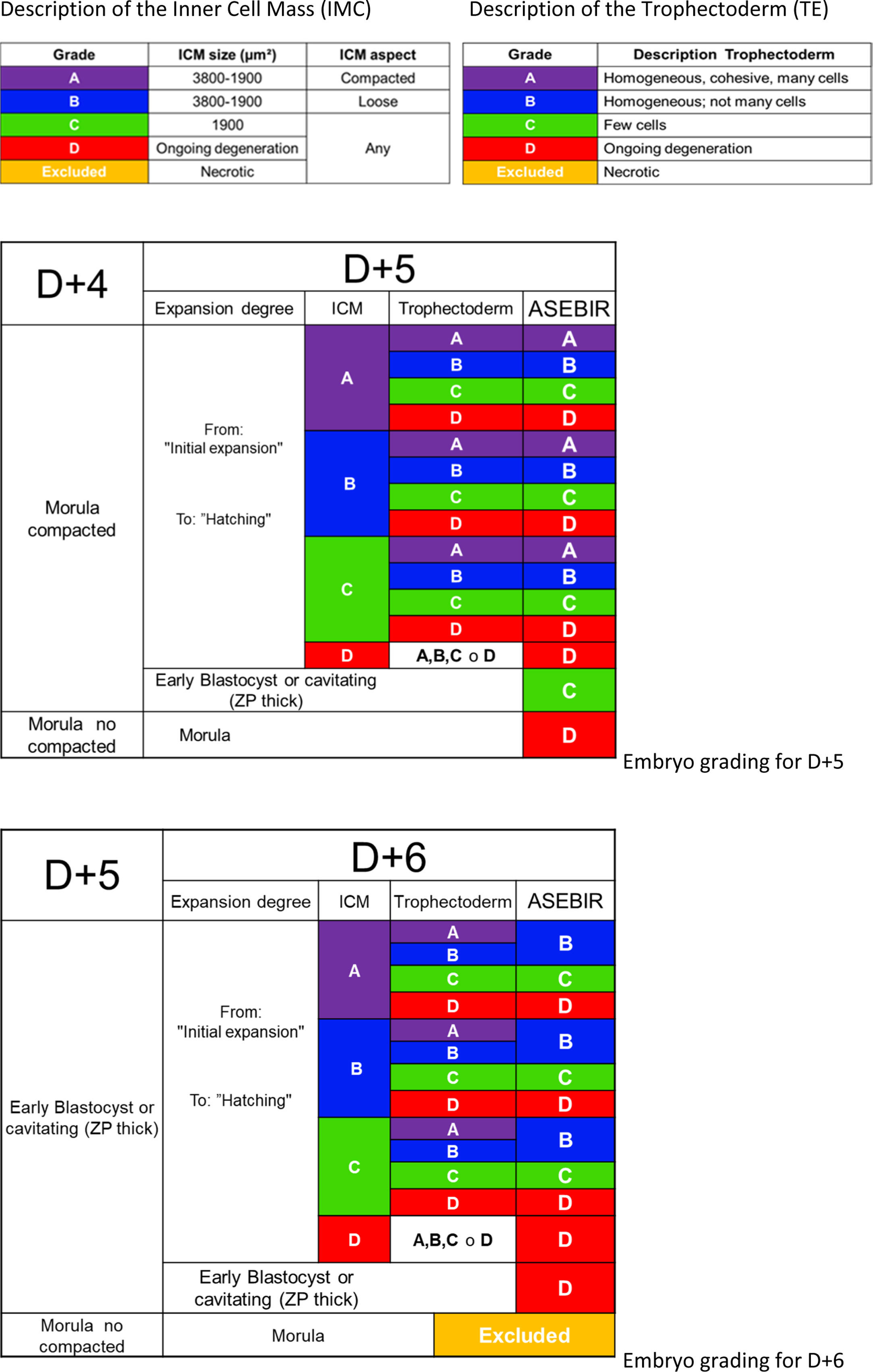
Days 5 and 6: morphological evaluation of the blastocystThe observation interval for day 5 (D+5) is set between 114 and 118 hpi, and between 136 and 140 hpi for day 6 (D+6), in accordance with the recommendations of the Istanbul Consensus (Balaban et al., 2011).
Performing blastocyst culture and transfer in blastocyst stage, higher pregnancy and recent live birth rates are obtained than those observed in transfers in early stages (Papanikolaou et al., 2006; Styer et al., 2008).
According to the current bibliography, only between 40% and 60% of oocytes fertilised in vitro reach the blastocyst stage (Gardner et al., 1998; Behr et al., 1999; Costa-Borges et al., 2016). Most of the embryos reach it on D+5, although embryos that divide more slowly may reach blastocyst on D+6 or even day 7 (D+7), a situation that implies lower, but not negligible, implantation rates (Khorram et al., 2000; Richter et al., 2001; Shapiro et al., 2001; Kovalevsky et al., 2013).
ASEBIR proposes a scoring system very similar to Gardner's (Gardner et al., 1998), where the parameters taken into account are the degree of expansion of the blastocoel, the characteristics of the trophectoderm (TE) and of the inner cell mass (ICM) (Table 5).
The observation of the blastocoel is related to good implantation rates (Shoukir et al., 1998); its formation could be related to a proper process of trophoblastic maturation (Wiley, 1984; Watson et al., 1992). With the expansion of the blastocoel there is a thinning of the ZP, reaching a minimum thickness when the blastocyst is totally expanded. Some authors associate this thinning with an improvement in implantation rates (Balaban et al., 2000; Yoon et al., 2001; Racowsky et al., 2003).
Regarding the TE, the number, shape and degree of cohesion of the layer of cells will help to score the blastocyst in the different categories, from a better prognosis with a homogeneous epithelium with elliptical cells to an irregular epithelium with few cells or with degenerative foci.
The ICM must be oval and its cells must be compacted. The favourable size varies between 1900 and 3800μm2 while lower sizes would imply lower implantation potential (Richter et al., 2001).
The presence of vacuoles and fragmentation is indicative of the start of apoptosis, but there are no bibliographical references to connect it directly with implantation failures.
Later, based on a retrospective validation study performed by the GIE (data not published), a scoring system was proposed that consists of 4 categories; A, B, C and D (Table 5), giving greater importance to the morphology of the TE than the ICM, in agreement with other published studies (Ahlstrom et al., 2011; Hill et al., 2013).
Blastocysts are classified according to the characteristics of the blastocyst itself without taking into account the assessment and selection of the embryo on previous days. It will only be taken into account in selection between various blastocysts of equal quality.
Discussion and conclusionsThis work presents an updating of the embryo scoring system that is already implemented in most Spanish Assisted Reproduction Centres, and validated by retrospective studies. This makes it possible to unify criteria between centres and to perform multicentre studies with the same scoring criteria.
The ASEBIR scoring system, in early embryos, morulae and blastocysts, classifies the embryos in four categories: A, B, C and D.
In early embryos, D+3 score depends on the D+2 score while in blastocyst stage, its score is independent of the embryo evolution on earlier days.
The current ASEBIR scoring system is based exclusively on morphological parameters; however, being a dynamic scoring, the GIE continues working on updating criteria based on the scientific advances from different approaches.
Work is also being done on a scoring system of blastocysts on D+7 of culture, as the rapid expansion of blastocyst biopsy for preimplantational genetic diagnosis cycles is leading many laboratories to transfer and/or cryopreserve in this stage and it is considered beneficial to unify scoring criteria.
The GIE recommends the participation of IVF laboratories in external quality control programmes (Santos, 2007; Mantilla et al., 2015) that include morphological embryo assessment and selection, with the aim of ensuring minimum inter- and intralaboratory variability.
Conflicts of interestThe authors declare no conflict of interest.