COVID-19, caused by SARS-CoV-2, has spread around the world since 2019. In severe cases, COVID-19 can lead to hospitalization and death. Systemic arterial hypertension and other comorbidities are associated with serious COVID-19 infection. Literature is unclear whether antihypertensive therapy with angiotensin receptor blockers (ARBs) and angiotensin converting enzyme (ACE) inhibitors affect COVID-19 outcomes. We aim to assess whether ACEI/ARB therapy is a risk factor for worse respiratory outcomes related to COVID-19 in hospitalized patients.
MethodsRetrospective study enrolling admitted COVID-19-diagnosed patients by RT-PCR at the Hospital Geral de Fortaleza, Brazil, during 2021. Patient medical records, sociodemographic, and clinical data were analyzed. Chest CT images were analyzed using CAD4COVID-CT/Thirona™ software.
ResultsA total of 294 patients took part in the study. A cut-off point of 66% of pulmonary involvement was found by ROC curve, with patients having higher risk of death and intubation and lower 60-day survival. Advanced age (RR 1.025, P=0.001) and intubation (RR 16.747, P<0.001) were significantly associated with a higher risk of death. Advanced age (RR 1.023, P=0.001) and the use of noninvasive ventilation (RR 1.548, P=0.037) were associated with a higher risk of intubation. Lung involvement (>66%) increased the risk of death by almost 2.5-fold (RR 2.439, P<0.001) and by more than 2.3-fold the risk of intubation (RR 2.317, P<0.001).
ConclusionsAltogether, our findings suggest that ACEI or ARB therapy does not affect the risk of death and disease course during hospitalization.
La COVID-19, causada por el SARS-CoV-2, se ha extendido por todo el mundo desde 2019. En casos graves, la COVID-19 puede provocar hospitalización y muerte. La hipertensión arterial sistémica y otras comorbilidades se asocian con una infección grave por COVID-19. La literatura no está clara si la terapia antihipertensiva con bloqueadores de los receptores de angiotensina (BRA) e inhibidores de la enzima convertidora de angiotensina (ECA) afecta los resultados de la COVID-19. Nuestro objetivo fue evaluar si la terapia BRA/ECA es un factor de riesgo de peores resultados respiratorios relacionados con COVID-19 en pacientes hospitalizados.
MétodosEstudio retrospectivo que incluyó pacientes ingresados con diagnóstico de COVID-19 mediante RT-PCR en el Hospital General de Fortaleza, Brasil, durante 2021. Se analizaron las historias clínicas de los pacientes, datos sociodemográficos y clínicos. Las imágenes de TC de tórax se analizaron utilizando el software CAD4COVID-CT/ThironaTM.
ResultadosParticiparon en el estudio un total de 294 pacientes. Mediante curva ROC se encontró un punto de corte del 66% de afectación pulmonar, teniendo los pacientes mayor riesgo de muerte e intubación y menor supervivencia a 60 días. La edad avanzada (RR 1,025; P=0,001) y la intubación (RR 16,747; P<0,001) se asociaron significativamente con un mayor riesgo de muerte. La edad avanzada (RR 1,023; P=0,001) y el uso de ventilación no invasiva (RR 1,548; P=0,037) se asociaron con un mayor riesgo de intubación. La afectación pulmonar (>66%) aumentó el riesgo de muerte casi 2,5 veces (RR 2,439; P<0,001) y más de 2,3 veces el riesgo de intubación (RR 2,317, P<0,001).
ConclusionesSe concluyó que el tratamiento con BRA o ECA no afecta el riesgo de muerte y el curso de la enfermedad durante la hospitalización.
A novel coronavirus, the etiologic agent of a severe acute respiratory syndrome, SARS-CoV-2, was discovered in December 2019 in Wuhan, China, and a worldwide epidemic of coronavirus 2019 (COVID-19) has spread. Data published in May, 2022 by the World Health Organization points to over 500 million confirmed cases and over six million deaths reported globally.1 The clinical spectrum of COVID-19 ranges from asymptomatic cases and upper respiratory infection to severe pneumonia associated with acute respiratory distress syndrome (ARDS).2
Many comorbidities are associated with a more severe course of the disease and worse clinical outcomes, such as diabetes, cardiovascular disease, and hypertension.3 Initial Chinese case reports have indicated that systemic arterial hypertension was the most frequent condition among COVID-19 diagnosed patients, with a percentage ranging from 15 to 30%.2,4 Given that hypertension is often treated with angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARBs), and the discovery that SARS-CoV-2 binds to ACE2 in the lung to enter respiratory cells,5,6 it is still unclear whether these agents could either be beneficial or harmful in hospitalized COVID-19-patients.
Previous animal studies have shown an increase in ACE2 expression with the use of ARBs/ACEI, which may lead to a risk of severe COVID-19 infection and complications.7 Conversely, ACE2 is a negative regulator of the renin–angiotensin pathway, that inhibits angiotensin II effects, thereby negatively regulating vasoconstriction, cell proliferation, and inflammation, altogether could be favorable for patients.8 The use of ACEI/ARBs in COVID-19 infection remains a subject of debate. Although some studies have already shown that the therapy with ARBs/ACEI is not associated with an increased risk of in-hospital death by COVID-19,9,10 more studies are warranted to better understand their role in COVID-19 outcomes in hospitalized patients.
No studies so far have explored whether hypertensive patients under ACEI/ARBs therapy have a greater chance of COVID-19 associated pulmonary involvement assessed by chest computer tomography (CT) imaging, with mortality and risk for intubation and mechanical ventilation correlations. Therefore, this study aimed to assess whether ACEI/ARB therapy is associated to worse COVID-19-related respiratory outcomes in hospitalized patients.
Material and methodsThis is a retrospective cohort study analyzing patients diagnosed with COVID-19 admitted at Hospital Geral de Fortaleza. The study protocol was approved by the Institutional Ethics Board of the General Hospital of Fortaleza (protocol no. 4.365.121). During the study period, March until October of 2021, the gamma variant (P.1) was the most prevalent in the state of Ceará, where the city of Fortaleza is located. By 2022, more than 1.2 million COVID-19 cases have already been recorded with more than 26,000 deaths in Ceará.
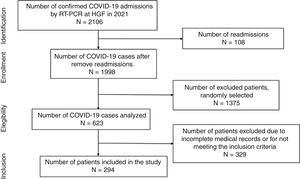
All included patients were symptomatic with respiratory distress and had COVID-19 diagnosed upon hospital admission using real-time reverse transcription-polymerase chain reaction (RT-PCR) of standard nasopharyngeal and oropharyngeal swab specimens. The study inclusion criteria were age ≥18 years, both genders, and diagnosed with COVID-19 by RT-PCR. Exclusion criteria were patients’ diagnosis of COVID-19 by another method, patients with incomplete medical records or without chest tomography images within 48h of hospital admission. Patients’ data were analyzed according to 3 groups: hypertensive users of ACEI/ARB, hypertensive users of other classes of antihypertensive drugs, and non-hypertensive patients. Classification into groups was made according to the use of medications prior to admission, not considering the introduction of medications after hospital admission. After recruiting participants who met the study's inclusion criteria, they were placed on a list and a simple randomization was performed using the website www.random.org. Of all hospitalized patients, 623 were randomly selected for medical chart analysis. After patient enrolment, sociodemographic and clinical data were collected. Clinical characteristics included comorbidities and treatment therapies, symptoms, and time from illness onset to admission, length of hospital stay, hospital discharge or death. All information was collected from the medical chart by trained researchers. COVID-19-related pulmonary involvement was scored with the aid of an artificial intelligence software using chest CT images, which were digitally analyzed using the CAD4COVID-CT software (Thirona™, 2020, The Netherlands). Data were expressed as percentage of COVID-19-related pulmonary damage using artificial intelligence algorithm.
All patients were submitted to a routine chest CT within 48h from admission to the hospital. Chest CTs were obtained using either a 8 channel-Toshiba or a 64 channel-Philips CT scanners (Toshiba, JP; Phillips, NL). Infection control and prevention were taken into account in all cases.
CT quantification of pulmonary parenchyma was performed using the CAD4COVID-CT software. CAD4COVID-CT is an artificial intelligence (AI)-based software package that was offered free-of-charge during the COVID-19 pandemic to assist healthcare professionals in their daily tasks. The software automatically quantifies the pulmonary extent of COVID-19 severity from CT scans using state-of-the-art deep learning techniques. The AI software identifies the lobar regions affected by COVID-19 pneumonia and quantifies them as the percentage of total lobe volume. All CT data were collected by two trained researchers.
Primary outcomes were defined as death and intubation/mechanical ventilation. Secondary outcome was defined as the percentage of pulmonary involvement. Independent analyzes were performed for each of these outcomes. Categorical data were expressed as absolute counts and relative frequencies as percentages. For all outcomes, univariate analyses were performed using the Chi-square test or Fisher's exact test when considering categorical exposure variables. The distribution of continuous variables was assessed by the Kolmogorov–Smirnov test and by visual assessment of the histogram. Normal data were expressed as mean±standard deviations, and asymmetrical data as median and interquartile ranges. Continuous variables with normal distribution were compared using the unpaired t-test for independent samples. Variables without normal distribution were compared using the Mann–Whitney test.
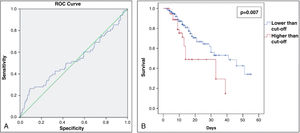
To evaluate predictive capacity for death considering the percentage of pulmonary involvement, a ROC curve was constructed, and the better cut-off was determined using higher Youden index (Youden Index=sensitivity+specificity−1). Then, the selected cut-off was used to build the new groups, which were used to evaluate the survival behavior through Kaplan–Meier analysis for a 2-month survival chance. The log-rank test was used to evaluate the statistical difference between the two based on cut-off groups.
In addition, bi- and multivariate analyses were applied to compare each outcome and the exposure variables. Therefore, Poisson regression with robust variance was applied to estimate the relative risk (for death and intubation outcomes), prevalence ratio (for the percentage of pulmonary involvement outcome), and their respective 95% confidence intervals. Wald tests were used to estimate statistical significance, using the level of significance of 5%. Assumptions for Poisson models were evaluated using the Pearson Chi-square goodness-of-fit test. In all multivariate analyses, no overdispersion was detected. Moreover, to verify multicollinearity, values of “tolerance”>0.1 and “variance inflation factor”<10.0 were used.
For all analyses, the construction of the final multivariate model used the “backwards” strategy, considering, in the initial model, only those variables that presented a value of P<0.20 in the bivariate analysis. Statistical significance and analysis of effect modifications were used to determine the final multivariate models. The independent variable “use of antihypertensive drugs” was defined as the primary exposure and was included in all multivariate models, regardless of the detected P-value.
Since intubation is a human intervention, this study also assessed the impact of this intervention on the death outcome. Therefore, an additional multivariate analysis was performed excluding this independent variable from the final model. In all analyses, a P<0.05 value was determined to be statistically significant. Data were analyzed using SPSS software version 26.0 for Mac (Armonk, NY: IBM Corp.).
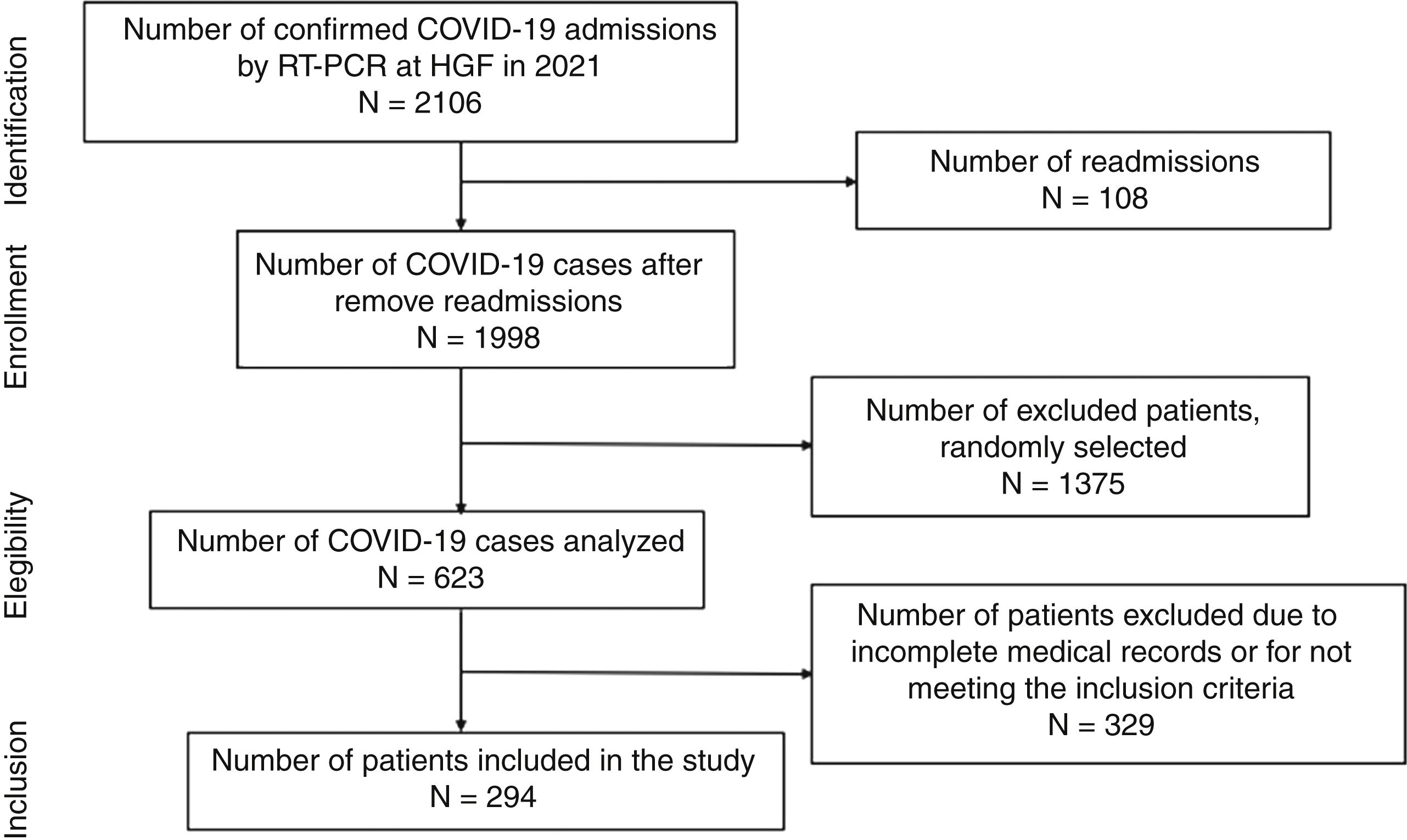
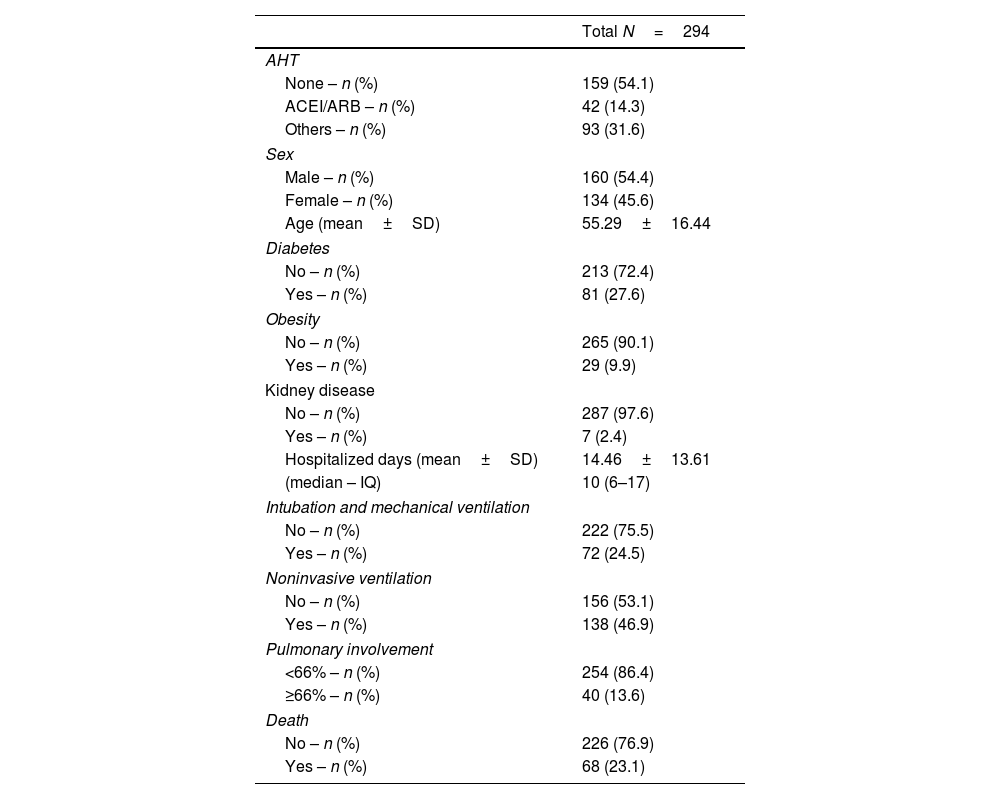
ResultsDuring the study period, there were 2106 hospital admissions due to COVID-19. After removing readmissions, exclusion of cases with incomplete medical records or cases with no CT images, a total of 294 patients were included in the study (shown Fig. 1). The average age was 55.2 years old. In the study population, male patients were more frequent (55.4%) than female (45.6%). Forty-two patients were hypertensive under ACEI/ARB treatment (14.3%), 93 patients were hypertensive users of other classes of antihypertensive drugs (31.6%), and 159 non-hypertensive patients, with other comorbidities. Regarding the clinical evolution of the patients, the mean number of days hospitalized was 14.46±13.61, 138 patients (46.9%) required non-invasive ventilation (NIV) and 72 patients (24.5%) were submitted to orotracheal intubation and mechanical ventilation (Table 1).
Distribution of the relative frequency for the different variables.
| Total N=294 | |
|---|---|
| AHT | |
| None – n (%) | 159 (54.1) |
| ACEI/ARB – n (%) | 42 (14.3) |
| Others – n (%) | 93 (31.6) |
| Sex | |
| Male – n (%) | 160 (54.4) |
| Female – n (%) | 134 (45.6) |
| Age (mean±SD) | 55.29±16.44 |
| Diabetes | |
| No – n (%) | 213 (72.4) |
| Yes – n (%) | 81 (27.6) |
| Obesity | |
| No – n (%) | 265 (90.1) |
| Yes – n (%) | 29 (9.9) |
| Kidney disease | |
| No – n (%) | 287 (97.6) |
| Yes – n (%) | 7 (2.4) |
| Hospitalized days (mean±SD) | 14.46±13.61 |
| (median – IQ) | 10 (6–17) |
| Intubation and mechanical ventilation | |
| No – n (%) | 222 (75.5) |
| Yes – n (%) | 72 (24.5) |
| Noninvasive ventilation | |
| No – n (%) | 156 (53.1) |
| Yes – n (%) | 138 (46.9) |
| Pulmonary involvement | |
| <66% – n (%) | 254 (86.4) |
| ≥66% – n (%) | 40 (13.6) |
| Death | |
| No – n (%) | 226 (76.9) |
| Yes – n (%) | 68 (23.1) |
Legend: ACEI: angiotensin-converting-enzyme inhibitors; AHT: antihypertensive therapy; ARB: angiotensin receptor clockers; IQ: interquartile range; SD: standard deviation.
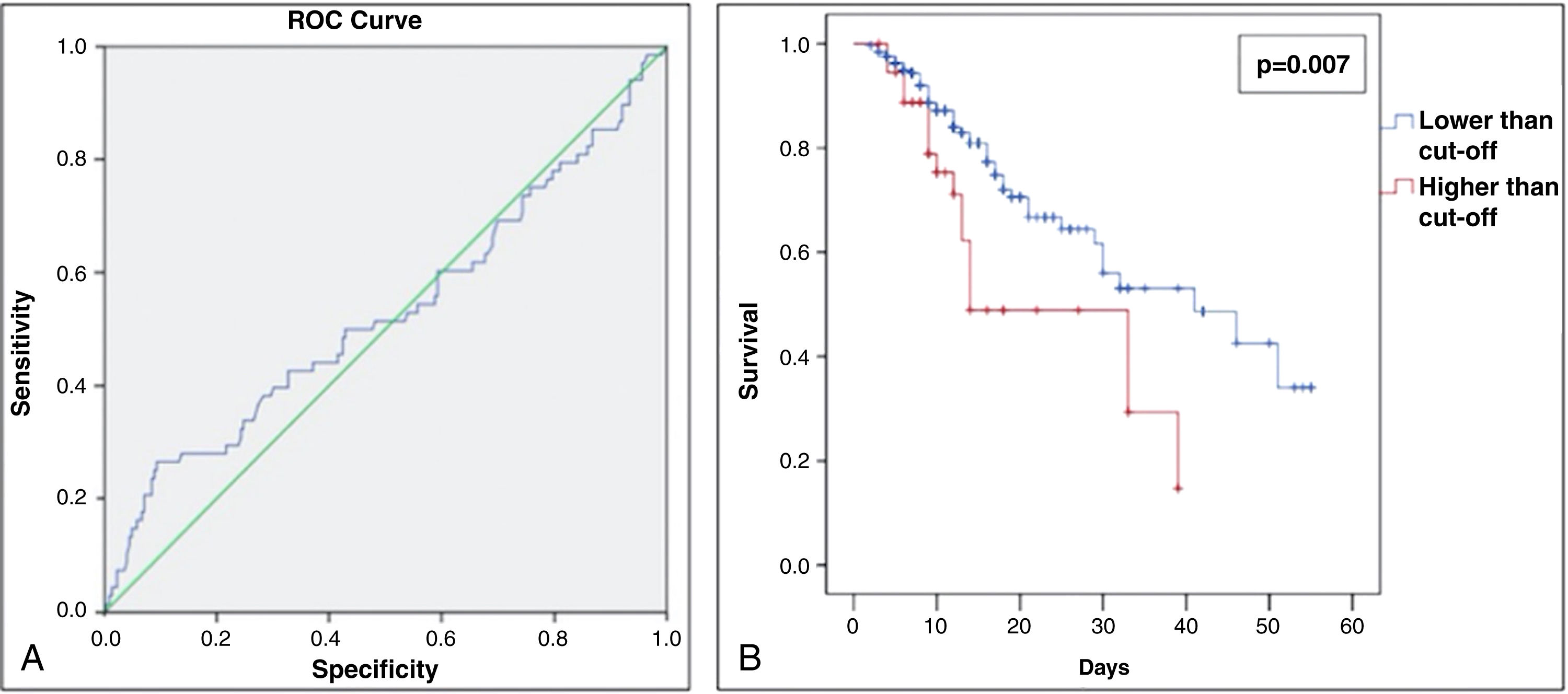
The group of patients who had more than 66% of pulmonary involvement presented a lower survival rate in 60 days than the other patients (Fig. 2A and B). Moreover, only 13.6% of the study population had more than 66% of lung involvement due to COVID-19 (shown in Table 1). No statistical significance was found between the pulmonary involvement and any other variable assessed. Therefore, being a hypertensive patient or the use of ACEI/ARBs was not significantly associated with a higher percentage of COVID-19-related pulmonary lesions.
(A) Receiver operating characteristic (ROC) curve for % of COVID-19-related lung involvement from the study patients admitted at the Hospital Geral de Fortaleza in 2021. (B) Sixty day–survival curve from the study patients admitted at the Hospital Geral de Fortaleza in 2021, comparing lower and higher cut-off (%) of COVID-19-related lung involvement.
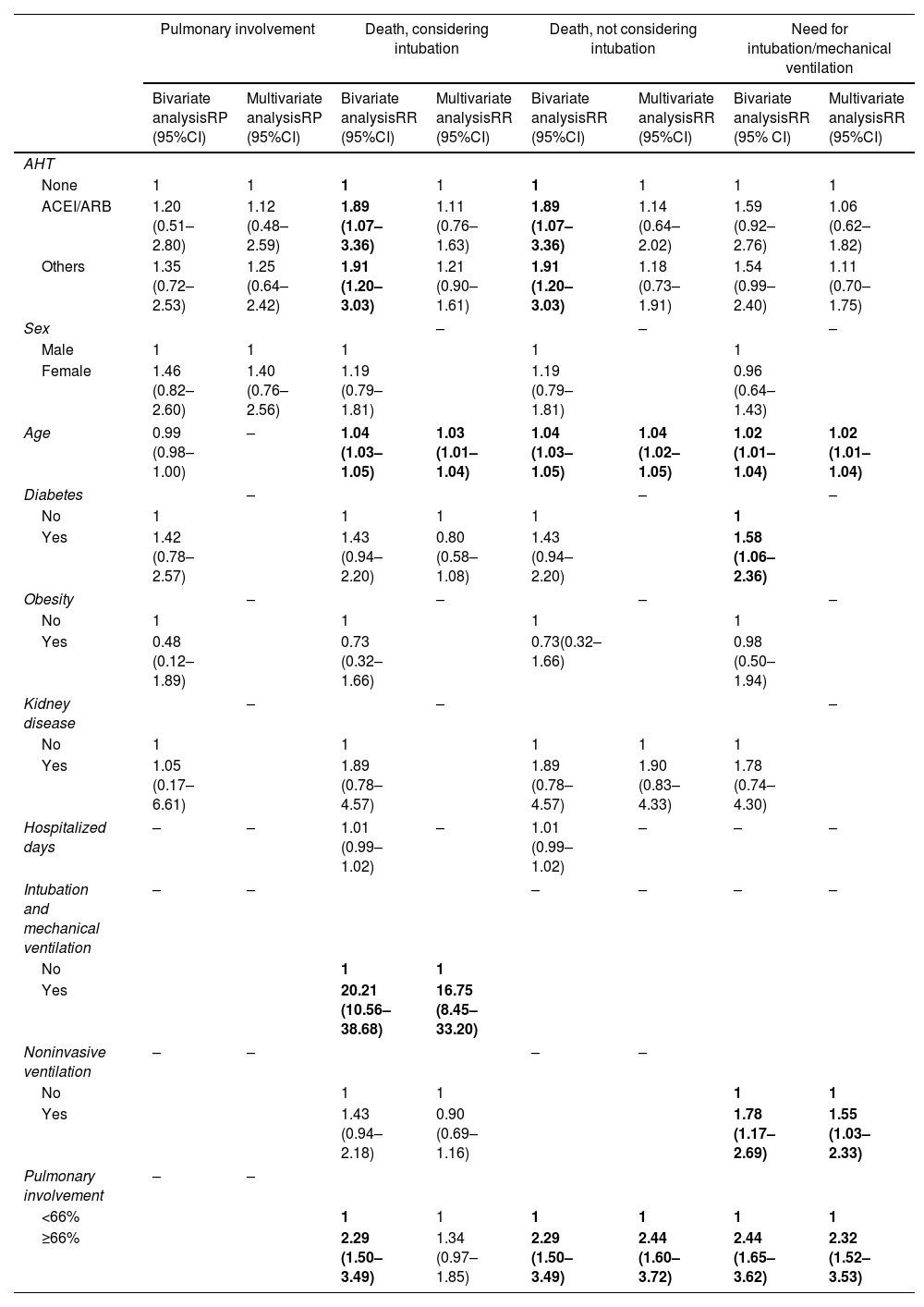
After that, the risk for death was analyzed according to independent variables, including lung involvement as an exposure variable. Only older age (relative risk [RR]: 1.03 [95% confidence interval (95% CI): 1.01–1.04] P=0.001) and intubation (RR: 16.75 [95% CI: 8.45–33.20] P<0.001) were significantly associated with a higher risk for death (shown in Table 2). It is important to highlight that the use of ACEI/ARB was not a risk factor for death. Since intubation is a human intervention for patients with severe COVID-19 infection (where we observed an 16-fold higher risk for death), intubation per se could suppress the effect of other variables. Therefore, it was decided to exclude this variable as shown in Table 2, where lung involvement>66% appeared as a significant risk factor for death, increasing its risk by almost 2.5 times (RR: 2.44 [95% CI: 1.60–3.72] P<0.001).
Association between independent variables and the outcomes: percentage of pulmonary involvement, death considering intubation as independent variable, death not considering intubation as independent variable, and need for intubation/mechanical ventilation.
| Pulmonary involvement | Death, considering intubation | Death, not considering intubation | Need for intubation/mechanical ventilation | |||||
|---|---|---|---|---|---|---|---|---|
| Bivariate analysisRP (95%CI) | Multivariate analysisRP (95%CI) | Bivariate analysisRR (95%CI) | Multivariate analysisRR (95%CI) | Bivariate analysisRR (95%CI) | Multivariate analysisRR (95%CI) | Bivariate analysisRR (95% CI) | Multivariate analysisRR (95%CI) | |
| AHT | ||||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ACEI/ARB | 1.20 (0.51–2.80) | 1.12 (0.48–2.59) | 1.89 (1.07–3.36) | 1.11 (0.76–1.63) | 1.89 (1.07–3.36) | 1.14 (0.64–2.02) | 1.59 (0.92–2.76) | 1.06 (0.62–1.82) |
| Others | 1.35 (0.72–2.53) | 1.25 (0.64–2.42) | 1.91 (1.20–3.03) | 1.21 (0.90–1.61) | 1.91 (1.20–3.03) | 1.18 (0.73–1.91) | 1.54 (0.99–2.40) | 1.11 (0.70–1.75) |
| Sex | – | – | – | |||||
| Male | 1 | 1 | 1 | 1 | 1 | |||
| Female | 1.46 (0.82–2.60) | 1.40 (0.76–2.56) | 1.19 (0.79–1.81) | 1.19 (0.79–1.81) | 0.96 (0.64–1.43) | |||
| Age | 0.99 (0.98–1.00) | – | 1.04 (1.03–1.05) | 1.03 (1.01–1.04) | 1.04 (1.03–1.05) | 1.04 (1.02–1.05) | 1.02 (1.01–1.04) | 1.02 (1.01–1.04) |
| Diabetes | – | – | – | |||||
| No | 1 | 1 | 1 | 1 | 1 | |||
| Yes | 1.42 (0.78–2.57) | 1.43 (0.94–2.20) | 0.80 (0.58–1.08) | 1.43 (0.94–2.20) | 1.58 (1.06–2.36) | |||
| Obesity | – | – | – | – | ||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.48 (0.12–1.89) | 0.73 (0.32–1.66) | 0.73(0.32–1.66) | 0.98 (0.50–1.94) | ||||
| Kidney disease | – | – | – | |||||
| No | 1 | 1 | 1 | 1 | 1 | |||
| Yes | 1.05 (0.17–6.61) | 1.89 (0.78–4.57) | 1.89 (0.78–4.57) | 1.90 (0.83–4.33) | 1.78 (0.74–4.30) | |||
| Hospitalized days | – | – | 1.01 (0.99–1.02) | – | 1.01 (0.99–1.02) | – | – | – |
| Intubation and mechanical ventilation | – | – | – | – | – | – | ||
| No | 1 | 1 | ||||||
| Yes | 20.21 (10.56–38.68) | 16.75 (8.45–33.20) | ||||||
| Noninvasive ventilation | – | – | – | – | ||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.43 (0.94–2.18) | 0.90 (0.69–1.16) | 1.78 (1.17–2.69) | 1.55 (1.03–2.33) | ||||
| Pulmonary involvement | – | – | ||||||
| <66% | 1 | 1 | 1 | 1 | 1 | 1 | ||
| ≥66% | 2.29 (1.50–3.49) | 1.34 (0.97–1.85) | 2.29 (1.50–3.49) | 2.44 (1.60–3.72) | 2.44 (1.65–3.62) | 2.32 (1.52–3.53) | ||
ACEI: angiotensin-converting-enzyme inhibitors; AHT: antihypertensive therapy; ARB: angiotensin receptor blockers; IQ: interquartile range; PR: prevalence ratio; RR: risk ratio; SAH: systemic arterial hypertension; SD: standard deviation. Bold estimates mean statistical significance (P<0.05).
For the outcome intubation/mechanical ventilation, older age (RR: 1.02 [95% CI: 1.01–1.04] P=0.001) and the use of non-invasive ventilation (RR: 1.55 [95% CI: 1.03–2.33] P=0.037) were associated to higher risk for intubation (shown in Table 2). In addition, severe lung involvement (>66%) increases more than 2.3 times the risk for intubation (RR: 2.32 [95% CI: 1.52–3.53] P<0.001). Noteworthy, the use of ACEI/ARBs was not associated with increased risk for intubation.
DiscussionThis study evaluated whether ACEI/ARB therapy represents a risk factor for worse respiratory outcomes related to COVID-19 in hospitalized patients. Our results corroborate the literature indicating that treatment with ACE inhibitors or ARBs was not associated with an increased risk of in-hospital death or mechanical intubation/ventilation. A cut-off value of 66% for pulmonary involvement on chest CT was identified, above the 66% cut-off, patients showed a higher risk of in-hospital death and intubation, with a lower 60-day survival compared to patients with an affected lung area below 66%. Regarding the variables that influence the risk of death and intubation/mechanical ventilation for hospitalized patients with COVID-19, we found that advanced age and intubation were significantly associated with a higher risk of in-hospital death. The use of non-invasive ventilation and older age were associated with an increased risk of intubation.
The outbreak of SARS-CoV-2 has been a global catastrophe. Although the scientific community is making an extraordinary effort to fight against the related morbimortality, it is still far from fully understanding whether the overactivation of the immune system and the state of hyperinflammation can be ameliorated by medications. After the discovery that the SARS-CoV-2 spike protein (S1) binds to ACE-2 to enter human lung cells, studies have explored whether the imbalance in renin–angiotensin–aldosterone system (RAS) axis is implicated in a more severe COVID-19 inflammatory response, especially in the older adults and people with comorbidities, such as diabetes and hypertension.6,11
In humans, despite the structural homology between ACE and ACE2, these receptors have divergent physiological functions. ACE has as its final product angiotensin II (Ang II), which is associated with vasoconstriction, endothelial damage, and thrombosis formation. ACE2 counterbalances the deleterious effects of the ACE/RAS since it cleaves Ang I and II into Ang (1–9) and Ang (1–7), respectively, with protective vasodilatory and antiproliferative effects.11,12
Since ACE2 tissue expression has been shown to be upregulated by ACEI and ARBs treatment and hypertension is commonly treated with these medications, initial reports speculated that ACEI and ARBs could increase the risk of severe SARS-CoV-2 infectivity by facilitating viral load.13,14 SARS-CoV-2-driven release of inflammatory cytokines, such as interferons (IFNs), could increase ACE2 expression and potentiate COVID-19 infection.15 In contrast, other studies with ARDS lung injury have already demonstrated a possible benefit of ACE2, protecting against acute lung injury in several experimental models.16,17 However, the results of the present study do not support the deleterious roles of ACEI and ARB in COVID-19 infection among hospitalized patients, as it was not detected an increased risk of in-hospital death, intubation, or more percentage of pulmonary involvement under these anti-hypertensive therapies.
In this study, it was proposed a threshold (>66%) for the percentage of pulmonary involvement by COVID-19, as assessed by CAD4COVID-CT software, beyond which patients are more at risk of death with lower 60-days survival. Of note, this IA-based software was designed to track COVID-19-related lung injury and no other potential causes of pulmonary damage, such as chronic obstructive pulmonary disease. To our knowledge, this is the first study to address a measurable level of COVID-19-related lung injury and the association with clinical outcomes in hospitalized patients.
A randomized clinical trial with 659 hospitalized patients also found mild to moderate COVID-19 in patients undertaking ACEIs or ARBs before hospital admission. No significant difference in the mean number of days alive and out of the hospital was found.18 Another large cohort study assessing big data from the American Health System, with more than 800,000 hypertensive patients, concluded that neither ACEI nor ARB use was associated with an increased likelihood of COVID-19 infection.19 A recent Italian study also supported this evidence, showing that treatment with ACEI or ARB was not an independent predictor neither for in-hospital death nor for the combination of in-hospital death/need for ICU.9 Contrary to these results, a Spanish study that analyzed data from 849 patients showed that RAAS inhibitors may play a protective role in hypertensive patients with COVID-19, since the overall mortality in hypertensive patients was 28.4%, but lower among those with prescribed RAAS inhibitors before (odds ratio −0.167 [95% CI −0.220, −0.114] P<0.10) and during hospitalization (odds ratio 0.090 [95% CI −0.008,0.188] P<0.10).20
The literature has already identified groups of patients at greater risk of worse outcomes in COVID-19, such as patients with hypertension, diabetes, advanced age, and patients with cardiovascular disease.21–23 Our study also documented that older age is associated with an increased risk for death and intubation in COVID-19 hospitalized patients. Each year of age increases the chance of death by 2.5% and the chance of intubation by 2.3%. Moreover, patients with COVID-19-lung affected area>66% could be 2.5 times more prone to death and 2.3 times more at risk for intubation and mechanical ventilation.
In agreement with current evidence, our findings support that ACEI/ARB treatment does not worsen the outcomes of hypertensive patients with COVID-19. The last bulletin of the American College of Cardiology keeps supporting ACEI/ARB therapy for COVID-19 patients until otherwise.24
This study has important limitations. Our study enrolled a limited number of patients under ACRI/ARB treatment. Distinct anti-hypertensive treatment characteristics (e.g., duration, posology, compliance etc.) were not covered by our statistical analyses. Although the CAD4COVID-CT software picks COVID-19-related lung injury, we cannot rule out other previous causes of pulmonary involvement.
Our findings corroborate with literature that older age is a risk factor for worse COVID-19 outcomes and that ACEI or ARB therapy does not affect the risk of death and disease course during hospitalization.
Authors’ contributionsFelipe B. Lima designed and supervised the study, obtained authorization for CAD4COVID-CTTM software research use, wrote the manuscript, revised and approved the final version of the manuscript.
Francisco Wilker Mustafa Gomes Muniz, Gdayllon C. Meneses performed statistical analysis and conducted the results interpretation, revised and approved the final version of the manuscript.
Karine C. Bezerra, Carolyne N. Moreira contributed for data analysis and interpretation, revised and approved the final version of the manuscript.
André P. Aguiar, collected the data, revised the literature, revised and approved the final version of the manuscript.
José Carlos R. Nascimento, Tainá Veras de S. Freitas, Eanes Delgado B. Pereira, Elizabeth de F. Daher contributed for data analysis and interpretation, revised and approved the final version of the manuscript.Pedro Felipe C. de Bruin, Reinaldo B. Oriá designed and supervised the study, revised and approved the final version of the manuscript.
FundingThis study was supported by FUNCAP INOVA FIOCRUZ FIO-0167-00070.01.00/20.
Ethical considerationsThe study protocol was approved by the Institutional Ethics Board of the General Hospital of Fortaleza (protocol no. 4.365.121).
Conflict of interestThe authors declare no conflict of interest related to this study.
We are thankful to Brazilian CNPq, FUNCAP and CAPES funding agencies. The authors are thankful to all patients and family members and hospital staff for supporting this study.