Major Depressive Disorder (MDD) is a highly prevalent psychiatric disorder that impairs the cognitive function of individuals. Aerobic exercise stands out as a promising non-pharmacological intervention for enhancing cognitive function and promoting brain health.
While positive impacts of aerobic exercise on executive function in adults with depression have been documented, a comprehensive understanding of its benefits on overall cognitive function, including memory, attention, and processing speed, along with key moderating factors in adults with MDD, remains unexplored. The purpose of the systematic review and meta-analysis was to investigate the effects of aerobic exercise on overall cognitive function in adults with MDD, and to explore whether cognitive sub-domains, aerobic exercise characteristics, and study and sample variables modify the effects of aerobic exercise on cognition.
MethodsSix English electronic databases (Embase, Cochrane Central, Scopus, APA PsycInfo, PubMed, Web of Science) were searched from inception to 2 April 2023. Randomized trials, including adults aged 18 years or above with a diagnosis of clinical depression, of the effects of aerobic exercise on cognitive function in adults with MDD compared to non-aerobic exercise groups were included. A three-level meta-analysis was conducted utilizing a random-effects model in R. The quality of the studies was evaluated using the Physiotherapy Evidence Database (PEDro) scale. The PROSPERO registration number is CRD42022367350.
ResultsTwelve randomized trials including 945 adults with MDD were included. Results indicated that aerobic exercise significantly improved overall cognitive function (g = 0.21; 95 % confidence intervals [CI] = 0.07, 0.34), and the sub-domains of memory (g = 0.25; 95 % CI = 0.06, 0.44) and executive function (g = 0.12; 95 % CI = 0.04, 0.20). Significant benefits in cognitive function were found from moderate-to-vigorous (mixed) intensity (g = 0.19; 95 % CI = 0.02, 0.37), aerobic exercise conducted 3 times per week (g = 0.23; 95 % CI = 0.10, 0.38), in sessions < 45 min (g = 0.59; 95 % CI = 0.28, 0.90), and 45–60 min (g = 0.16; 95 % CI = 0.07, 0.26), in aerobic exercise intervention ≤ 12 weeks (g = 0. 26; 95 % CI = 0.08, 0.44).
LimitationsThis review only included peer-reviewed English-language studies, which may lead to a language bias. The results of the Egger's test suggested a potential publication bias.
ConclusionsAerobic exercise is efficacious in improving overall cognitive function and the sub-domains of memory and executive function in adults with major depressive disorder.
Depression has emerged as a leading cause of worldwide illness burden, disability, and suicide (Birnie et al., 2022; Kawilapat et al., 2022; Li et al., 2023). However, roughly two-thirds of adults diagnosed with depression do not engage in therapy actively (Heissel et al., 2023). Major Depressive Disorder (MDD), as one of the costliest psychiatric disorders (Patel et al., 2023), is a devastating, recurrent, and highly prevalent psychiatric disorder (Lan et al., 2023; Lee et al., 2020) affecting approximately 5 % of adults worldwide (Kowalec et al., 2022). MDD is responsible for an annual economic loss of billions of dollars globally (Greenberg et al., 2021; Sepehrmanesh et al., 2017), and is associated with a lower quality of life (Hammar et al., 2022; Irwin et al., 2023), poor academic performance, marital issues, and a higher rate of unemployment and absenteeism (Iancu et al., 2020; Lépine & Briley, 2011; Sepehrmanesh et al., 2017). MDD symptoms include negative emotions, hopelessness (Chai et al., 2019), insomnia, suicidal ideation, and cognitive dysfunction (Zhao et al., 2020).
Cognitive impairments have been widely reported to be a core deficit of MDD individuals (Kriesche et al., 2022; Pan et al., 2019), and meta-analyses have demonstrated that these individuals have deficits in several cognitive domains, such as processing speed, attention, memory, and executive function (EF) (Kriesche et al., 2022; Rock et al., 2014; Snyder, 2013). There is evidence that cognitive impairment is observed in both the acute and remission phases of depression (Kriesche et al., 2022; Legemaat et al., 2022), and cognitive impairment may deteriorate over time and with recurrent episodes when untreated (Hammar et al., 2022; Maramis et al., 2021). Moreover, cognitive impairment negatively impacts quality of life, social, interpersonal functioning (Hammar et al., 2022), daily activities, education and work (Keefe et al., 2022) in individuals with depression. Antidepressants, despite being the first-line treatment for MDD (Zhdanov et al., 2020), have shown minimal and limited effects on cognitive function (Rosenblat et al., 2016). In addition, antidepressants can cause side effects, such as nausea, headaches, and sleepiness (Du et al., 2020). Thus, non-pharmacological interventions to enhance cognitive function in MDD participants have scientific and practical relevance.
Aerobic exercise, a low-cost non-pharmacological treatment, has been shown to reduce depressive symptoms (standardized mean difference [SMD] = -1.156; p < 0.001) in adults (18 years or older) with diagnosed or indicated depression (Heissel et al., 2023) and alleviate cognitive fatigue (Zhang et al., 2023). Further, aerobic exercise has also shown significant benefits to cognitive function in the healthy population (McSween et al., 2019; Nanda et al., 2013), people with Alzheimer's Disease (Zhang et al., 2022), participants with schizophrenia (Shimada et al., 2022), children with preterm birth (Ren, Feng, et al., 2023), and adults aged 50 years and older (Northey et al., 2018). Several randomized trials have suggested that aerobic exercise produces positive effects on attention (Buschert et al., 2018), memory (Buschert et al., 2018; Khatri et al., 2001; Krogh et al., 2012), and executive function (Imboden et al., 2020; Khatri et al., 2001) in participants with MDD. However, other studies have suggested that aerobic exercise does not have a significant effect on cognitive outcomes in participants with MDD (Brush et al., 2020; Krogh et al., 2009). In addition, several meta-analyses have explored the effects of physical exercise on cognitive function (Brondino et al., 2017; Sun et al., 2018) or executive function (Contreras-Osorio et al., 2022; Ren, Alderman, et al., 2023) in depressed adults. Brondino et al. (2017) found that exercise did not enhance processing speed, attention/vigilance, global cognition, verbal learning and memory, reasoning/problem-solving, and working memory in depressed adults (Brondino et al., 2017). Further, Sun et al. (2018) also found that exercise did not significantly improve these same outcomes; however, interventions that included both physical and cognitive activities, and exercise with low-intensity did produce significant improvements in cognition (Sun et al., 2018). Contreras-Osorio et al. (2022) suggested that exercise selectively improved working memory (effect size [ES] = 0.33), but was unrelated to inhibitory control and cognitive flexibility in adults with depression (Contreras-Osorio et al., 2022), suggesting differential effects across the different aspects of executive function. However, Ren et al. (2023) found that aerobic exercise broadly improved executive function (ES = 0.203) in adults with depression (Ren, Alderman, et al., 2023). In general, the findings from meta-analyses on the effects of exercise on cognitive function in depressed adults are mixed, and few meta-analyses have specifically focused on aerobic exercise. While randomized trials of different modes of exercise on cognitive function in depression have grown in recent years, most trials have used aerobic exercise as an intervention. Synthesizing results support the notion that aerobic exercise improves EF in this population. However, the effects of aerobic exercise on overall cognitive function and other cognitive sub-domains by meta-analysis remain unknow. Given the conflicting results observed in randomized trials regarding the effects of aerobic exercise on cognitive function in adults with MDD, it is necessary to conduct a comprehensive examination of the effects of aerobic exercise on overall cognitive function and its sub-domains of memory, attention, and processing speed to fill this gap. More importantly, the detailed prescription (frequency, intensity, session time, duration) for the effect of aerobic exercise on cognitive function in adults with MDD has not been determined. This may hinder the development of optimal aerobic exercise prescriptions. In addition, important moderators between aerobic exercise and cognition have not been examined, such as the characteristics of study design (aerobic exercise plus antidepressants; comparator/control group), and sample characteristics (proportion of female participants; mean age; clinical setting). In fact, these moderators are crucial factors to consider when scientific researchers try to understand how aerobic exercise improves cognitive function in adults with depression.
This review addresses the disparate outcomes in randomized trials examining aerobic exercise impact on cognitive function in MDD. Given the absence of comprehensive evidence from systematic reviews and meta-analyses, our objective is to scrutinize aerobic exercise effects on overall cognitive function. Additionally, we aim to explore potential moderation in cognitive sub-domains, exercise characteristics, and study/sample variables. This meta-analysis serves as a theoretical foundation for clinicians and depressed patients considering aerobic exercise as a non-pharmacological intervention.
MethodsDesign and eligibility criteriaThe meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). Its protocol was preregistered in the International Prospective Register of Systematic Reviews (PROSPERO) on 24 October 2022 (registration number CRD42022367350).
The criteria for inclusion were: (1) participants were adults (aged 18 years or older) with a diagnosis of MDD using recognized or established diagnostic criteria (e.g. the International Classification of Diseases, Tenth Revision (ICD-10), the Diagnostic and Statistical Manual of Mental Disorders, (DSM‑Ⅳ or DSM-5), or the Mini-International neuropsychiatric interview (MINI), and by applying a cut-off on reliable depression scales (such as Beck Depression Inventory, Beck Depression Inventory-II, the Montgomery-Åsberg Depression Rating Scale, and Hamilton Depression Rating Scale); (2) studies employed aerobic exercise as the experimental intervention, or aerobic exercise as main intervention combined with other intervention (such as antidepressant); (3) studies included a comparator/control conditions that performed non-aerobic exercises, standard care, active control, placebo pill, no treatment, or a wait list; (4) studies included an overall cognitive function task, and at least one measure of a cognitive sub-domain such as attention, processing speed, memory, and executive function; and (5) randomized trials published in English-language peer-reviewed journals.
The exclusion criteria were: (1) studies that employed a single bout of (acute) aerobic exercise; (2) experimental group used mind-body exercises (Yoga, Tai-Chi, and Qigong), resistance exercise or aerobic exercise combined with resistance exercise or multicomponent exercise; (3) both experimental and comparator groups used aerobic exercise; and (4) studies in which data could not be extracted for ES estimation even after contacting the authors.
Literature search and selectionSix English electronic databases (Embase, Cochrane Central, Scopus, APA PsycInfo, PubMed, Web of Science) were searched from inception to 12 October 2022, and an updated search was conducted on 2 April 2023. The search term (i.e., keywords) included medical subject headings (“depression” AND "aerobic exercise " AND “cognition” OR “executive function” AND "randomized controlled trial" [Publication Type]) and related free terms (see Supplementary Table 1). After removing duplicates, two authors (FFR and FTC) independently reviewed the titles and abstracts of the remaining literature. Next, the same two authors independently read and identified the full-text of studies that had been preliminarily accepted. In instances when the two authors disagreed on which studies should be included, a third author (YKC) was consulted to reach consensus.
Data extraction and coding strategyCochrane Collaboration Handbook methods and strategies were followed throughout the data extraction process (Higgins et al., 2019). Two authors (FFR and FTC) extracted data separately by using a pre-developed extraction form in Microsoft Excel. Extracted data and information included: first author, year of publication, depression diagnostic criteria and instruments, characteristics of groups and participants (including sample size of experimental and control groups, mean age, gender, severity of depression, clinical setting, percentage of antidepressant use of exercise group, exercise frequency, exercise intensity, session time of exercise, duration of exercise, cognitive function domains and tasks, mean values and standard deviations of post-test outcome. If raw data were unavailable or inaccessible in the included studies, FFR would contact the authors. When an included study presented several relevant outcome measures on the same cognitive sub-domain, all of them were included.
The cognitive sub-domains were categorized according to their features and previous meta-analysis (Northey et al., 2018; Sanders et al., 2019), these sub-domains included attention, processing speed, memory, and executive function (which together comprise overall cognitive function). The characteristics of aerobic exercise were coded: (1) intensity (moderate only, and moderate-to-vigorous (mixed)); (2) frequency (2 times per week, and 3 times per week); (3) session time (< 45 min, 45–60 min, and > 60 min) (Chen et al., 2020); (4) duration (≤ 12 weeks, and > 12 weeks). Study and sample characteristics were coded: (1) comparator/control group (active control group, placebo pill, and treatment as usual care), (2) exercise plus antidepressant (NO: participants of aerobic exercise group did not use antidepressant, YES: more than 95 % participants of aerobic exercise group used antidepressant, and MIXED: some participants of aerobic exercise group used antidepressant); (3) proportion of female (> 50 %, ≤ 50 %, and unclear); (4) mean age (young adults (18-44 years), middle adults (45-64 years), old adults (≥ 65 years), and unclear); (5) clinical setting (inpatients, and outpatients (Krogh et al., 2017; Lee et al., 2021).
Assessment of study qualityTwo authors (WSZ and FFR) independently assessed the study quality of all included studies using PEDro scale (Maher et al., 2003). Study quality was ranked as high (excellent: scoring 9–10 points), (good: scoring 6–8 points), moderate (scoring 4-5 points), and poor (scoring 0–3 points). If the two authors disagreed, the third author (YKC) was engaged to reach a consensus.
Statistical analysisThis statistical analysis was conducted using R software (R Core Team, 2013; Viechtbauer, 2010). To manage the dependency of effect sizes within studies, a three-level meta-analysis with a random-effects model was performed. This model considers the following three categories of effect size (ES) variability: sampling variance (level 1); within-study variance (level 2); and between-study variance (level 3) (Cheung, 2014). Standardized mean difference and its variance were calculated based on post-test means, standard deviations, and sample sizes of the intervention and comparator/control groups. With the use of restricted maximum-likelihood estimation (REML) method, pooled ES of aerobic exercise on cognitive function was determined using Hedges’ g and 95 % confidence intervals (CIs). A positive Hedges’ g indicated that aerobic exercise had a favorable effect for cognitive function compared to the comparator/control group. The Hedges’ g values were classified as follows: g = 0.20 (small), g = 0.20 to 0.49 (small-to- medium), g = 0.5 to 0.79 (medium), and g ≥ 0.80 (large) (Cohen, 1992). Testing for statistical significance in level 2 (within-study) and level 3 (between-study) variances were performed using likelihood ratio tests (LRT). 95 % prediction intervals (PIs) were calculated to determine the expected range in which an ES in future identical studies will fall. Statistical significance was defined as a p-value < 0.05.The I2 statistic was used to estimate of total between-study heterogeneity (1–49 % indicating low, 50–74 % indicating moderate, and 75–100 % indicating high) (Higgins et al., 2003). Funnel plots and multilevel Egger's regression tests were used to examine the possibility of publication bias (Egger et al., 1997). Sensitivity analyses were conducted to investigate if outliers and influential studies affect pooled ES (Viechtbauer & Cheung, 2010).
The steps comprising the three-level meta-analysis were: (1) all cognitive tests were utilized to calculate the effect of aerobic exercise on overall cognitive function in adults with MDD; and (2) moderator analyses were performed to explore potential moderators of the observed effects. The subgroup analyses included cognitive sub-domains (attention, processing speed, memory, and executive function), exercise intensity, frequency, session time, duration, comparator/control group, exercise plus antidepressant or not, proportion of female, mean age, and clinical setting. The meta-regression analyses included the proportion of female, mean age, session time of exercise (per time), duration of exercise, volume of exercise per week, total intervention time, and sample size.
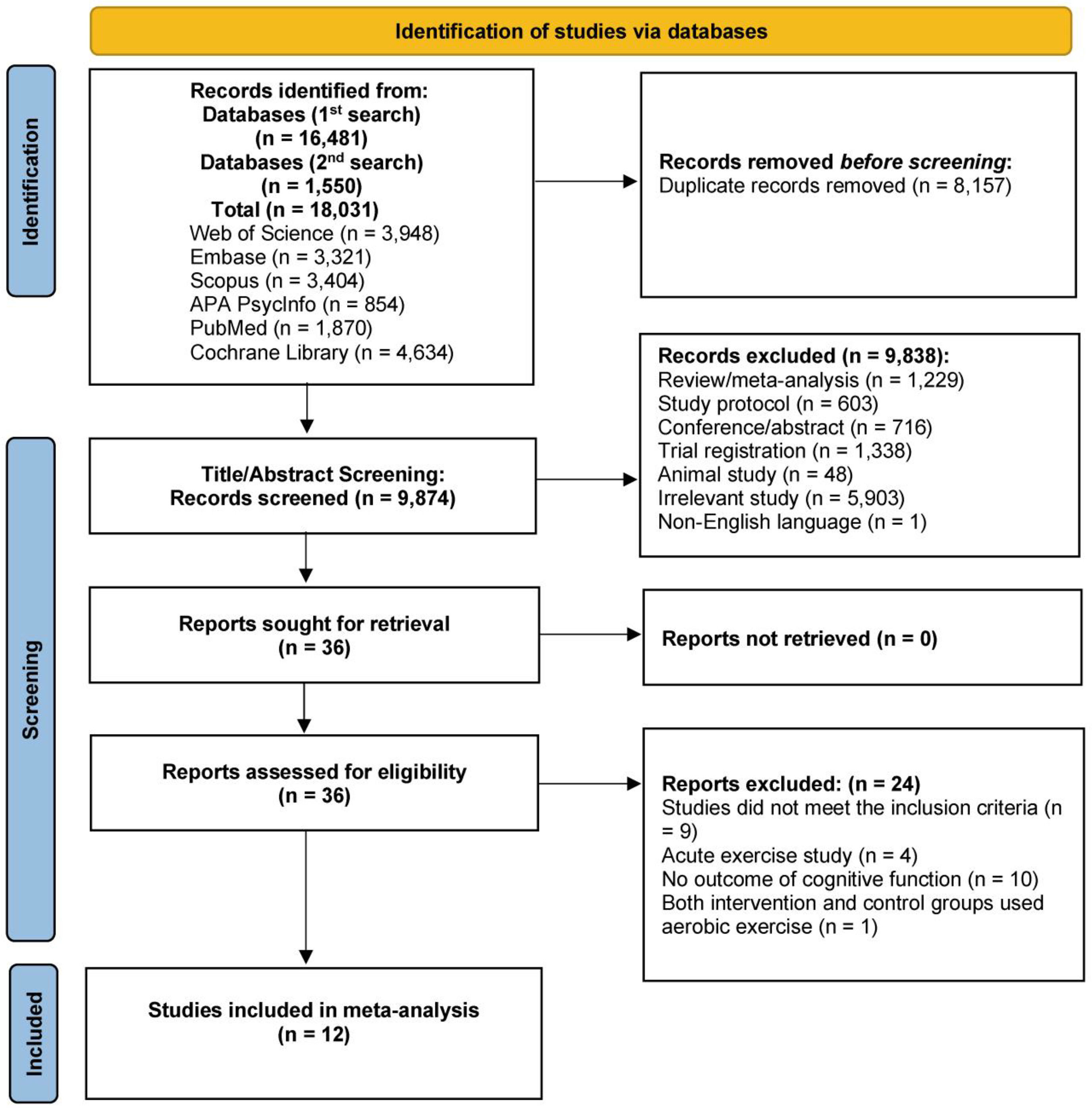
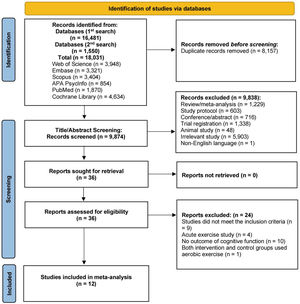
ResultsFig. 1 depicts the literature search and article selection procedure. The literature search yielded 18,031 records. Following the removal of duplicates (k = 8,157), 9,874 studies met the criteria for title and abstract screening. Subsequently, 9,838 studies were excluded. The next step included reading the full text of the remaining 36 studies. Following this step, 12 studies met the inclusion criteria for this analysis. The first author of one article (Brush et al., 2020) provided more detailed data via direct email request.
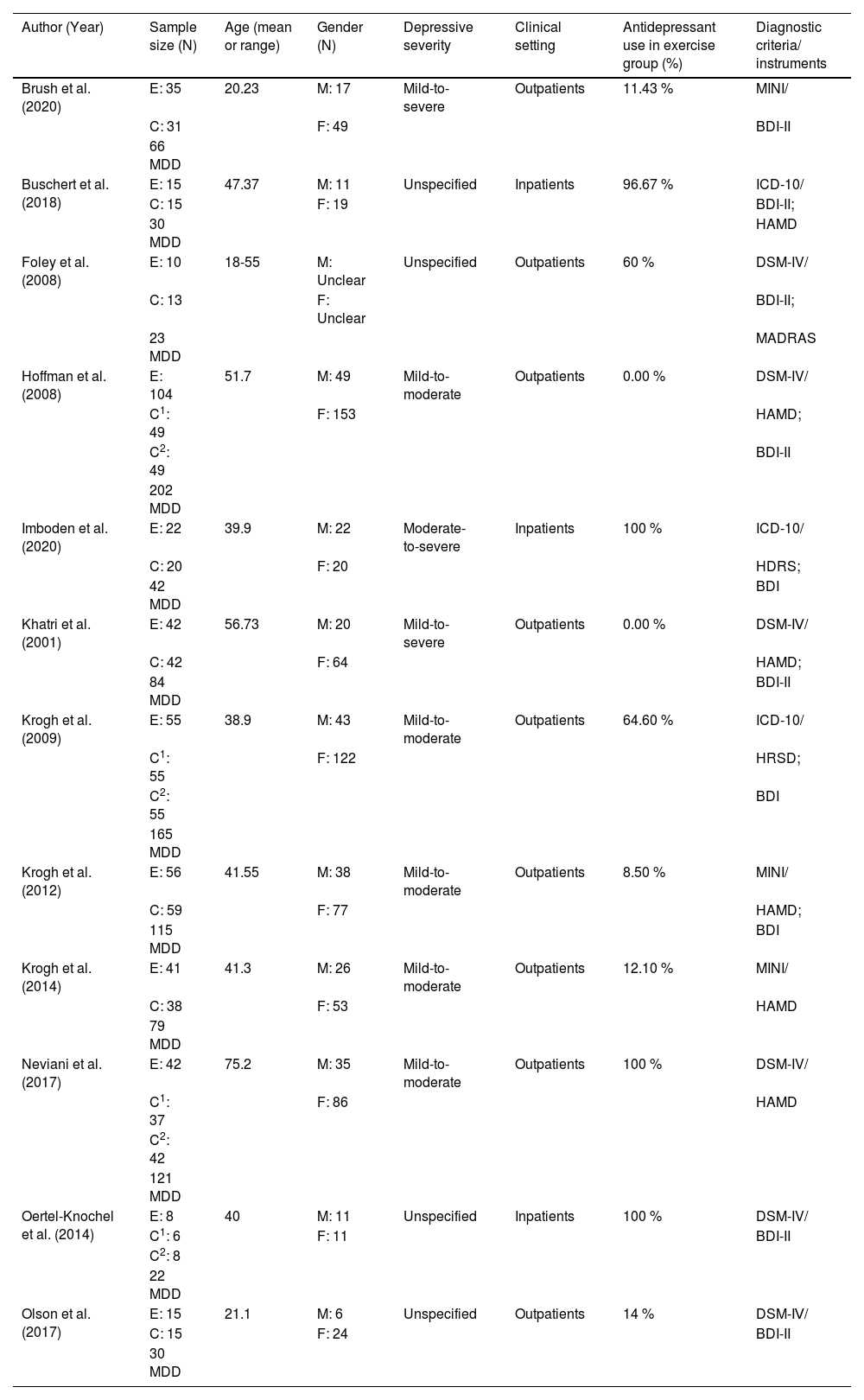
Tables 1 and 2 provide the study characteristics of the 12 included randomized trials published between 2001 and 2023 (Brush et al., 2020; Buschert et al., 2018; Foley et al., 2008; Hoffman et al., 2008; Imboden et al., 2020; Khatri et al., 2001; Krogh et al., 2014; Krogh et al., 2009; Krogh et al., 2012; Neviani et al., 2017; Oertel-Knöchel et al., 2014; Olson et al., 2017). In total, 979 participants (678 females) with MDD (mean age from 20.23 to 75.20 years) are included in this review, and the number of female participants ranged from 11 to 153. However, 945 participants completed posttest and are thus included in the meta-analysis, with 437 in aerobic exercise group and 508 in comparator/control group. Depressive severity of participants ranged from mild-to-moderate (five studies), mild-to-severe (two studies), moderate-to-severe (one study), and unspecified (four studies). Six studies had 8.50 % to 64.60 % participants, four studies had 96.67 % to 100 % participants using antidepressant medications in the exercise group, and two studies did not use antidepressant medications in the exercise group. There were 9 studies that examined outpatients. Within the 12 included studies, diagnostic criteria for MDD were based on the DSM-IV in six studies, ICD-10 in three studies, and MINI in three studies. The scores of baseline depression were measured using the Beck Depression Inventory (BDI) or Beck Depression Inventory-II (BDI-II) in 10 studies, the Hamilton Depression Rating Scale (HAMD or HDRS or HRSD) in 8 studies, and the Montgomery-Åsberg Depression Rating Scale (MADRAS) in one study. Regarding the comparator/control groups, ten studies used active comparators (e.g., stretching, occupational or art therapy, relaxation, and non-aerobic progressive physical activity such as flexibility, resistance training, and balance training), four studies used treatment as usual care (antidepressants, including one study that used antidepressants while serving as a waiting-list control), and one study used a placebo medication group. The frequency of aerobic exercise ranged from 2 to 3 times per week, and each session time lasted from 30 to 90 min. Five studies utilized moderate only intensity, while another seven studies used moderate-to-vigorous (mixed) intensity exercise. Duration of the aerobic exercise intervention varied from 3 to 24 weeks. As for the cognitive sub-domains, six studies assessed processing speed, eight studies assessed memory, six studies assessed attention, and nine studies assessed executive function.
Overview of participants’ characteristics of included studies (k = 12).
| Author (Year) | Sample size (N) | Age (mean or range) | Gender (N) | Depressive severity | Clinical setting | Antidepressant use in exercise group (%) | Diagnostic criteria/ instruments |
|---|---|---|---|---|---|---|---|
| Brush et al. (2020) | E: 35 | 20.23 | M: 17 | Mild-to-severe | Outpatients | 11.43 % | MINI/ |
| C: 31 | F: 49 | BDI-II | |||||
| 66 MDD | |||||||
| Buschert et al. (2018) | E: 15 | 47.37 | M: 11 | Unspecified | Inpatients | 96.67 % | ICD-10/ |
| C: 15 | F: 19 | BDI-II; | |||||
| 30 MDD | HAMD | ||||||
| Foley et al. (2008) | E: 10 | 18-55 | M: Unclear | Unspecified | Outpatients | 60 % | DSM-IV/ |
| C: 13 | F: Unclear | BDI-II; | |||||
| 23 MDD | MADRAS | ||||||
| Hoffman et al. (2008) | E: 104 | 51.7 | M: 49 | Mild-to-moderate | Outpatients | 0.00 % | DSM-IV/ |
| C1: 49 | F: 153 | HAMD; | |||||
| C2: 49 | BDI-II | ||||||
| 202 MDD | |||||||
| Imboden et al. (2020) | E: 22 | 39.9 | M: 22 | Moderate-to-severe | Inpatients | 100 % | ICD-10/ |
| C: 20 | F: 20 | HDRS; | |||||
| 42 MDD | BDI | ||||||
| Khatri et al. (2001) | E: 42 | 56.73 | M: 20 | Mild-to-severe | Outpatients | 0.00 % | DSM-Ⅳ/ |
| C: 42 | F: 64 | HAMD; | |||||
| 84 MDD | BDI-II | ||||||
| Krogh et al. (2009) | E: 55 | 38.9 | M: 43 | Mild-to-moderate | Outpatients | 64.60 % | ICD-10/ |
| C1: 55 | F: 122 | HRSD; | |||||
| C2: 55 | BDI | ||||||
| 165 MDD | |||||||
| Krogh et al. (2012) | E: 56 | 41.55 | M: 38 | Mild-to-moderate | Outpatients | 8.50 % | MINI/ |
| C: 59 | F: 77 | HAMD; | |||||
| 115 MDD | BDI | ||||||
| Krogh et al. (2014) | E: 41 | 41.3 | M: 26 | Mild-to-moderate | Outpatients | 12.10 % | MINI/ |
| C: 38 | F: 53 | HAMD | |||||
| 79 MDD | |||||||
| Neviani et al. (2017) | E: 42 | 75.2 | M: 35 | Mild-to-moderate | Outpatients | 100 % | DSM-IV/ |
| C1: 37 | F: 86 | HAMD | |||||
| C2: 42 | |||||||
| 121 MDD | |||||||
| Oertel-Knochel et al. (2014) | E: 8 | 40 | M: 11 | Unspecified | Inpatients | 100 % | DSM-IV/ |
| C1: 6 | F: 11 | BDI-II | |||||
| C2: 8 | |||||||
| 22 MDD | |||||||
| Olson et al. (2017) | E: 15 | 21.1 | M: 6 | Unspecified | Outpatients | 14 % | DSM-Ⅳ/ |
| C: 15 | F: 24 | BDI-II | |||||
| 30 MDD |
Note. k = number of included studies; N = number; % = percentage; E = experimental group; C = control group; C1 = first control group; C2 = second control group; MDD = Major depressive disorder; M = male; F = female; MINI = The Mini-International neuropsychiatric interview; DSM-5 = The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; DSM‑Ⅳ = The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ICD-10 = International Classification of Diseases, Tenth Revision; BDI = Beck Depression Inventory; BDI-II = Beck Depression Inventory-II; MADRAS = the Montgomery-Åsberg Depression Rating Scale; HAMD/HDRS/HRSD = Hamilton Depression Rating Scale; Diagnostic criteria/instruments = Diagnostic criteria and depression scales.
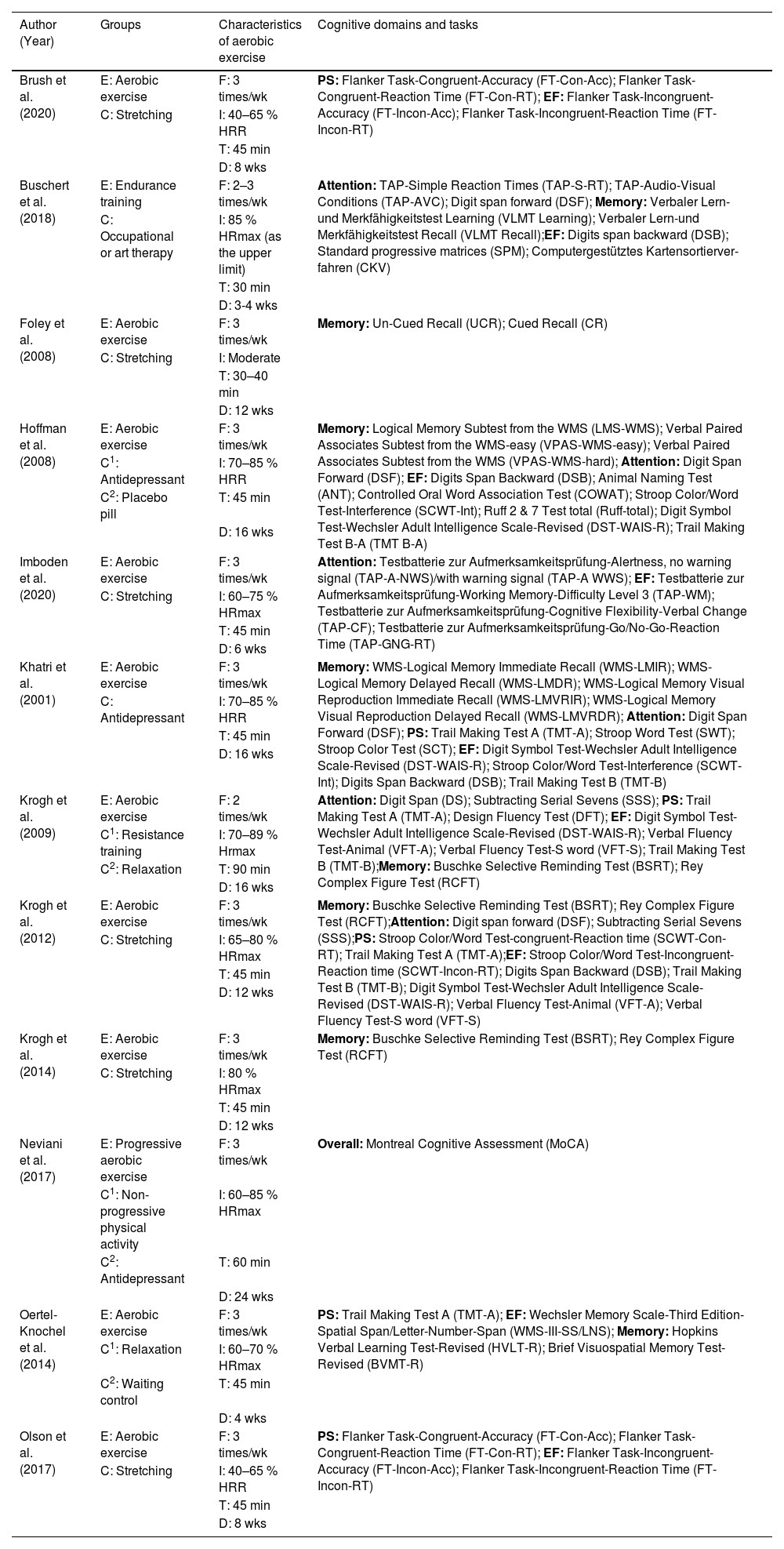
Overview of groups, characteristics of aerobic exercise and cognitive domains and tasks of included studies (k = 12).
| Author (Year) | Groups | Characteristics of aerobic exercise | Cognitive domains and tasks |
|---|---|---|---|
| Brush et al. (2020) | E: Aerobic exercise | F: 3 times/wk | PS: Flanker Task-Congruent-Accuracy (FT-Con-Acc); Flanker Task-Congruent-Reaction Time (FT-Con-RT); EF: Flanker Task-Incongruent-Accuracy (FT-Incon-Acc); Flanker Task-Incongruent-Reaction Time (FT-Incon-RT) |
| C: Stretching | I: 40–65 % HRR | ||
| T: 45 min | |||
| D: 8 wks | |||
| Buschert et al. (2018) | E: Endurance training | F: 2–3 times/wk | Attention: TAP-Simple Reaction Times (TAP-S-RT); TAP-Audio-Visual Conditions (TAP-AVC); Digit span forward (DSF); Memory: Verbaler Lern-und Merkfähigkeitstest Learning (VLMT Learning); Verbaler Lern-und Merkfähigkeitstest Recall (VLMT Recall);EF: Digits span backward (DSB); Standard progressive matrices (SPM); Computergestütztes Kartensortierver-fahren (CKV) |
| C: Occupational or art therapy | I: 85 % HRmax (as the upper limit) | ||
| T: 30 min | |||
| D: 3-4 wks | |||
| Foley et al. (2008) | E: Aerobic exercise | F: 3 times/wk | Memory: Un-Cued Recall (UCR); Cued Recall (CR) |
| C: Stretching | I: Moderate | ||
| T: 30–40 min | |||
| D: 12 wks | |||
| Hoffman et al. (2008) | E: Aerobic exercise | F: 3 times/wk | Memory: Logical Memory Subtest from the WMS (LMS-WMS); Verbal Paired Associates Subtest from the WMS-easy (VPAS-WMS-easy); Verbal Paired Associates Subtest from the WMS (VPAS-WMS-hard); Attention: Digit Span Forward (DSF); EF: Digits Span Backward (DSB); Animal Naming Test (ANT); Controlled Oral Word Association Test (COWAT); Stroop Color/Word Test-Interference (SCWT-Int); Ruff 2 & 7 Test total (Ruff-total); Digit Symbol Test-Wechsler Adult Intelligence Scale-Revised (DST-WAIS-R); Trail Making Test B-A (TMT B-A) |
| C1: Antidepressant | I: 70–85 % HRR | ||
| C2: Placebo pill | T: 45 min | ||
| D: 16 wks | |||
| Imboden et al. (2020) | E: Aerobic exercise | F: 3 times/wk | Attention: Testbatterie zur Aufmerksamkeitsprüfung-Alertness, no warning signal (TAP-A-NWS)/with warning signal (TAP-A WWS); EF: Testbatterie zur Aufmerksamkeitsprüfung-Working Memory-Difficulty Level 3 (TAP-WM); Testbatterie zur Aufmerksamkeitsprüfung-Cognitive Flexibility-Verbal Change (TAP-CF); Testbatterie zur Aufmerksamkeitsprüfung-Go/No-Go-Reaction Time (TAP-GNG-RT) |
| C: Stretching | I: 60–75 % HRmax | ||
| T: 45 min | |||
| D: 6 wks | |||
| Khatri et al. (2001) | E: Aerobic exercise | F: 3 times/wk | Memory: WMS-Logical Memory Immediate Recall (WMS-LMIR); WMS-Logical Memory Delayed Recall (WMS-LMDR); WMS-Logical Memory Visual Reproduction Immediate Recall (WMS-LMVRIR); WMS-Logical Memory Visual Reproduction Delayed Recall (WMS-LMVRDR); Attention: Digit Span Forward (DSF); PS: Trail Making Test A (TMT-A); Stroop Word Test (SWT); Stroop Color Test (SCT); EF: Digit Symbol Test-Wechsler Adult Intelligence Scale-Revised (DST-WAIS-R); Stroop Color/Word Test-Interference (SCWT-Int); Digits Span Backward (DSB); Trail Making Test B (TMT-B) |
| C: Antidepressant | I: 70–85 % HRR | ||
| T: 45 min | |||
| D: 16 wks | |||
| Krogh et al. (2009) | E: Aerobic exercise | F: 2 times/wk | Attention: Digit Span (DS); Subtracting Serial Sevens (SSS); PS: Trail Making Test A (TMT-A); Design Fluency Test (DFT); EF: Digit Symbol Test-Wechsler Adult Intelligence Scale-Revised (DST-WAIS-R); Verbal Fluency Test-Animal (VFT-A); Verbal Fluency Test-S word (VFT-S); Trail Making Test B (TMT-B);Memory: Buschke Selective Reminding Test (BSRT); Rey Complex Figure Test (RCFT) |
| C1: Resistance training | I: 70–89 % Hrmax | ||
| C2: Relaxation | T: 90 min | ||
| D: 16 wks | |||
| Krogh et al. (2012) | E: Aerobic exercise | F: 3 times/wk | Memory: Buschke Selective Reminding Test (BSRT); Rey Complex Figure Test (RCFT);Attention: Digit span forward (DSF); Subtracting Serial Sevens (SSS);PS: Stroop Color/Word Test-congruent-Reaction time (SCWT-Con-RT); Trail Making Test A (TMT-A);EF: Stroop Color/Word Test-Incongruent-Reaction time (SCWT-Incon-RT); Digits Span Backward (DSB); Trail Making Test B (TMT-B); Digit Symbol Test-Wechsler Adult Intelligence Scale-Revised (DST-WAIS-R); Verbal Fluency Test-Animal (VFT-A); Verbal Fluency Test-S word (VFT-S) |
| C: Stretching | I: 65–80 % HRmax | ||
| T: 45 min | |||
| D: 12 wks | |||
| Krogh et al. (2014) | E: Aerobic exercise | F: 3 times/wk | Memory: Buschke Selective Reminding Test (BSRT); Rey Complex Figure Test (RCFT) |
| C: Stretching | I: 80 % HRmax | ||
| T: 45 min | |||
| D: 12 wks | |||
| Neviani et al. (2017) | E: Progressive aerobic exercise | F: 3 times/wk | Overall: Montreal Cognitive Assessment (MoCA) |
| C1: Non-progressive physical activity | I: 60–85 % HRmax | ||
| C2: Antidepressant | T: 60 min | ||
| D: 24 wks | |||
| Oertel-Knochel et al. (2014) | E: Aerobic exercise | F: 3 times/wk | PS: Trail Making Test A (TMT-A); EF: Wechsler Memory Scale-Third Edition-Spatial Span/Letter-Number-Span (WMS-III-SS/LNS); Memory: Hopkins Verbal Learning Test-Revised (HVLT-R); Brief Visuospatial Memory Test-Revised (BVMT-R) |
| C1: Relaxation | I: 60–70 % HRmax | ||
| C2: Waiting control | T: 45 min | ||
| D: 4 wks | |||
| Olson et al. (2017) | E: Aerobic exercise | F: 3 times/wk | PS: Flanker Task-Congruent-Accuracy (FT-Con-Acc); Flanker Task-Congruent-Reaction Time (FT-Con-RT); EF: Flanker Task-Incongruent-Accuracy (FT-Incon-Acc); Flanker Task-Incongruent-Reaction Time (FT-Incon-RT) |
| C: Stretching | I: 40–65 % HRR | ||
| T: 45 min | |||
| D: 8 wks |
Note. k = number of included studies; E = experimental group; C = control group; C1 = first control group; C2 = second control group; F = frequency; I = intensity; T = time; D = duration; /wk = per week; % = percentage; HRR = Heart rate reserve; HRmax = maximum heart rate; min = minutes; wks = weeks; PS = processing speed; EF = executive function
The results of the PEDro scale evaluation of 12 randomized studies revealed that two studies received 4–5 points, indicating moderate quality, while ten scored 6–8 points, indicating high quality. The included studies averaged 6.2 (see Supplementary Table 2).
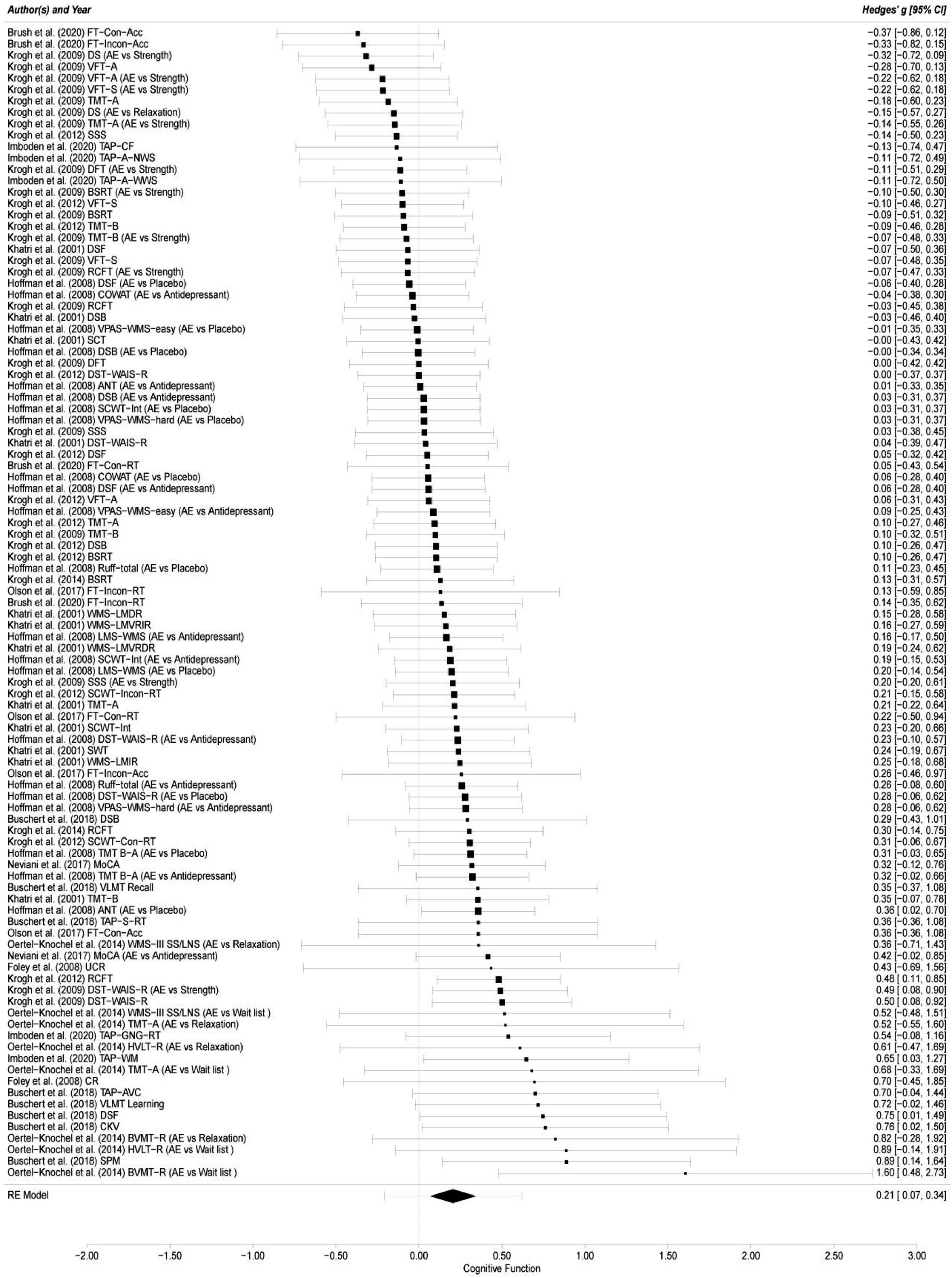
Overall cognitive functionThe results of this meta-analysis from the 12 included studies with 101 effect sizes indicated that the effect of aerobic exercise on overall cognitive function of adults with MDD was significant (g = 0.21; 95 % CI = 0.07, 0.34; p = 0.003) compared with the comparator/control group (see Fig. 2). The 95 % prediction intervals (PIs) suggested that a future ES will fall between -0.21 and 0.62 (Hedges’ g). The likelihood ratio tests indicated that the influence of within-study variance was not statistically significant (LRT = 0; p > 0.05), while the effect of between-study variance was significant (LRT = 16.03; p < 0.001), explaining 44.74 % of the variance in level 3. Statistical heterogeneity in the pooled ES was low (I2 = 44.74 %).
Forest plot of aerobic exercise on cognitive function.
FT-Con-Acc = Flanker Task-Congruent-Accuracy; FT-Incon-Acc = Flanker Task-Incongruent-Accuracy; DS = Digit Span; AE = aerobic exercise; vs.= versus.; VFT-A = Verbal Fluency Test-Animal; VFT-S = Verbal Fluency Test-S word; TMT-A = Trail Making Test A; SSS = Subtracting Serial Sevens; TAP-CF = Testbatterie zur Aufmerksamkeitsprüfung-Cognitive Flexibility; TAP-A-NWS = Testbatterie zur Aufmerksamkeitsprüfung-Alertness, no warning signal; DFT = Design Fluency Test; TAP-A-WWS = Testbatterie zur Aufmerksamkeitsprüfung-Alertness, with warning signal; BSRT = Buschke Selective Reminding Test; TMT-B = Trail Making Test B; DSF = Digit Span Forward; RCFT = Rey Complex Figure Test; COWAT = Controlled Oral Word Association Test; DSB = Digits Span Backward; VPAS-WMS-easy = Verbal Paired Associates Subtest from the WMS-easy; SCT = Stroop Color Test; DST-WAIS-R = Digit Symbol Test-Wechsler Adult Intelligence Scale-Revised; ANT = Animal Naming Test; SCWT-Int = Stroop Color/Word Test-Interference; VPAS-WMS-hard = Verbal Paired Associates Subtest from the WMS; FT-Con-RT = Flanker Task-Congruent-Reaction Time; FT-Incon-RT = Flanker Task-Incongruent-Reaction Time; WMS-LMDR = WMS-Logical Memory Delayed Recall; WMS-LMVRIR = WMS-Logical Memory Visual Reproduction Immediate Recall; LMS-WMS = Logical Memory Subtest from the WMS; WMS-LMVRDR = WMS-Logical Memory Visual Reproduction Delayed Recall; SCWT-Incon-RT = Stroop Color/Word Test-Incongruent-Reaction time; SWT = Stroop Word Test; WMS-LMIR = WMS-Logical Memory Immediate Recall; SCWT-Con-RT = Stroop Color/Word Test-congruent-Reaction time; TMT B-A = Trail Making Test B-A; MoCA = Montreal Cognitive Assessment; VLMT Recall = Verbaler Lern-und Merkfähigkeitstest Recall; TAP-S-RT = Testbatterie zur Aufmerksamkeitsprüfung-Simple Reaction Times; WMS-III-SS/LNS = Wechsler Memory Scale-Third Edition-Spatial Span/Letter-Number-Span; UCR = Un-Cued Recall; TAP-GNG-RT = Testbatterie zur Aufmerksamkeitsprüfung-Go/No-Go-Reaction Time; HVLT-R = Hopkins Verbal Learning Test-Revised; TAP-WM = Testbatterie zur Aufmerksamkeitsprüfung-Working Memory-Difficulty Level 3; CR = Cued Recall; TAP-AVC = Testbatterie zur Aufmerksamkeitsprüfung-Audio-Visual Conditions; VLMT Learning = Verbaler Lern-und Merkfähigkeitstest Learning; CKV = Computergestütztes Kartensortierver-fahren; BVMT-R = Brief Visuospatial Memory Test-Revised; SPM = Standard progressive matrices; RE Model = random-effects model.
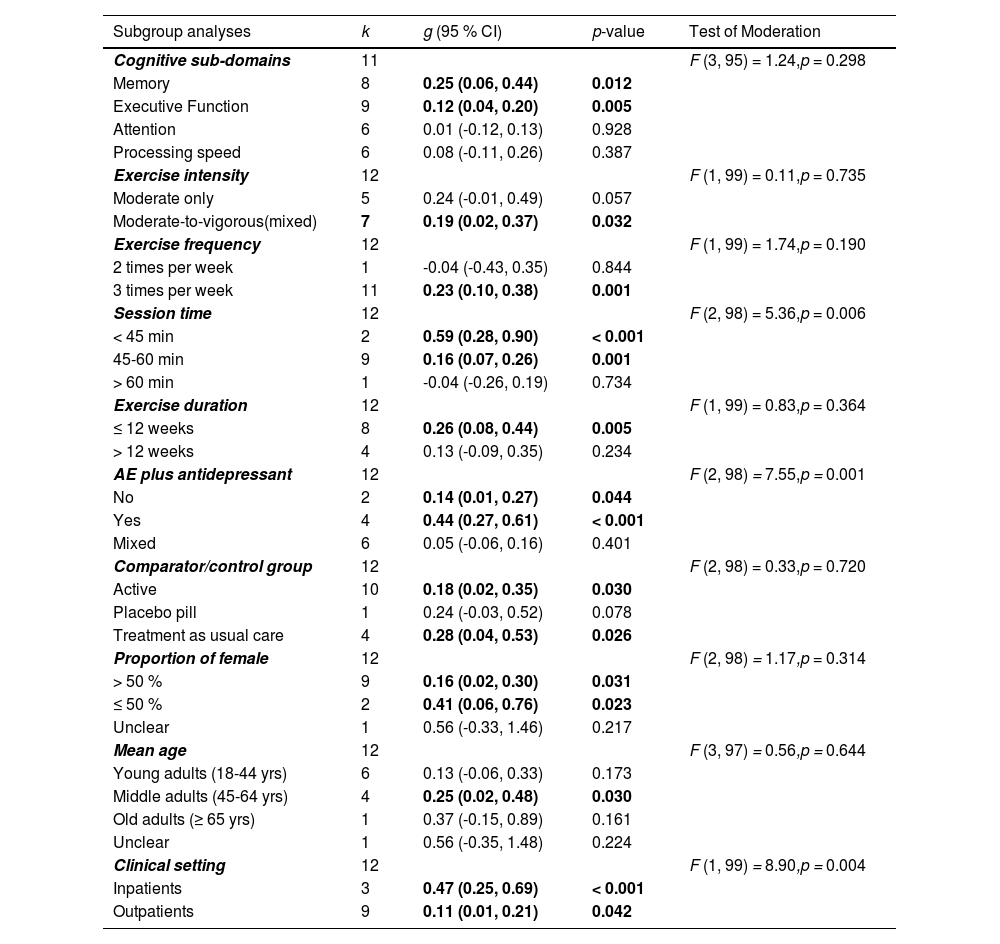
Table 3 summarized the results of all subgroup analyses. The test for moderators indicated that there were no statistically significant subgroup differences in ES for cognitive sub-domains (F = 1.24, p = 0.298). The results indicated that aerobic exercise only had statistically significant effects on memory (g = 0.25; 95 % CI = 0.06, 0.44; p = 0.012; 95 % PIs = -0.20, 0.69) and executive function (g = 0.12; 95 % CI = 0.04, 0.20; p = 0.005; 95 % PIs = -0.02, 0.26) of adults with MDD compared with a comparator/control group. However, there were no significant effects on attention (g = 0.01; 95 % CI = -0.12, 0.13; p = 0.928; 95 % PIs = -0.12, 0.13) and processing speed (g = 0.08; 95 % CI = -0.11, 0.26; p = 0.387; 95 % PIs = -0.25, 0.40) following aerobic exercise.
Categorical moderator analyses of aerobic exercise on cognition.
Note. k = number of included studies; g = Hedges’ g; CI = confidence interval, AE = aerobic exercise; yrs = years
The results of the test of moderation for aerobic exercise characteristics revealed that there were no statistically significant subgroup differences in ES for exercise intensity (F = 0.11, p = 0.735), exercise frequency (F = 1.74, p = 0.190), and exercise duration (F = 0.83, p = 0.364), but there was a statistically significant subgroup differences in ES for session time (F = 5.36, p = 0.006). Studies with session time < 45 min yielded a larger effect (g = 0.59; 95 % CI = 0.28, 0.90; p < 0.001) than session time between 45–60 min (g = 0.16; 95 % CI = 0.07, 0.26; p = 0.001), and session time > 60 min (g = -0.04; 95 % CI = -0.26, 0.19; p = 0.734) on cognitive function.
Study design and sample characteristicsThe results of the test of moderation for study design and sample characteristics indicated that there were no statistically significant subgroup differences in ES for comparator/control group (F = 0.33, p = 0.720), proportion of females in the study sample (F = 1.17, p = 0.314), and mean age (F = 0.56, p = 0.644); however, there were statistically significant subgroup differences in ES for aerobic exercise plus antidepressant use (F = 7.55, p = 0.001), and clinical setting (F = 8.90, p = 0.004). The findings of aerobic exercise plus antidepressant use indicated that the ES was larger for YES (which represents participants of aerobic exercise group used antidepressant) (g = 0.44; 95 % CI = 0.27, 0.61; p < 0.001) than ESs for NO (which represents participants of aerobic exercise group did not use antidepressant) (g = 0.14; 95 % CI = 0.01, 0.27; p = 0.044) and MIXED (which represents some participants of aerobic exercise group used antidepressant) (g = 0.05; 95 % CI = -0.06, 0.16; p = 0.401) when compared with a comparator/control group. For clinical setting, the results indicated that aerobic exercise produced a larger effect on cognitive function for inpatients (g = 0.47; 95 % CI = 0.25, 0.69; p < 0.001) than outpatients (g = 0.11; 95 % CI = 0.01, 0.21; p = 0.042).
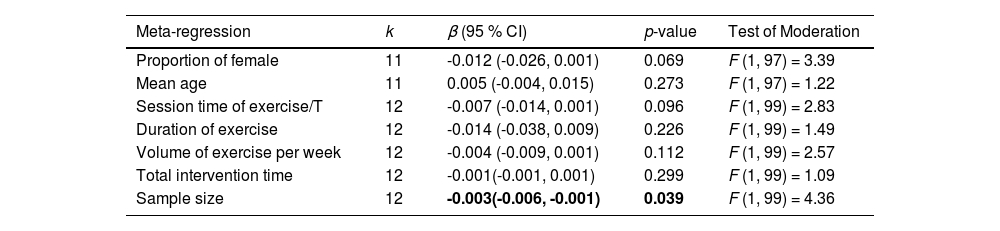
Table 4 summarizes the results of all meta-regressions analyses. The results suggested that the influence of aerobic exercise on cognitive function was significantly moderated by the sample size of included studies (β = -0.003; 95 % CI = -0.006, -0.001; p = 0.039), but not significantly moderated by proportion of female (β = -0.012; 95 % CI = -0.026, 0.001; p = 0.069), mean age (β = 0.005; 95 % CI = -0.004, 0.015; p = 0.273), session time of exercise per time (β = -0.007; 95 % CI = -0.014, 0.001; p = 0.096), duration of exercise (β = -0.014; 95 % CI = -0.038, 0.009; p = 0.226), volume of exercise per week (β = -0.004; 95 % CI = -0.009, 0.001; p = 0.112), and total intervention time (β = -0.001; 95 % CI = -0.001, 0.001; p = 0.299).
Continuous moderator analyses of aerobic exercise on cognition.
Note. k = number of included studies; β = regression coefficient; CI = confidence interval; /T = per time.
In one randomized trial (Oertel-Knöchel et al., 2014), the residual for one ES (Oertel-Knochel et al. used the brief visuospatial memory test-revised) was more than three standard deviations from the mean. Removing this outlier reduced the overall ES of aerobic exercise on cognitive function by 0.02 (g = 0.19; 95 % CI = 0.07, 0.32; p = 0.003). In total, thirteen effect sizes had a Cook's distance more than three standard deviations from the mean. These ES's included Brush et al. (2020) FT-Con-ACC; Buschert et al. (2018) SPM and CKV; Imboden et al.(2020) TAP-A-NWS, TAP-A-WWS, TAP-WM, TAP-CF, and TAP-GNG-RT; Oertel-Knochel et al. (2014) HVLT-R, and BVMT-R; Neviani et al. (2017) MoCA; Foley et al. (2008) CR; Krogh et al. (2014) BSRT (Brush et al., 2020; Buschert et al., 2018; Foley et al., 2008; Imboden et al., 2020; Krogh et al., 2014; Neviani et al., 2017; Oertel-Knöchel et al., 2014), see Table 2 for the full names of the task abbreviations. After removing those outliers, the overall ES of aerobic exercise on cognitive function decreased by 0.05 and remained significant (g = 0.16; 95 % CI = 0.05, 0.28; p = 0.005).
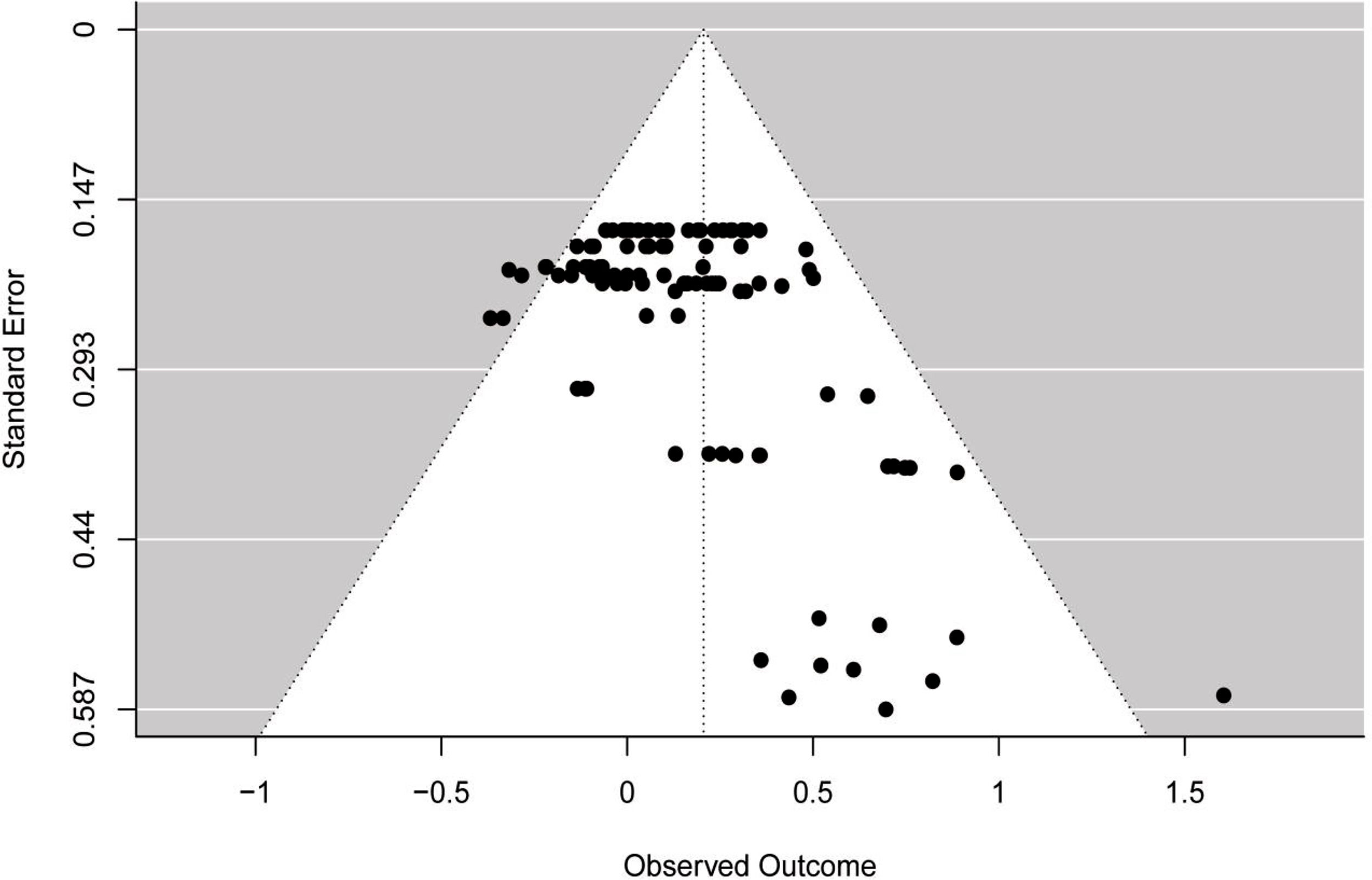
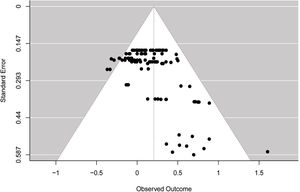
Publication biasThe funnel plot (see Fig. 3) showed asymmetry across ES, which was confirmed by the multilevel extension of Egger's test (F = 13.36; p = 0.0004). The findings suggested a possibility of publication bias in the included studies.
DiscussionThis meta-analytic review investigated evidence strictly from randomized controlled trials on the effects of aerobic exercise on cognitive function in adults with MDD. Twelve randomized trials were included, and the key results revealed that aerobic exercise significantly improved overall cognitive function, and its sub-domains of memory and executive function in adults with MDD when compared with a comparator/control group. In addition, the effects of aerobic exercise on overall cognitive function were moderated by several factors including session time, aerobic exercise plus antidepressant use, clinical setting, and sample size. These results may contribute to the development of non-pharmacological treatments to enhance cognitive function in adults with MDD.
Overall cognitive functionOur findings suggested a small to moderate effect on overall cognitive function in adults with MDD following aerobic exercise. However, this result was not consistent with prior meta-analyses (Brondino et al., 2017; Sun et al., 2018), which find nonsignificant effects of physical exercise on cognitive function in depressed adults. Such disparity is not unexpected as the current meta-analysis deviated from previous ones in several meaningful ways. For example, our review focused on aerobic exercise, while the two previous reviews included aerobic, non-aerobic, and mind-body exercises, underscoring potential disparate outcomes when contrasting aerobic exercise with alternative modes of physical activity. As such, our review is the first to confirm that aerobic exercise is effective for improving cognitive function in adults with MDD. Researchers, clinicians, and health professionals can contemplate aerobic exercise as a viable alternative or adjunctive therapy to augment cognitive function in individuals with MDD. Additionally, our finding that aerobic exercise in conjunction with anti-depressant therapy makes it especially apparent that aerobic exercise may not only be complimentary to standard therapies but raises the possibility that it may also be synergistic. Future research will need to unpack that question. Interesting, our finding is also consonant with previous meta-analyses that found aerobic exercise had significant improvements on cognitive function in other mental health disorders such as schizophrenia (Shimada et al., 2022) and dementia (Balbim et al., 2022), as well as in healthy individuals including adults older than 50 years (Northey et al., 2018).
Cognitive sub-domainsOur meta-analysis yielded a small-to-moderate ES for memory, which is in line with a previous meta-analysis (Brondino et al., 2017) that found physical exercise had a significant effect on visual learning and memory in adults with depression. However, our finding contradicts another prior meta-analysis (Sun et al., 2018), which found physical exercise had no significant benefit on verbal/visual learning and memory in individuals with depression. These conflicting results may be due to differences in inclusion criteria (i.e., severity of depression/depression symptoms), as well as the type of exercise intervention, as the previous meta-analysis included aerobic, resistance, and mind-body exercise, and our meta-analysis specifically focused on aerobic exercise. Further, the previous meta-analysis analyzed verbal learning, visual learning, and memory separately. However, we performed an overall analysis of memory, and found that aerobic exercise had a small-to-moderate ES in adults with MDD.
Regarding executive function, the present findings confirmed the results from a recent meta-analysis (Ren, Alderman, et al., 2023), which observed that aerobic exercise significantly enhanced executive function in depressed adults. Notably, the ES in the present meta-analysis was smaller than that reported by (Ren, Alderman, et al., 2023). Inclusion criteria and the number of included studies may have contributed to the discrepancy in results. The previous meta-analysis (Ren, Alderman, et al., 2023) included both English and Chinese language published studies (k =10); however, the current study only included English language published studies (k = 9). Importantly, these results support aerobic exercise as a promising intervention that has positive benefits for executive function in adults with MDD.
Unfortunately, no significant effects were observed for attention or processing speed in this review. Prior meta-analyses (Brondino et al., 2017; Sun et al., 2018) indicated that physical exercise had no significant effect on attention and processing speed in people with depression, similar to our results. In addition, our results are in agreement with a previous meta-analysis, which found that aerobic exercise had non-significant effects on attention and processing speed in participants with schizophrenia (Xu et al., 2022). Furthermore, a non-significant ES on processing speed in participants with ischemic cerebrovascular disorder after aerobic exercise has been observed (Shu et al., 2020). However, it should be noted that Smith et al. (2010) has found positive benefits for attention and processing speed in individuals without depression after aerobic exercise (Smith et al., 2010). Since only six randomized trials have investigated the effects of aerobic exercise on attention and processing speed in adults with MDD. Therefore, more studies are needed in the future to investigate the effects of aerobic exercise on these cognitive sub-domains in adults with MDD.
Aerobic exercise characteristicsExercise intervention characteristics, including intensity, frequency, session time, and duration, play significant roles in the cognitive benefits of aerobic exercise (Shimada et al., 2022). Our findings suggest that the optimal dose for the effects of aerobic exercise on cognitive function in adults with MDD are as follows: moderate-to-vigorous (mixed), frequency of 3 times per week, each session time < 45 min, and intervention duration of ≤ 12 weeks. However, these findings are from a small number of studies, and future work is needed to better understand how to optimize the exercise ‘dose’ to best improve MDD symptoms.
Regarding intensity, our results showed that moderate-to-vigorous (mixed) aerobic exercise induced a significant small-to-moderate ES on cognitive function in adults with MDD. However, moderate intensity aerobic exercise produced a favorable but non-significant ES on cognitive function. These findings are meaningful, since both moderate intensity and moderate-to-vigorous intensity exercise were analyzed as a whole in previous meta-analyses (Brondino et al., 2017; Ren, Alderman, et al., 2023; Sun et al., 2018). Accordingly, we separated them into two groups for analysis, and these results may provide a fresh perspective for formulating the aerobic exercise prescription. In addition, further investigation into moderate intensity aerobic exercise using well-designed randomized controlled trials is crucial to understanding the relationship of aerobic exercise as a treatment for adults with MDD.
As for frequency, session time, and duration, our meta-analysis found that aerobic exercise performed 3 times per week, for session time < 45 min and between 45–60 min, and for a duration of ≤ 12 weeks had significant benefits on cognitive function; however, aerobic exercise performed 2 times per week, for session time > 60 minute, and for a duration of > 12 weeks did not. Our results of frequency and session time align with a recent meta-analysis (Ren, Alderman, et al., 2023), which found that exercise training (resistance, mind-body and aerobic) conducted 3 times per week, with a session time ≤ 60 min resulted in significant effects on executive function in depressed adults. However, our results for duration contradict the results of the previous meta-analysis (Ren, Alderman, et al., 2023), which indicated that exercise training practiced with a duration of ≥ 13 weeks significantly promoted executive function in depressed adults, but 3-12 weeks did not. What cannot ignore the non-significant result of 2 times per week (k = 1), session time > 60 minute (k = 1), and durations > 12 weeks (k = 4) are clearly due to a lack of statistical power. Therefore, further research is needed to determine if aerobic exercise at less frequent, longer session time and duration can also lead to significant improvements in cognitive function in adults with MDD.
Study design and sample characteristicsThe present meta-analysis explored two study design characteristics (aerobic exercise plus antidepressant use, and comparator/control group), and three sample characteristics (proportion of females, mean age, and clinical setting) as moderators. The test of moderation indicated that the effect of aerobic exercise on cognitive function was moderated by antidepressant use as well as clinical setting in adults with MDD. Regarding antidepressant use, a small beneficial ES on cognitive function was found when the aerobic exercise group did not use antidepressant medication (NO), although the ES just reached the threshold for statistical significance, this suggests that aerobic exercise intervention may have a positive impact on cognitive function in adults with MDD. In addition, a small-to-moderate benefit on cognitive function was observed when the aerobic exercise group used antidepressant medication (YES). This pattern of results suggests that aerobic exercise combined with antidepressant medication is a more effective intervention for improving cognitive function in adults with MDD. The combination of both interventions may produce a synergistic effect, yielding a better treatment effect. The specific mechanisms behind the observed synergistic effect of antidepressants and aerobic exercise remain unclear; nonetheless, existing evidence supports the idea that both interventions contribute to the promotion of neuroplasticity (Andrade & Rao, 2010; Mellow et al., 2020). The integration of these two interventions might be the reason underlying a greater cognitive effect via the facilitation of neuroplasticity. However, a significant ES did not extend to the aerobic exercise group when only some of the participants used antidepressants (MIXED). The lack of statistical significance in the ES may be due to the use of different comparison/control groups, study design, exercise dosage, and sample size. Considering that only 8.5 % to 64.6 % of participants used aerobic exercise combined with antidepressant across 6 studies, the result requires further investigation, as our results are likely underpowered. For the comparator/control group, the findings suggest that aerobic exercise produced significant effects on cognitive function when compared to an active group or a treatment as usual group, but did not produce significant effects on cognitive function when compared with placebo medication. Our results are partially consistent with the findings of (Ren, Alderman, et al., 2023), which found that exercise had significant effects on executive function when compared with usual medical treatment and placebo medication, but had no significant effects on executive function when compared to an active control. However, our results suggest aerobic exercise may have more beneficial effects on cognition than active comparator/control groups (stretching, occupational or art therapy, non-progressive physical activity, relaxation, resistance training), and treatment as usual groups (antidepressant).
Results from included trials with more than 50 % and less than or equal to 50 % female participants showed that aerobic exercise significantly enhanced cognitive function, implying that aerobic exercise is an effective intervention to improve cognition regardless of sex. For age, the results indicate that aerobic exercise only produces significant benefits on cognition in middle-aged depressed adults (45–64 years), but not in younger depressed (18–44 years old) and older depressed adults (≥ 65 years old). Our findings were partly comparable to a previous review (Ren, Alderman, et al., 2023). However, it is worth noting that there is only one study that examined older adults with MDD. As such, more studies are required for older participants to properly power this question. Lastly, although both groups demonstrated a significant effect, we found preliminary evidence for greater improvements in cognitive function among inpatient relative to outpatient participants following aerobic exercise.
Aerobic exercise may enhance cognitive function in adults with MDD via different underlying mechanisms. First, aerobic exercise has been shown to increase cerebral blood flow (Kleinloog et al., 2019; Tomoto et al., 2021), which plays a critical role in maintaining proper brain function and cognition (Abdelsaid et al., 2016; Cheng et al., 2022). Second, brain-derived neurotrophic factor (BDNF), a protein that plays an important role in the development and survival of nerve cells (Nowak et al., 2015), is closely associated with cognitive functions (White & Castellano, 2008). Decreased BDNF levels in the brain are associated with impaired cognitive function (Canivet et al., 2017). Depressed participants have evidenced lower serum BDNF levels when compared with healthy controls, and have been found to negatively correlate with depression severity (Chiou & Huang, 2017). Aerobic exercise has been shown to increase the production of BDNF (Kim et al., 2022; Salehi et al., 2016), which support the growth and survival of neurons and may enhance cognitive function. In addition, a recent meta-analysis (da Cunha et al., 2023) suggested that exercise interventions (including aerobic exercise) led to a significantly increase in circulating BDNF in adults with MDD when compared with a control group. Lastly, the hippocampus is a complicated region inside the brain that serves as a pivotal component in the process of memory formation and retrieval (Xiu et al., 2023), and it appears to be highly plastic to intervention (Wilckens et al., 2021). Hippocampal atrophy has been associated with cognitive impairments (De Crescenzo et al., 2016), and decreased hippocampal volume in individuals with depression has been documented (Campbell et al., 2004; Sheline et al., 1996). Previous studies have shown that exercise interventions significantly increase hippocampal volume in typically aging humans (Erickson et al., 2011; Ten Brinke et al., 2015; Wilckens et al., 2021). Therefore, increased hippocampal volume after aerobic exercise may enhance cognition in individuals with MDD. Overall, the mechanisms by which aerobic exercise improves cognitive function in adults with MDD are complex. More studies are needed to examine the potential mechanisms that underlie aerobic exercise effects on cognitive function in depressed individuals.
LimitationsThere are several limitations to the present systematic review and meta-analysis. First, the current review only included those studies published in peer-reviewed English-language journals, which may lead to a language bias. Second, the results of the Egger's test suggested a potential publication bias, despite the fact that we used a rigorous selection process and criteria to determine which studies we included; therefore, it is still necessary to interpret the findings of the current meta-analysis with caution, especially given the small number of studies that exist in this field of investigation, which further led to multiple instances of underpowered results. Lastly, only two of the 12 included trials underwent follow-up evaluation on cognitive function in adults with MDD. As a consequence, we did not conduct follow-up analysis, and further randomized trials are needed to determine how long the positive effects of aerobic exercise on cognition may last following intervention.
ConclusionsThe present systematic review and meta-analysis concludes that aerobic exercise is an effective approach to improve cognitive function, as well as the sub-domains of memory and executive function, in adults with MDD. Aerobic exercise may be a promising non-pharmacological treatment for cognitive function that supplements standard, pharmacological treatments in this population. More studies are needed to examine the extent and dosage of exercise intervention, as well as the potential mechanisms that underlie the benefits of aerobic exercise on cognitive function in adults with MDD.